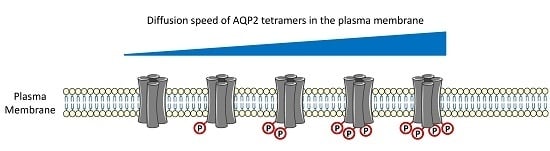
AQP2 Plasma Membrane Diffusion Is Altered by the Degree of AQP2-S256 Phosphorylation
Abstract
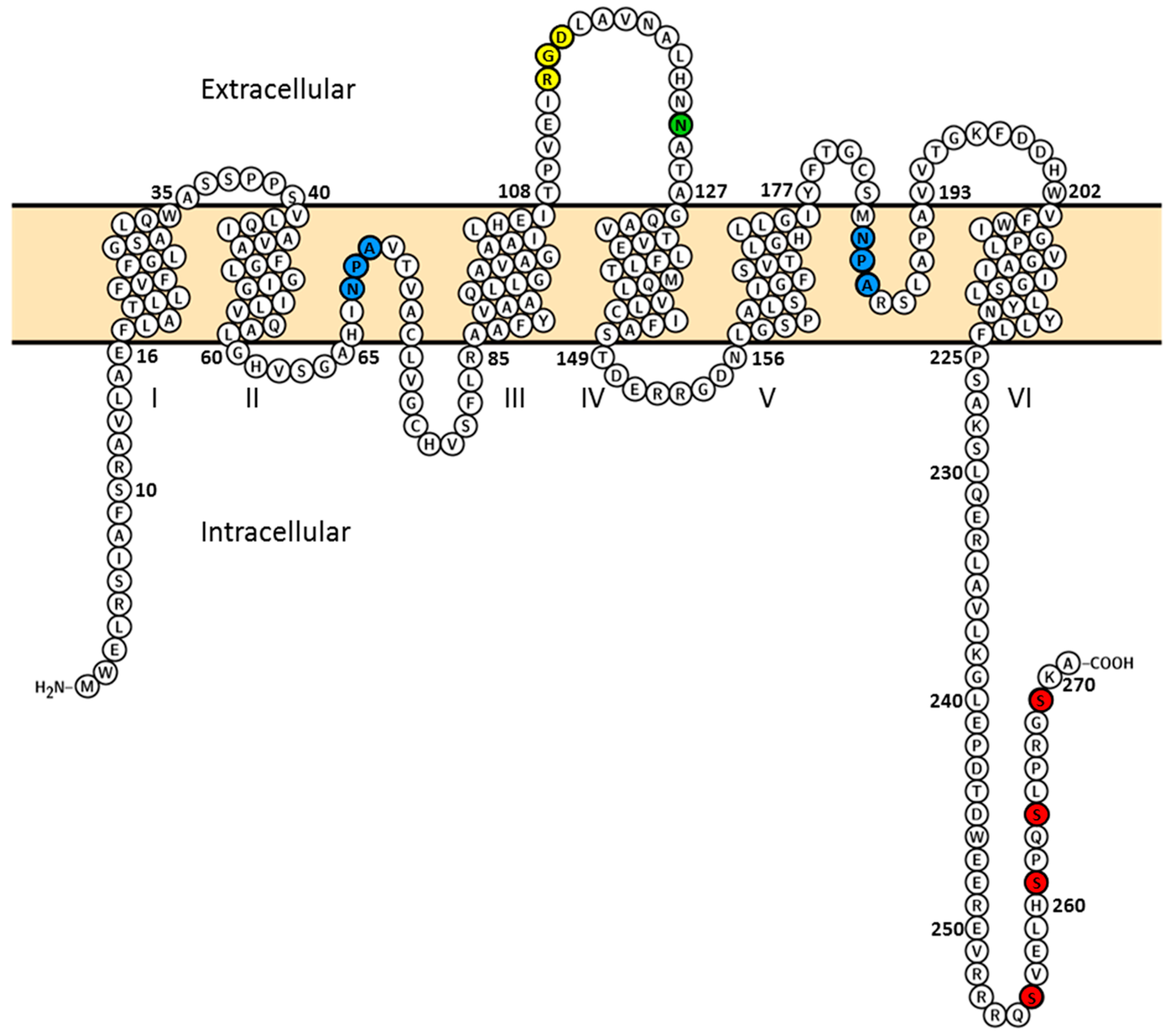
:1. Introduction
2. Results and Discussion
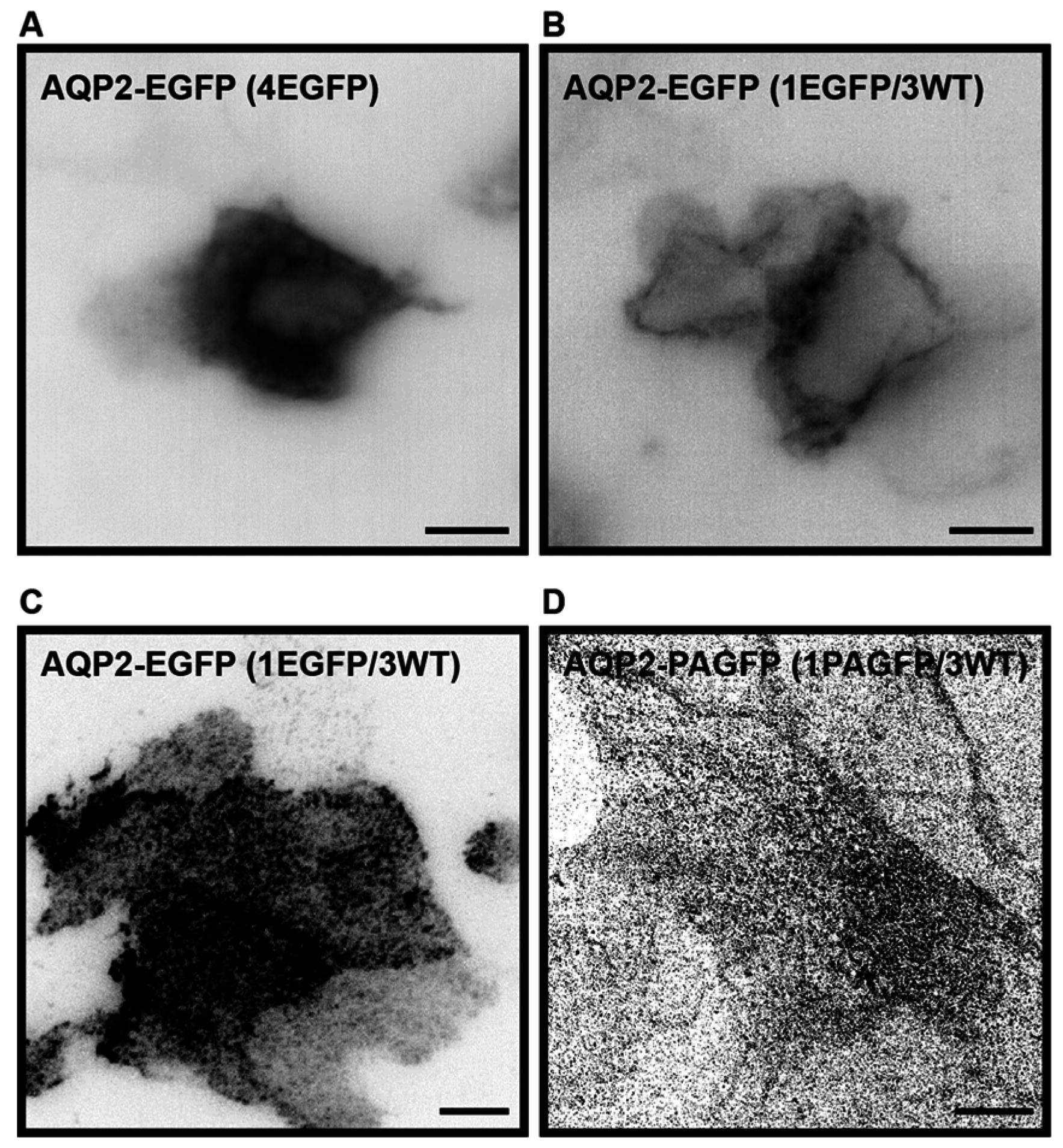
2.1. Establishment of a Cell Culture System for Measurements of Average Diffusion Coefficients of Aquaporin-2
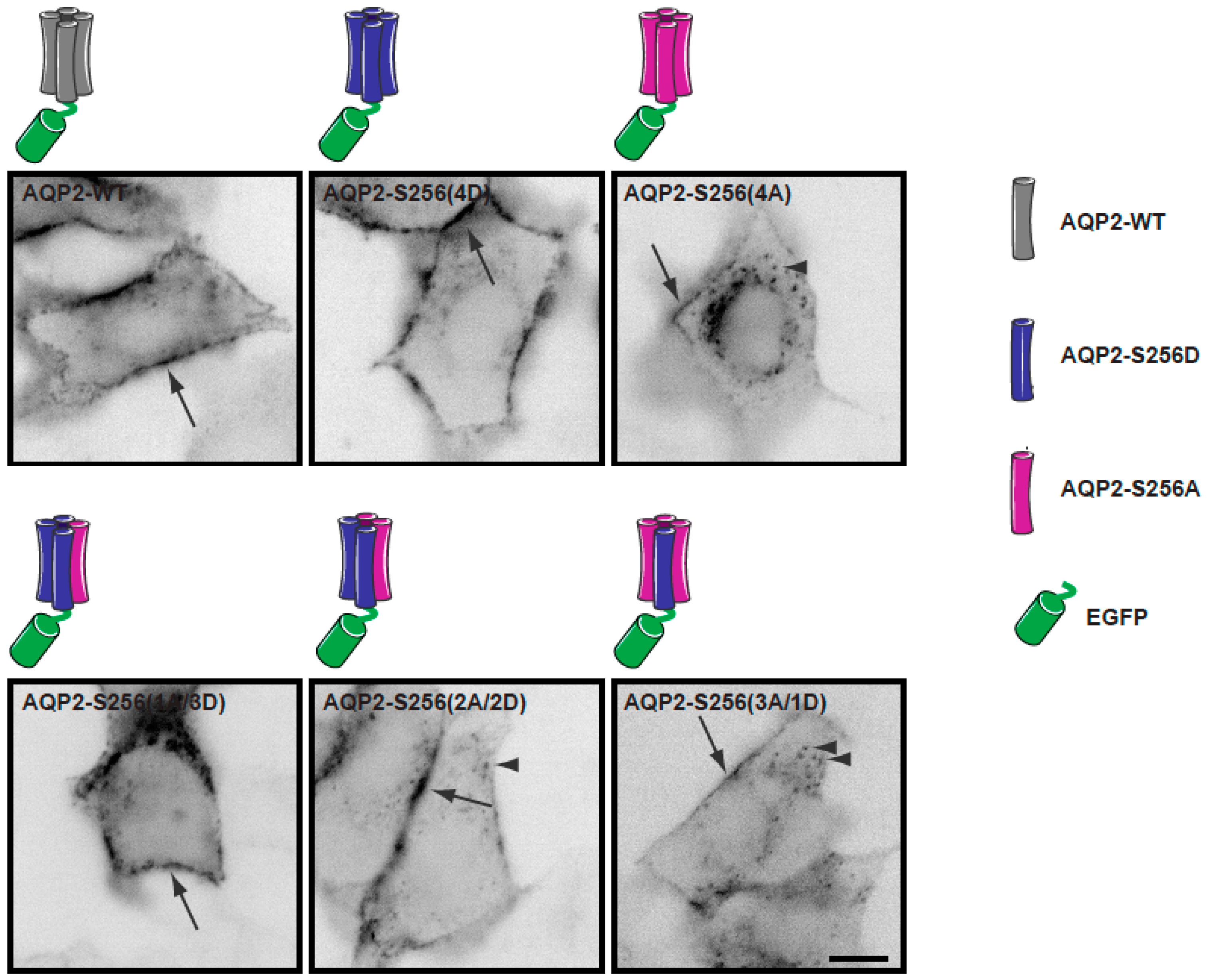
2.2. Plasma Membrane Diffusion of AQP2 Changes with the Degree of S256 Phosphorylation
3. Materials and Methods
3.1. Plasmids, Bacterial Growth Conditions, and Cell Culture
3.2. Microscopy
3.3. Sample Preparation for Photoactivated Localization Microscopy (PALM) Imaging
3.4. Photoactivatable Localization Microscopy (PALM) Imaging
3.5. Measurement of Diffusion Coefficients by k-Space Image Correlation Spectroscopy (kICS) Analysis
3.6. Statistics
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Raina, S.; Preston, G.M.; Guggino, W.B.; Agre, P. Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J. Biol. Chem. 1995, 270, 1908–1912. [Google Scholar] [PubMed]
- Nejsum, L.N.; Kwon, T.H.; Jensen, U.B.; Fumagalli, O.; Frokiaer, J.; Krane, C.M.; Menon, A.G.; King, L.S.; Agre, P.C.; Nielsen, S. Functional requirement of aquaporin-5 in plasma membranes of sweat glands. Proc. Natl. Acad. Sci. USA 2002, 99, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Frigeri, A.; Gropper, M.A.; Umenishi, F.; Kawashima, M.; Brown, D.; Verkman, A.S. Localization of MIWC and GLIP water channel homologs in neuromuscular epithelial and glandular tissues. J. Cell Sci. 1995, 108, 2993–3002. [Google Scholar] [PubMed]
- Nejsum, L.N. The renal plumbing system: Aquaporin water channels. Cell. Mol. Life Sci. 2005, 62, 1692–1706. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; DiGiovanni, S.R.; Christensen, E.I.; Knepper, M.A.; Harris, H.W. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc. Natl. Acad. Sci. USA 1993, 90, 11663–11667. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Song, Y.; Yang, B.; Gillespie, A.; Carlson, E.J.; Epstein, C.J.; Verkman, A.S. Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc. Natl. Acad. Sci. USA 2000, 97, 4386–4391. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Yang, B.; Gillespie, A.; Carlson, E.J.; Epstein, C.J.; Verkman, A.S. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J. Clin. Investig. 1997, 100, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.L.; Ma, T.; Yang, B.; Knepper, M.A.; Verkman, A.S. Fourfold reduction of water permeability in inner medullary collecting duct of aquaporin-4 knockout mice. Am. J. Physiol. 1998, 274, C549–C554. [Google Scholar] [PubMed]
- Christensen, B.M.; Wang, W.; Frokiaer, J.; Nielsen, S. Axial heterogeneity in basolateral AQP2 localization in rat kidney: Effect of vasopressin. Am. J. Physiol. Ren. Physiol. 2003, 284, F701–F717. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, M.; Fushimi, K.; Terada, Y.; Bai, L.; Marumo, F.; Sasaki, S. cAMP-dependent phosphorylation stimulates water permeability of aquaporin-collecting duct water channel protein expressed in Xenophus oocytes. J. Biol. Chem. 1995, 270, 10384–10387. [Google Scholar] [PubMed]
- Marples, D.; Knepper, M.A.; Christensen, E.I.; Nielsen, S. Redistribution of aquaporin-2 water channels induced by vasopressin in rat kidney inner medullary collecting duct. Am. J. Physiol. 1995, 269, C655–C664. [Google Scholar] [PubMed]
- Yamamoto, T.; Sasaki, S.; Fushimi, K.; Ishibashi, K.; Yaoita, E.; Kawasaki, K.; Marumo, F.; Kihara, I. Vasopressin increases AQP-CD water channel in apical membrane of collecting duct cells in Brattleboro rats. Am. J. Physiol. 1995, 268, C1546–C1551. [Google Scholar] [PubMed]
- Omasits, U.; Ahrens, C.H.; Müller, S.; Wollscheid, B. Protter: Interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 2014, 30, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Fushimi, K.; Sasaki, S.; Marumo, F. Structure of aquaporin-2 vasopressin water channel. J. Biol. Chem. 1996, 271, 5171–5176. [Google Scholar] [PubMed]
