Assessment of Photodynamic Inactivation against Periodontal Bacteria Mediated by a Chitosan Hydrogel in a 3D Gingival Model
Abstract
:1. Introduction
2. Results
2.1. Mucoadhesion Studies of Chitosan Hydrogel
2.2. Effect of Formulations and Postincubation Time on Photodynamic Inactivation (PDI) against Staphylococcus aureus (S. aureus) Biofilms in 3D Gingival Model
2.3. Effect of Illumination Site and Non-Illumination Site on PDI against S. aureus Biofilm
2.4. Adjustment of PDI Treatment
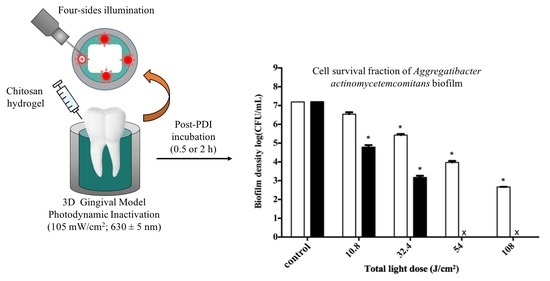
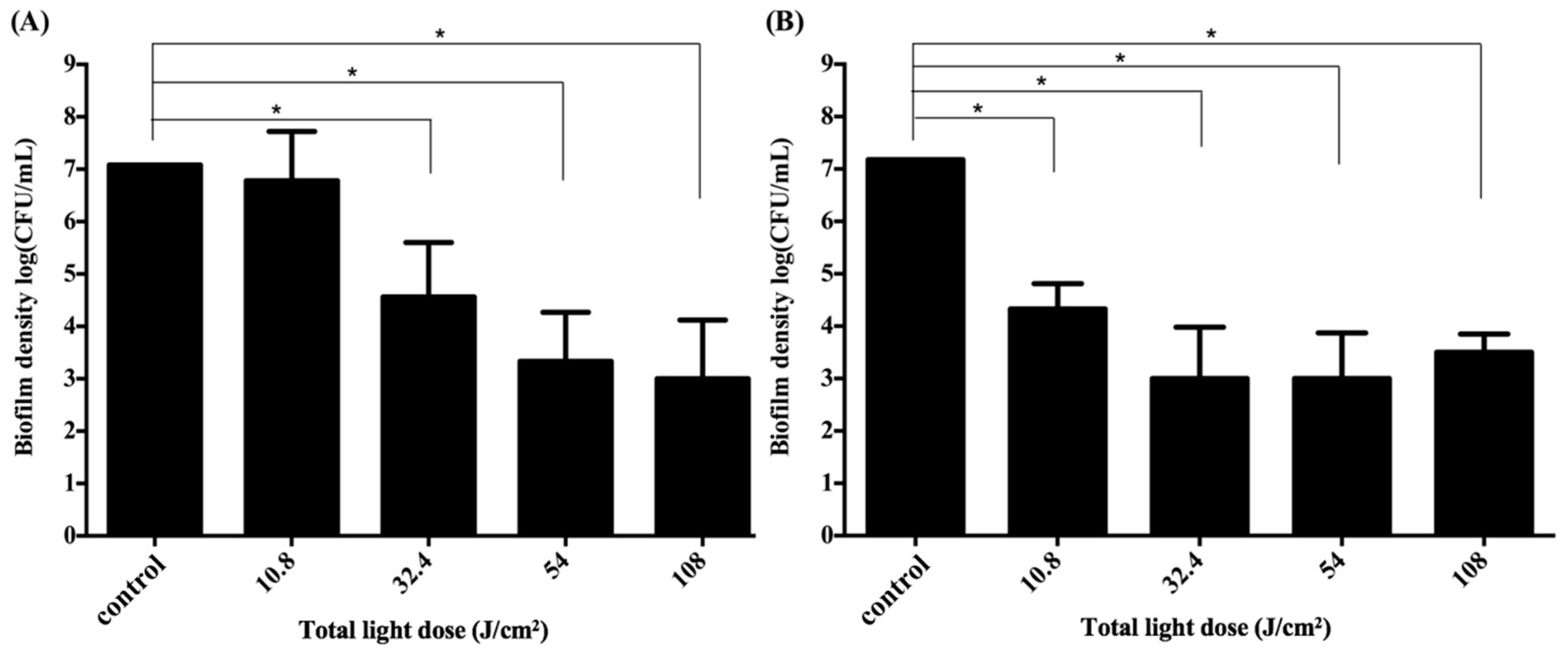
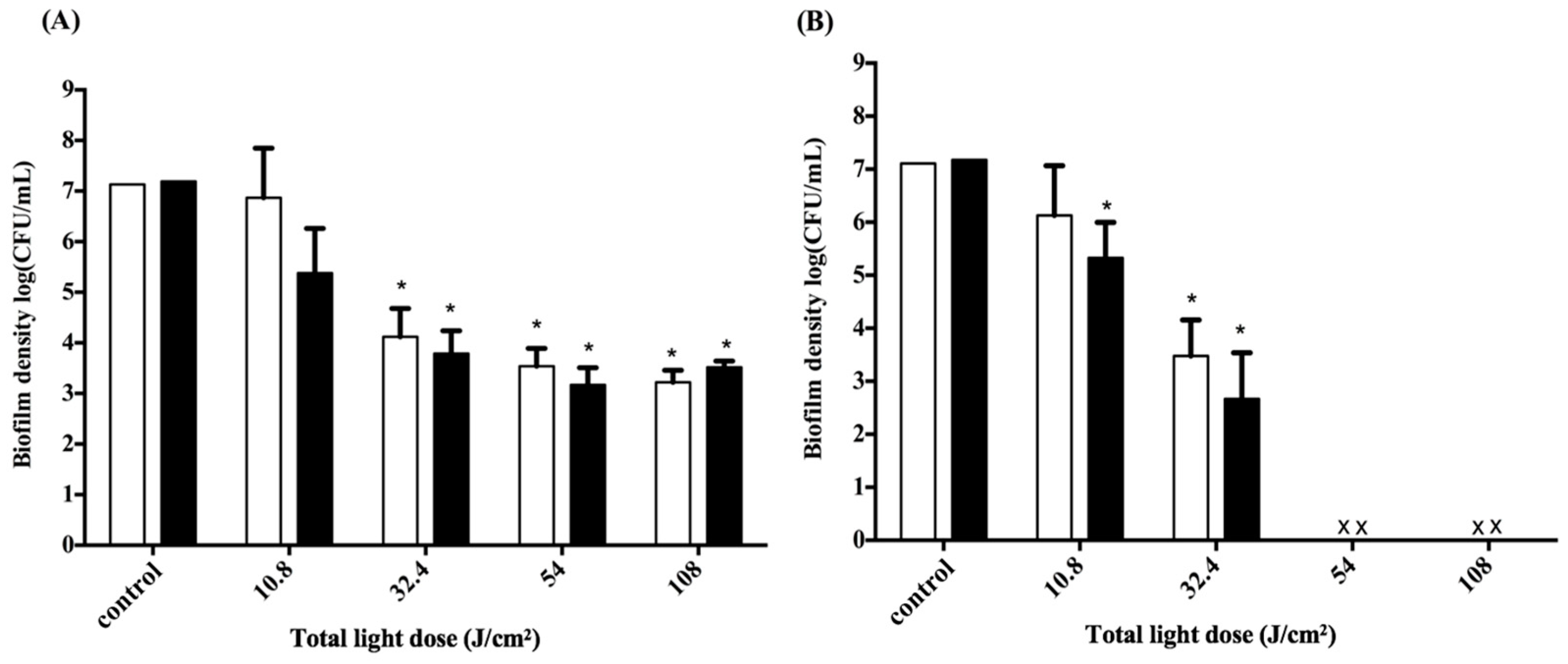
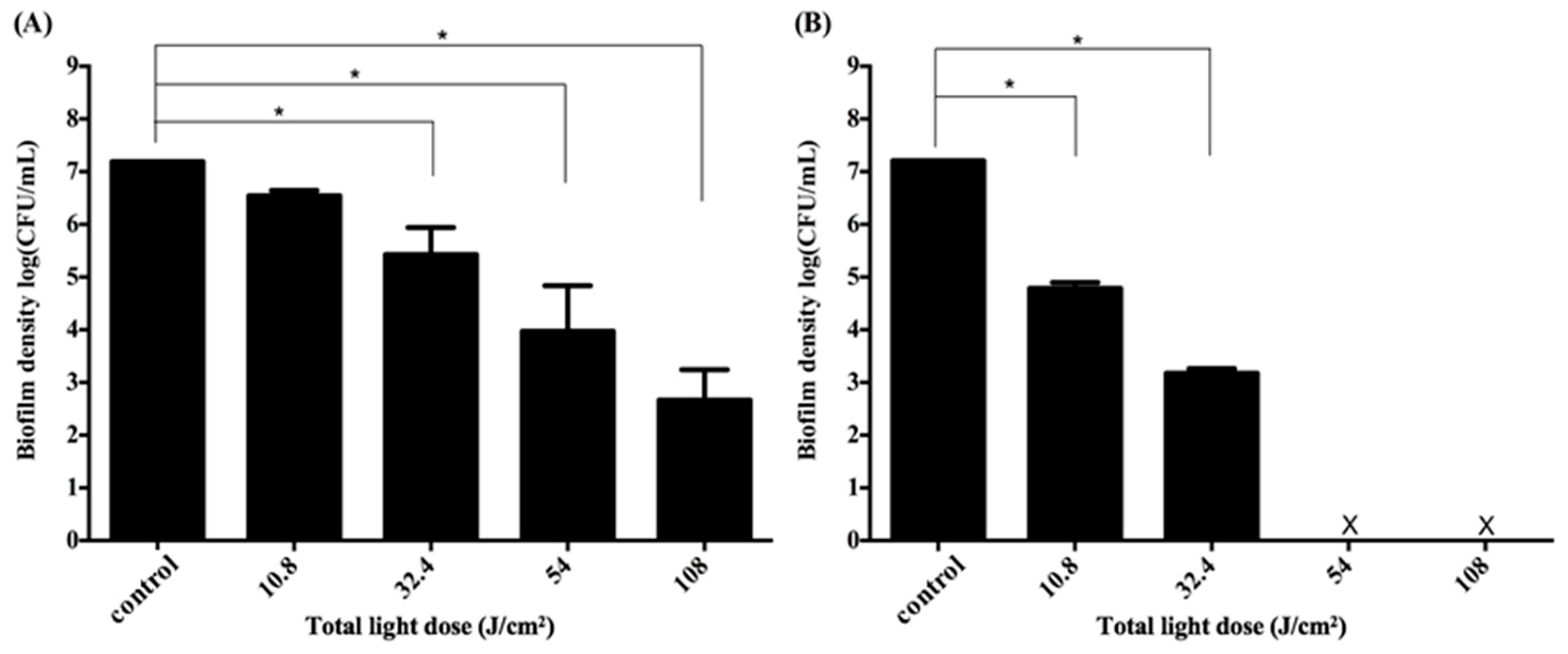
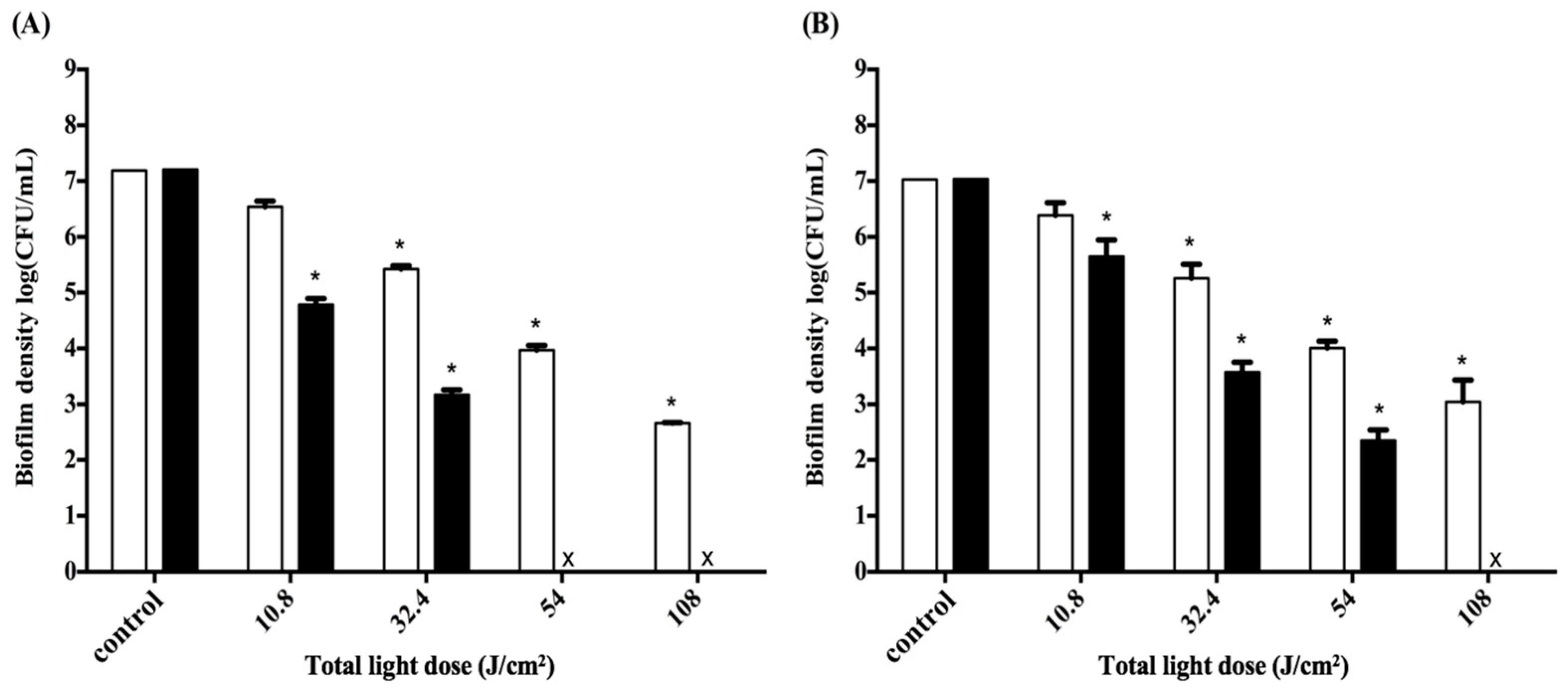
2.5. Impact of the Light Dose on PDI against Periodontal Strain Biofilms
3. Discussion
4. Experimental Section
4.1. Preparation of Chitosan Hydrogel and Mucoadhesion Studies
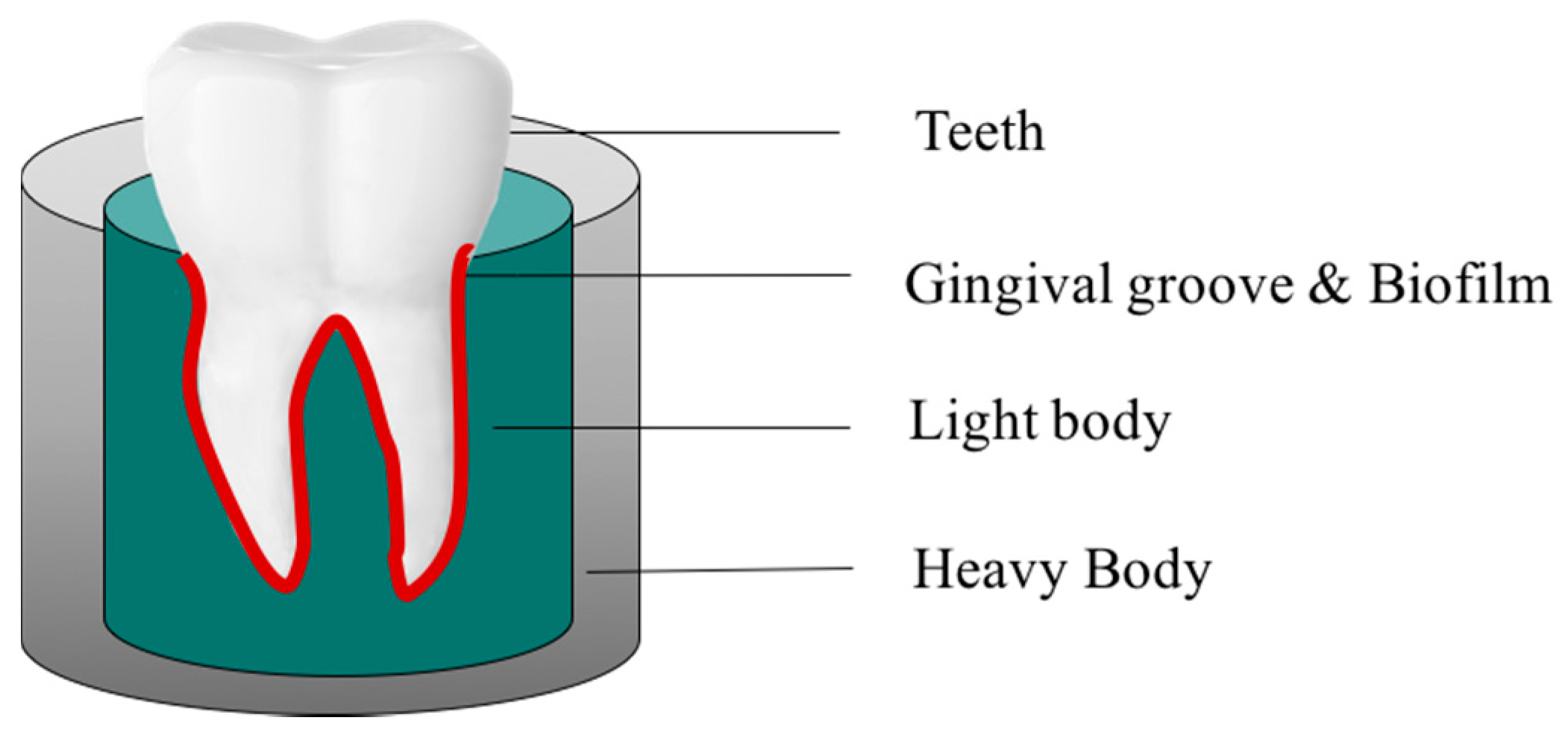
4.2. Development of the 3D Gingival Model
4.3. Biofilm Formation and EPS Staining in Biofilms
4.4. Chitosan Hydrogel-Mediated PDI in Biofilm Cells
4.5. Statistics Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jepsen, K.; Jepsen, S. Antibiotics/antimicrobials: Systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontology 2000 2016, 71, 82–112. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Perez, A.; Moya-Villaescusa, M.J. Periodontal disease affecting tooth furcations. A review of the treatments available. Med. Oral Patol. Oral Cir. Bucal 2009, 14, 554–557. [Google Scholar] [CrossRef]
