Characterization, Quantification, and Determination of the Toxicity of Iron Oxide Nanoparticles to the Bone Marrow Cells
Abstract
:1. Introduction
2. Results and Discussion
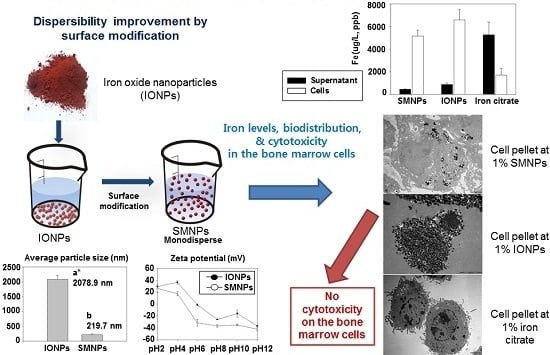
2.1. Preparation of SMNPs (Surface-Modified Iron Oxide Nanoparticles)
2.2. Characterization of IONPs (Iron Oxide Nanoparticles) and SMNPs
2.2.1. Particle Size Distribution

2.2.2. Zeta Potential

2.3. Iron Levels in the Bone Marrow Cells


2.4. Effect of SMNPs, IONPs, and Iron Citrate on the Cytotoxicity of Various Kinds of Eukaryotic Cells

2.5. Effect of SMNPs, IONPs, and Iron Citrate on the Differentiation of Murine Bone Marrow-Derived Dendritic Cells

2.6. Effect of SMNPs, IONPs, and Iron Citrate on the Secretion of Cytokines by BMDCs

3. Experimental Section
3.1. Materials
3.2. Preparation of SMNPs
3.3. Characterization of IONPs and SMNPs
3.3.1. Particle Size Measurements Using Differential Light Scattering
3.3.2. Measurement of Zeta Potential
3.3.3. TEM Measurements
3.4. Cell Lines and Cell Culture
3.5. Determination of Iron Levels
3.6. Generation and Culture of DCs
3.7. Analysis of Cytotoxicity
3.8. Fluorescence-Activated Cell Sorting
3.9. ELISA
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- CDC. Recommendations to Prevent and Control Iron Deficiency in the United States; MMWR: Atlanta, GA, USA, 1998. [Google Scholar]
- Cheney, K.; Gumbiner, C.; Benson, B.; Tenenbein, M. Survival after a severe iron poisoning treated with intermittent infusions of deferoxamine. Clin. Toxicol. 1995, 33, 61–66. [Google Scholar] [CrossRef]
- Aditya, N.P.; Sanghoon Ko, S. Solid lipid nanoparticles (SLNs) delivery vehicles for food bioactives. RSC Adv. 2015, 5, 30902–30911. [Google Scholar] [CrossRef]
- Aditya, N.P.; Shim, M.; Lee, I.; Lee, Y.; Im, M.H.; Ko, S. Curcumin and genistein coloaded nanostructured lipid carriers: In vitro digestion and antiprostate cancer activity. J. Agric. Food Chem. 2013, 61, 1878–1883. [Google Scholar] [CrossRef] [PubMed]
- Aditya, N.P.; Macedo, A.S.; Doktorovova, S.; Souto, E.B.; Kim, S.; Chang, P.-S.; Ko, S. Development and evaluation of lipid nanocarriers for quercetin delivery: A comparative study of solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and lipid nanoemulsions (LNE). LWT Food Sci. Technol. 2014, 59, 115–121. [Google Scholar] [CrossRef]
- Aditya, N.P.; Yang, H.; Kim, S.; Ko, S. Fabrication of amorphous curcumin nanosuspensions using β-lactoglobulin to enhance solubility, stability, and bioavailability. Colloids Surf. B Biointerfaces 2015, 127, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O.; Ha, T.V.A.; Choi, Y.J.; Ko, S. Optimization of homogenization-evaporation process for lycopene nanoemulsion production and its beverage applications. J. Food Sci. 2014, 79, N1604–N1610. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.V.A.; Kim, S.; Choi, Y.; Kwak, H.S.; Lee, S.; Wen, J.; Oey, I.; Ko, S. Antioxidant activity and bioaccessibility of size-different nanoemulsions for lycopene-enriched tomato extract. Food Chem. 2015, 178, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Sozer, N.; Kokini, J.L. Nanotechnology and its applications in the food sector. Trends Biotechnol. 2009, 27, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Chau, C.-F.; Wu, S.-H.; Yen, G.-C. The development of regulations for food nanotechnology. Trends Food Sci. Technol. 2007, 18, 269–280. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Simchi, A.; Imani, M. Recent advances in surface engineering of superparamagnetic iron oxide nanoparticles for biomedical applications. J. Iran. Chem. Soc. 2010, 7, S1–S27. [Google Scholar] [CrossRef]