- Tamma, G.; Lasorsa, D.; Ranieri, M.; Mastrofrancesco, L.; Valenti, G.; Svelto, M. Integrin signaling modulates AQP2 trafficking via Arg-Gly-Asp (RGD) motif. Cell. Physiol. Biochem. 2011, 27, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Rice, W.; Gu, Z.; Li, J.; Huang, J.; Brenner, M.B.; van Hoek, A.; Xiong, J.; Gundersen, G.G.; Norman, J.C.; et al. Aquaporin 2 promotes cell migration and epithelial morphogenesis. J. Am. Soc. Nephrol. 2012, 23, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Hasler, U.; Nunes, P.; Bouley, R.; Lu, H.A. Phosphorylation events and the modulation of aquaporin 2 cell surface expression. Curr. Opin. Nephrol. Hypertens. 2008, 17, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Fenton, R.A.; Moeller, H.B.; Hoffert, J.D.; Yu, M.J.; Nielsen, S.; Knepper, M.A. Acute regulation of aquaporin-2 phosphorylation at Ser-264 by vasopressin. Proc. Natl. Acad. Sci. USA 2008, 105, 3134–3139. [Google Scholar] [CrossRef] [PubMed]
- Hoffert, J.D.; Fenton, R.A.; Moeller, H.B.; Simons, B.; Tchapyjnikov, D.; McDill, B.W.; Yu, M.J.; Pisitkun, T.; Chen, F.; Knepper, M.A. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J. Biol. Chem. 2008, 283, 24617–24627. [Google Scholar] [CrossRef] [PubMed]
- Nejsum, L.N.; Zelenina, M.; Aperia, A.; Frokiaer, J.; Nielsen, S. Bidirectional regulation of AQP2 trafficking and recycling: Involvement of AQP2-S256 phosphorylation. Am. J. Physiol. Ren. Physiol. 2005, 288, F930–F938. [Google Scholar] [CrossRef] [PubMed]
- Van Balkom, B.W.; Savelkoul, P.J.; Markovich, D.; Hofman, E.; Nielsen, S.; van der Sluijs, P.; Deen, P.M. The role of putative phosphorylation sites in the targeting and shuttling of the aquaporin-2 water channel. J. Biol. Chem. 2002, 277, 41473–41479. [Google Scholar] [CrossRef] [PubMed]
- Moeller, H.B.; Praetorius, J.; Rutzler, M.R.; Fenton, R.A. Phosphorylation of aquaporin-2 regulates its endocytosis and protein–protein interactions. Proc. Natl. Acad. Sci. USA 2010, 107, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Deen, P.M.; Croes, H.; van, A.R.A.; Ginsel, L.A.; van, O.C.H. Water channels encoded by mutant aquaporin-2 genes in nephrogenic diabetes insipidus are impaired in their cellular routing. J. Clin. Investig. 