- Oberoi, S.S.; Dhingra, C.; Sharma, G.; Sardana, D. Antibiotics in dental practice: How justified are we. Int. Dent. J. 2015, 65, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Veloo, A.C.; Seme, K.; Raangs, E.; Rurenga, P.; Singadji, Z.; Wekema-Mulder, G.; van Winkelhoff, A.J. Antibiotic susceptibility profiles of oral pathogens. Int. J. Antimicrob. Agents 2012, 40, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Voos, A.C.; Kranz, S.; Tonndorf-Martini, S.; Voelpel, A.; Sigusch, H.; Staudte, H.; Albrecht, V.; Sigusch, B.W. Photodynamic antimicrobial effect of safranine O on an Ex Vivo periodontal biofilm. Lasers Surg. Med. 2014, 46, 235–243. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Kishen, A. Advanced noninvasive light-activated disinfection: Assessment of cytotoxicity on fibroblast versus antimicrobial activity against enterococcus faecalis. J. Endod. 2007, 33, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z. A review of progress in clinical photodynamic therapy. Technol. Cancer Res. Treat. 2005, 4, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Amann, C.; Yaroslavsky, A.N.; Title, C.; Smink, D.; Zarins, B.; Kochevar, I.E.; Redmond, R.W. Photochemical repair of achilles tendon rupture in a rat model. J. Surg. Res. 2005, 124, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Bliss, J.M.; Bigelow, C.E.; Foster, T.H.; Haidaris, C.G. Susceptibility of candida species to photodynamic effects of photofrin. Antimicrob. Agents Chemother. 2004, 48, 2000–2006. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Mia, N. Sensitisation of candida albicans to killing by low-power laser light. J. Oral Pathol. Med. 1993, 22, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Fontana, C.R.; Abernethy, A.D.; Som, S.; Ruggiero, K.; Doucette, S.; Marcantonio, R.C.; Boussios, C.I.; Kent, R.; Goodson, J.M.; Tanner, A.C.; et al. The antibacterial effect of photodynamic therapy in dental plaque-derived