- Sun, R.; Dittrich, J.; Le-Huu, M.; Mueller, M.M.; Bedke, J.; Kartenbeck, J.; Lehmann, W.D.; Krueger, R.; Bock, M.; Huss, R.; et al. Physical and biological characterization of superparamagnetic iron oxide- and ultrasmall superparamagnetic iron oxide-labeled cells: A comparison. Investig. Radiol. 2005, 40, 504–513. [Google Scholar] [CrossRef]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Laird Forrest, M.; Stroeve, P.; Mahmoudi, M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Hofmann, H.; Rothen-Rutishauser, B.; Petri-Fink, A. Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles. Chem. Rev. 2012, 112, 2323–2338. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Paik, S.-Y.-R.; Ryu, J.; Choi, K.-O.; Kang, T.S.; Lee, J.K.; Song, C.W.; Ko, S. Dynamic light scattering-based method to determine primary particle size of iron oxide nanoparticles in simulated gastrointestinal fluid. Food Chem. 2014, 161, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Wells, S. Surface-modified superparamagnetic nanoparticles for drug delivery: Preparation, characterization, and cytotoxicity studies. NanoBiosci. IEEE Trans. 2004, 3, 66–73. [Google Scholar] [CrossRef]
- Jafari, M.; Xu, W.; Naahidi, S.; Chen, B.; Chen, P. A new amphipathic, amino-acid-pairing (aap) peptide as sirna delivery carrier: Physicochemical characterization and in vitro uptake. J. Phys. Chem. B 2012, 116, 13183–13191. [Google Scholar] [CrossRef] [PubMed]
- Bourlinos, A.B.; Bakandritsos, A.; Georgakilas, V.; Petridis, D. Surface modification of ultrafine magnetic iron oxide particles. Chem. Mater. 2002, 14, 3226–3228. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Wang, C.C.; Yang, W.L.; Deng, Y.H.; Fu, S.K. Encapsulation of nanosized magnetic iron oxide by polyacrylamide via inverse miniemulsion polymerization. J. Magn. Magn. Mater. 2004, 277, 136–143. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, W.S.; Choi, H.-G.; Jang, B.; Lee, K.; Park, J.-H.; Kim, H.-J.; Cho, S.-N.; Shin, S.J. Mycobacterium tuberculosis RpfB drives Th1-type T cell immunity via a TLR4-dependent activation of dendritic cells. J. Leukoc. Biol. 2013, 94, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Marcato, P.D. Nanobiotechnology perspectives. Role of nanotechnology in the food industry: A review. Int. J. Food Sci. Technol. 2013, 48, 1127–1134. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paik, S.-Y.-R.; Kim, J.-S.; Shin, S.J.; Ko, S. Characterization, Quantification, and Determination of the Toxicity of Iron Oxide Nanoparticles to the Bone Marrow Cells. Int. J. Mol. Sci. 2015, 16, 22243-22257. https://doi.org/10.3390/ijms160922243
Paik S-Y-R, Kim J-S, Shin SJ, Ko S. Characterization, Quantification, and Determination of the Toxicity of Iron Oxide Nanoparticles to the Bone Marrow Cells. International Journal of Molecular Sciences. 2015; 16(9):22243-22257. https://doi.org/10.3390/ijms160922243
Chicago/Turabian StylePaik, Sae-Yeol-Rim, Jong-Seok Kim, Sung Jae Shin, and Sanghoon Ko. 2015. "Characterization, Quantification, and Determination of the Toxicity of Iron Oxide Nanoparticles to the Bone Marrow Cells" International Journal of Molecular Sciences 16, no. 9: 22243-22257. https://doi.org/10.3390/ijms160922243