1995, 95, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, W.; Seibold, A.; Antaramian, A.; Lonergan, M.; Arthus, M.F.; Hendy, G.N.; Birnbaumer, M.; Bichet, D.G. Molecular identification of the gene responsible for congenital nephrogenic diabetes insipidus. Nature 1992, 359, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Nejsum, L.N.; Christensen, T.M.; Robben, J.H.; Milligan, G.; Deen, P.M.; Bichet, D.G.; Levin, K. Novel mutation in the AVPR2 gene in a Danish male with nephrogenic diabetes insipidus caused by ER retention and subsequent lysosomal degradation of the mutant receptor. NDT Plus 2011, 4, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Metzenberg, A.; Das, S.; Jing, B.; Gitschier, J. Mutations in the V2 vasopressin receptor gene are associated with X-linked nephrogenic diabetes insipidus. Nat. Genet. 1992, 2, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Van den Ouweland, A.M.; Dreesen, J.C.; Verdijk, M.; Knoers, N.V.; Monnens, L.A.; Rocchi, M.; van Oost, B.A. Mutations in the vasopressin type 2 receptor gene (AVPR2) associated with nephrogenic diabetes insipidus. Nat. Genet. 1992, 2, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.H.; Frokiaer, J.; Fernandez-Llama, P.; Knepper, M.A.; Nielsen, S. Reduced abundance of aquaporins in rats with bilateral ischemia-induced acute renal failure: Prevention by α-MSH. Am. J. Physiol. 1999, 277, F413–F427. [Google Scholar] [PubMed]
- Kwon, T.H.; Frokiaer, J.; Knepper, M.A.; Nielsen, S. Reduced AQP1, -2, and -3 levels in kidneys of rats with CRF induced by surgical reduction in renal mass. Am. J. Physiol. 1998, 275, F724–F741. [Google Scholar] [PubMed]
- Xu, D.L.; Martin, P.Y.; Ohara, M.; St. John, J.; Pattison, T.; Meng, X.; Morris, K.; Kim, J.K.; Schrier, R.W. Upregulation of aquaporin-2 water channel expression in chronic heart failure rat. J. Clin. Investig. 1997, 99, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Katsura, T.; Gustafson, C.E.; Ausiello, D.A.; Brown, D. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am. J. Physiol. 1997, 272, F817–F822. [Google Scholar] [PubMed]