biofilms. J. Periodontal Res. 2009, 44, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Foschi, F.; Fontana, C.R.; Ruggiero, K.; Riahi, R.; Vera, A.; Doukas, A.G.; Pagonis, T.C.; Kent, R.; Stashenko, P.P.; Soukos, N.S. Photodynamic inactivation of enterococcus faecalis in dental root canals In Vitro. Lasers Surg. Med. 2007, 39, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M.; Crossley, K.B. Photosensitizing agents—Circumventing resistance and breaking down biofilms: A review. Int. Biodeterior. Biodegrad. 2004, 53, 119–126. [Google Scholar] [CrossRef]
- Giuliani, F.; Martinelli, M.; Cocchi, A.; Arbia, D.; Fantetti, L.; Roncucci, G. In Vitro resistance selection studies of RLP068/Cl, a new Zn(II) phthalocyanine suitable for antimicrobial photodynamic therapy. Antimicrob. Agents Chemother. 2010, 54, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Soukos, N.S.; Som, S.; Abernethy, A.D.; Ruggiero, K.; Dunham, J.; Lee, C.; Doukas, A.G.; Goodson, J.M. Phototargeting oral black-pigmented bacteria. Antimicrob. Agents Chemother. 2005, 49, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D.; Moter, A.; Devine, D.A. Dental plaque biofilms: Communities, conflict and control. Periodontology 2000 2011, 55, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Auschill, T.M.; Hellwig, E.; Sculean, A.; Hein, N.; Arweiler, N.B. Impact of the intraoral location on the rate of biofilm growth. Clin. Oral Investig. 2004, 8, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.J., Jr. Supragingival and subgingival plaque: Paradigm of biofilms. Compend. Contin. Educ. Dent. 2010, 31, 104–106. [Google Scholar] [PubMed]
- Varum, K.M.; Anthonsen, M.W.; Grasdalen, H.; Smidsrod, O. 13C-Nmr studies of the acetylation sequences in partially N-deacetylated chitins (chitosans). Carbohydr. Res. 1991, 217, 19–27. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Gurny, R. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 35–52. [Google Scholar] [CrossRef]
- Chien, H.F.; Chen, C.P.; Chen, Y.C.; Chang, P.H.; Tsai, T.; Chen, C.T. The use of chitosan to enhance photodynamic inactivation against candida albicans and its drug-resistant clinical isolates. Int. J. Mol. Sci. 2013, 14, 7445–7456. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, R.M.; Abdel-Mohsen, A.M.; Hrdina, R.; Burgert, L.; Fohlerova, Z.; Pavlinak, D.; Sayed, O.N.; Jancar, J. Wound dressing based on chitosan/hyaluronan/nonwoven fabrics: Preparation, characterization and medical applications. Int. J. Biol. Macromol. 2016, 89, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Bigucci, F.; Abruzzo, A.; Vitali, B.; Saladini, B.; Cerchiara, T.; Gallucci, M.C.; Luppi, B. Vaginal inserts based on chitosan and carboxymethylcellulose complexes for local delivery of chlorhexidine: Preparation, characterization and antimicrobial activity. Int. J. Pharm. 2015, 478, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Singh, D.; Kothiyal, N.C.; Saini, A.K.; Singh, V.P.; Pathania, D. Synthesis of chitosan-g-poly(acrylamide)/ZnS nanocomposite for controlled drug delivery and antimicrobial activity. Int. J. Biol. Macromol. 2015, 74, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.F.; Facchi, S.P.; Follmann, H.D.; Pereira, A.G.; Rubira, A.F.; Muniz, E.C. Antimicrobial activity of chitosan derivatives containing N-quaternized moieties in its backbone: A review. Int. J. Mol. Sci. 2014, 15, 20800–20832. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.; Chien, H.F.; Wang, T.H.; Huang, C.T.; Ker, Y.B.; Chen, C.T. Chitosan augments photodynamic inactivation of gram-positive and gram-negative bacteria. Antimicrob. Agents Chemother. 2011, 55, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Hsieh, C.M.; Tsai, T.; Yang, J.C.; Chen, C.T. Optimization and evaluation of a chitosan/hydroxypropyl methylcellulose hydrogel containing toluidine blue O for antimicrobial photodynamic inactivation. Int. J. Mol. Sci. 2015, 16, 20859–20872. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.; Ward, J.M.; Ready, D. Aggregatibacter (Actinobacillus) actinomycetemcomitans: A triple A* periodontopathogen? Periodontology 2000 2010, 54, 78–105. [Google Scholar] [CrossRef] [PubMed]
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas gingivalis: Major periodontopathic pathogen overview. J. Immunol. Res. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Rolim, J.P.; de-Melo, M.A.; Guedes, S.F.; Albuquerque-Filho, F.B.; de Souza, J.R.; Nogueira, N.A.; Zanin, I.C.; Rodrigues, L.K. The antimicrobial activity of photodynamic therapy against streptococcus mutans using different photosensitizers. J. Photochem. Photobiol. B 2012, 106, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Zanin, I.C.; Goncalves, R.B.; Junior, A.B.; Hope, C.K.; Pratten, J. Susceptibility of streptococcus mutans biofilms to photodynamic therapy: An In Vitro study. J. Antimicrob. Chemother. 2005, 56, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Araujo, N.C.; Fontana, C.R.; Bagnato, V.S.; Gerbi, M.E. Photodynamic antimicrobial therapy of curcumin in biofilms and carious dentine. Lasers Med. Sci. 2014, 29, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, S.; Koshi, E.; Philip, K.; Mohan, A. Antimicrobial photodynamic therapy: An overview. J. Indian Soc. Periodontol. 2011, 15, 323–327. [Google Scholar] [PubMed]