- Fushimi, K.; Sasaki, S.; Marumo, F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J. Biol. Chem. 1997, 272, 14800–14804. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Sun, T.X.; Bouley, R.; Blackburn, K.; McLaughlin, M.; Brown, D. Inhibition of endocytosis causes phosphorylation (S256)-independent plasma membrane accumulation of AQP2. Am. J. Physiol. Ren. Physiol. 2004, 286, F233–F243. [Google Scholar] [CrossRef] [PubMed]
- Rice, W.L.; Zhang, Y.; Chen, Y.; Matsuzaki, T.; Brown, D.; Lu, H.A. Differential, phosphorylation dependent trafficking of AQP2 in LLC-PK1 cells. PLoS ONE 2012, 7, e32843. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.M.; Zelenina, M.; Aperia, A.; Nielsen, S. Localization and regulation of PKA-phosphorylated AQP2 in response to V2-receptor agonist/antagonist treatment. Am. J. Physiol. Ren. Physiol. 2000, 278, F29–F42. [Google Scholar]
- Lu, H.J.; Matsuzaki, T.; Bouley, R.; Hasler, U.; Qin, Q.H.; Brown, D. The phosphorylation state of serine 256 is dominant over that of serine 261 in the regulation of AQP2 trafficking in renal epithelial cells. Am. J. Physiol. Ren. Physiol. 2008, 295, F290–F294. [Google Scholar] [CrossRef] [PubMed]
- Moeller, H.B.; Aroankins, T.S.; Slengerik-Hansen, J.; Pisitkun, T.; Fenton, R.A. Phosphorylation and ubiquitylation are opposing processes that regulate endocytosis of the water channel aquaporin-2. J. Cell Sci. 2014, 127, 3174–3183. [Google Scholar] [CrossRef] [PubMed]
- Saxton, M.J.; Jacobson, K. Single-particle tracking: Applications to membrane dynamics. Annu. Rev. Biophys. Biomol. Struct. 1997, 26, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Jaskolski, F.; Henley, J.M. Synaptic receptor trafficking: The lateral point of view. Neuroscience 2009, 158, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Kolin, D.L.; Ronis, D.; Wiseman, P.W. k-Space image correlation spectroscopy: A method for accurate transport measurements independent of fluorophore photophysics. Biophys. J. 2006, 91, 3061–3075. [Google Scholar] [CrossRef] [PubMed]
- Marlar, S.; Arnspang, E.C.; Koffman, J.S.; Locke, E.M.; Christensen, B.M.; Nejsum, L.N. Elevated cAMP increases aquaporin-3 plasma membrane diffusion. Am. J. Physiol. Cell Physiol. 2014, 306, C598–C606. [Google Scholar] [CrossRef] [PubMed]
- Marlar, S.; Arnspang, E.C.; Pedersen, G.A.; Koffman, J.S.; Nejsum, L.N. Measuring localization and diffusion coefficients of basolateral proteins in lateral versus basal membranes using functionalized substrates and kICS analysis. Biochim. Biophys. Acta 2014, 1838, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Arnspang, E.C.; Koffman, J.S.; Marlar, S.; Wiseman, P.W.; Nejsum, L.N. Easy measurement of diffusion coefficients of EGFP-tagged plasma membrane proteins using k-Space Image Correlation Spectroscopy. J. Vis. Exp. 2014, 87, e51074. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, C.E.; Levine, S.; Katsura, T.; McLaughlin, M.; Aleixo, M.D.; Tamarappoo, B.K.; Verkman, A.S.; Brown, D. Vasopressin regulated trafficking of a green fluorescent protein-aquaporin 2 chimera in LLC-PK1 cells. Histochem. Cell Biol. 1998, 110, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Kamsteeg, E.J.; Heijnen, I.; van Os, C.H.; Deen, P.M. The subcellular localization of an aquaporin-2 tetramer depends on the stoichiometry of phosphorylated and nonphosphorylated monomers. J. Cell Biol. 2000, 151, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; van Engelenburg, S.B.; Lippincott-Schwartz, J. Superresolution imaging of biological systems using photoactivated localization microscopy. Chem. Rev. 2014, 114, 3189–3202. [Google Scholar] [CrossRef] [PubMed]
- Patterson, G.H.; Lippincott-Schwartz, J. A photoactivatable GFP for selective photolabeling of proteins and cells. Science 2002, 297, 1873–1877. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.J.; Yeaman, C. Protein trafficking in the exocytic pathway of polarized epithelial cells. Trends Cell Biol. 2001, 11, 483–486. [Google Scholar] [CrossRef]
- Nejsum, L.N.; Nelson, W.J. A molecular mechanism directly linking E-cadherin adhesion to initiation of epithelial cell surface polarity. J. Cell Biol. 2007, 178, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Umenishi, F.; Verbavatz, J.M.; Verkman, A.S. cAMP regulated membrane diffusion of a green fluorescent protein-aquaporin 2 chimera. Biophys. J. 2000, 78, 1024–1035. [Google Scholar] [CrossRef]