- Tahmassebi, J.F.; Drogkari, E.; Wood, S.R. A study of the control of oral plaque biofilms via antibacterial photodynamic therapy. Eur. Arch. Paediatr. Dent. 2015, 16, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Soukos, N.S.; Goodson, J.M. Photodynamic therapy in the control of oral biofilms. Periodontology 2000 2011, 55, 143–166. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.; Guggenheim, B.; Schmidlin, P.R. Efficacy of gasiform ozone and photodynamic therapy on a multispecies oral biofilm In Vitro. Eur. J. Oral Sci. 2007, 115, 77–80. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, L.M.; Calixto, G.M.; Chorilli, M.; Giusti, J.S.; Bagnato, V.S.; Soukos, N.S.; Amiji, M.M.; Fontana, C.R. Polymeric nanoparticle-based photodynamic therapy for chronic periodontitis In Vivo. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Luan, X.; Bi, L.; He, G.; Bai, X.; Zhou, C.; Zhang, Z. Toluidine blue-mediated photoinactivation of periodontal pathogens from supragingival plaques. Lasers Med. Sci. 2008, 23, 49–54. [Google Scholar] [CrossRef] [PubMed]
- O‘Neill, J.F.; Hope, C.K.; Wilson, M. Oral bacteria in multi-species biofilms can be killed by red light in the presence of toluidine blue. Lasers Surg. Med. 2002, 31, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Tabenski, L.; Buchalla, W.; Maisch, T. Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Bansal, K.; Rawat, M.K.; Jain, A.; Rajput, A.; Chaturvedi, T.P.; Singh, S. Development of satranidazole mucoadhesive gel for the treatment of periodontitis. AAPS PharmSciTech 2009, 10, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.; Silva, S.; Tavaria, F.; Pintado, M. Antimicrobial