- Katsura, T.; Verbavatz, J.M.; Farinas, J.; Ma, T.; Ausiello, D.A.; Verkman, A.S.; Brown, D. Constitutive and regulated membrane expression of aquaporin 1 and aquaporin 2 water channels in stably transfected LLC-PK1 epithelial cells. Proc. Natl. Acad. Sci. USA 1995, 92, 7212–7216. [Google Scholar] [CrossRef] [PubMed]
- Gaush, C.R.; Hard, W.L.; Smith, T.F. Characterization of an established line of canine kidney cells (MDCK). Exp. Biol. Med. 1966, 122, 931–935. [Google Scholar] [CrossRef]
- Louvard, D. Apical Membrane Aminopeptidase Appears at Site of Cell–Cell Contact in Cultured Kidney Epithelial-Cells. Proc. Natl. Acad. Sci. USA 1980, 77, 4132–4136. [Google Scholar] [CrossRef] [PubMed]
- Betzig, E.; Patterson, G.H.; Sougrat, R.; Lindwasser, O.W.; Olenych, S.; Bonifacino, J.S.; Davidson, M.W.; Lippincott-Schwartz, J.; Hess, H.F. Imaging intracellular fluorescent proteins at nanometer resolution. Science 2006, 313, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnspang, E.C.; Login, F.H.; Koffman, J.S.; Sengupta, P.; Nejsum, L.N. AQP2 Plasma Membrane Diffusion Is Altered by the Degree of AQP2-S256 Phosphorylation. Int. J. Mol. Sci. 2016, 17, 1804. https://doi.org/10.3390/ijms17111804
Arnspang EC, Login FH, Koffman JS, Sengupta P, Nejsum LN. AQP2 Plasma Membrane Diffusion Is Altered by the Degree of AQP2-S256 Phosphorylation. International Journal of Molecular Sciences. 2016; 17(11):1804. https://doi.org/10.3390/ijms17111804
Chicago/Turabian StyleArnspang, Eva C., Frédéric H. Login, Jennifer S. Koffman, Prabuddha Sengupta, and Lene N. Nejsum. 2016. "AQP2 Plasma Membrane Diffusion Is Altered by the Degree of AQP2-S256 Phosphorylation" International Journal of Molecular Sciences 17, no. 11: 1804. https://doi.org/10.3390/ijms17111804
APA StyleArnspang, E. C., Login, F. H., Koffman, J. S., Sengupta, P., & Nejsum, L. N. (2016). AQP2 Plasma Membrane Diffusion Is Altered by the Degree of AQP2-S256 Phosphorylation. International Journal of Molecular Sciences, 17(11), 1804. https://doi.org/10.3390/ijms17111804