and antibiofilm activity of chitosan on the oral pathogen candida albicans. Pathogens 2014, 3, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Lv, H.; Yang, B.; Chen, X.; Sun, H. Physical properties, antioxidant and antimicrobial activity of chitosan films containing carvacrol and pomegranate peel extract. Molecules 2015, 20, 11034–11045. [Google Scholar] [CrossRef] [PubMed]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.M.; Silva, S.; Pina, C.; Tavaria, F.K.; Pintado, M.M. Evaluation and insights into chitosan antimicrobial activity against anaerobic oral pathogens. Anaerobe 2012, 18, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Sarasam, A.R.; Brown, P.; Khajotia, S.S.; Dmytryk, J.J.; Madihally, S.V. Antibacterial activity of chitosan-based matrices on oral pathogens. J. Mater. Sci. Mater. Med. 2008, 19, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Needleman, I.G.; Smales, F.C.; Martin, G.P. An investigation of bioadhesion for periodontal and oral mucosal drug delivery. J. Clin. Periodontol. 1997, 24, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Khanvilkar, K.H.; Huang, Y.; Moore, A.D. Influence of hydroxypropyl methylcellulose mixture, apparent viscosity, and tablet hardness on drug release using a 23 full factorial design. Drug Dev. Ind. Pharm. 2002, 28, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.; Irwin, C.R.; Woolfson, A.D.; Djokic, J.; Adams, V. Physicochemical characterization and preliminary In Vivo efficacy of bioadhesive, semisolid formulations containing flurbiprofen for the treatment of gingivitis. J. Pharm. Sci. 1999, 88, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Pitts, B.; Willse, A.; McFeters, G.A.; Hamilton, M.A.; Zelver, N.; Stewart, P.S. A repeatable laboratory method for testing the efficacy of biocides against toilet bowl biofilms. J. Appl. Microbiol. 2001, 91, 110–117. [Google Scholar] [CrossRef] [PubMed]


| Formulation | HPMC a (%, w/w) | Chitosan (%, w/w) | TBO (μM) | Mucoadhesive Strength b (N) ±SD |
|---|---|---|---|---|
| TBO | 0 | 0 | 20 | 0.77 ± 0.04 |
| Mixture of TBO and chitosan | 0 | 0.25 | 20 | 1.45 ± 0.07 |
| F-1 | 0.25 | 0.25 | 20 | 5.13 ± 0.09 |
| F-2 | 0.5 | 0.25 | 20 | 7.23 ± 0.34 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, P.-C.; Hsieh, C.-M.; Chen, C.-P.; Tsai, T.; Chen, C.-T. Assessment of Photodynamic Inactivation against Periodontal Bacteria Mediated by a Chitosan Hydrogel in a 3D Gingival Model. Int. J. Mol. Sci. 2016, 17, 1821. https://doi.org/10.3390/ijms17111821
Peng P-C, Hsieh C-M, Chen C-P, Tsai T, Chen C-T. Assessment of Photodynamic Inactivation against Periodontal Bacteria Mediated by a Chitosan Hydrogel in a 3D Gingival Model. International Journal of Molecular Sciences. 2016; 17(11):1821. https://doi.org/10.3390/ijms17111821
Chicago/Turabian StylePeng, Po-Chun, Chien-Ming Hsieh, Chueh-Pin Chen, Tsuimin Tsai, and Chin-Tin Chen. 2016. "Assessment of Photodynamic Inactivation against Periodontal Bacteria Mediated by a Chitosan Hydrogel in a 3D Gingival Model" International Journal of Molecular Sciences 17, no. 11: 1821. https://doi.org/10.3390/ijms17111821
APA StylePeng, P.-C., Hsieh, C.-M., Chen, C.-P., Tsai, T., & Chen, C.-T. (2016). Assessment of Photodynamic Inactivation against Periodontal Bacteria Mediated by a Chitosan Hydrogel in a 3D Gingival Model. International Journal of Molecular Sciences, 17(11), 1821. https://doi.org/10.3390/ijms17111821