The Effect of Everolimus in an In Vitro Model of Triple Negative Breast Cancer and Osteoclasts
Abstract
:1. Introduction
2. Results
2.1. Osteoclastogenesis Model Induced by SCP2
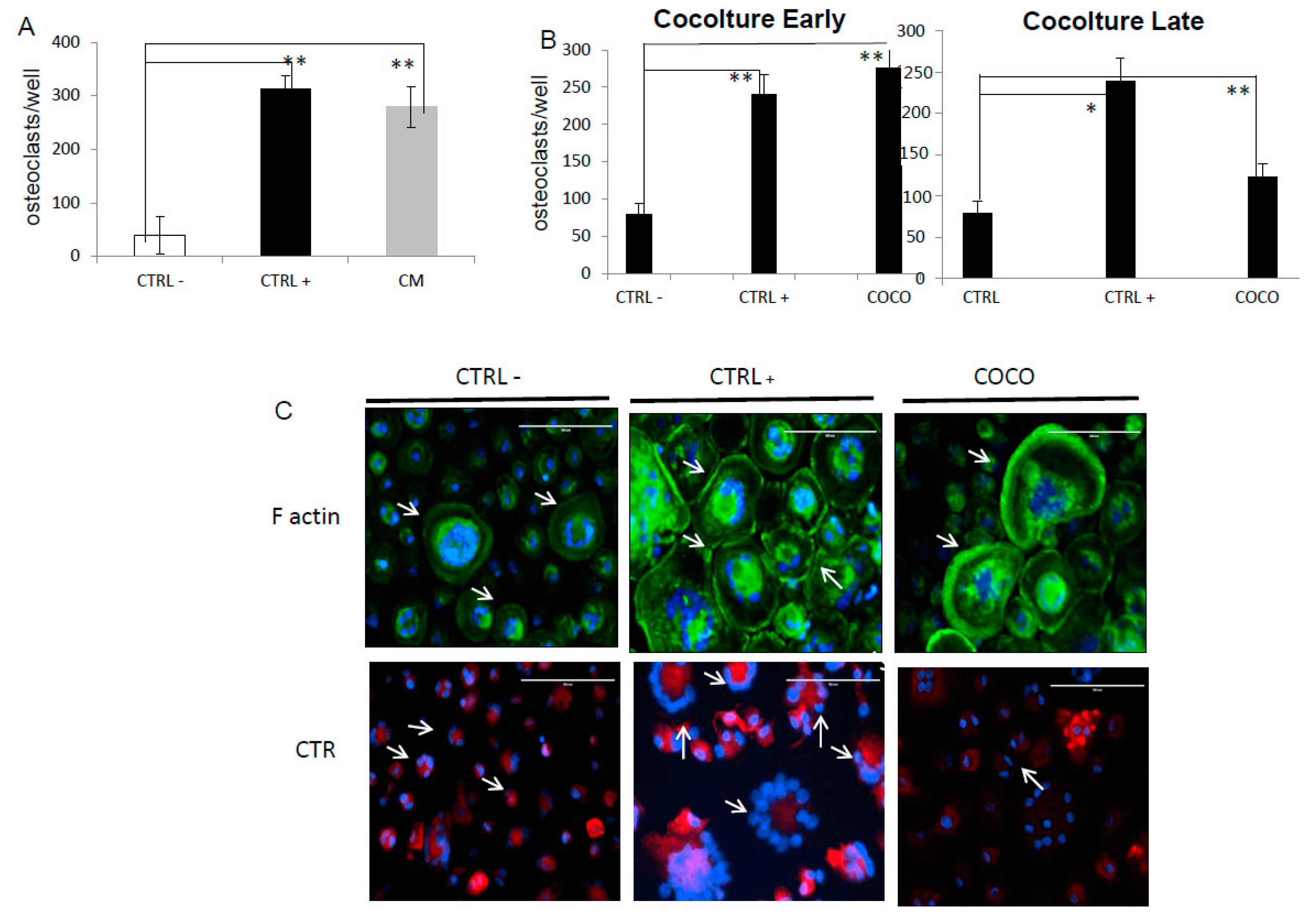
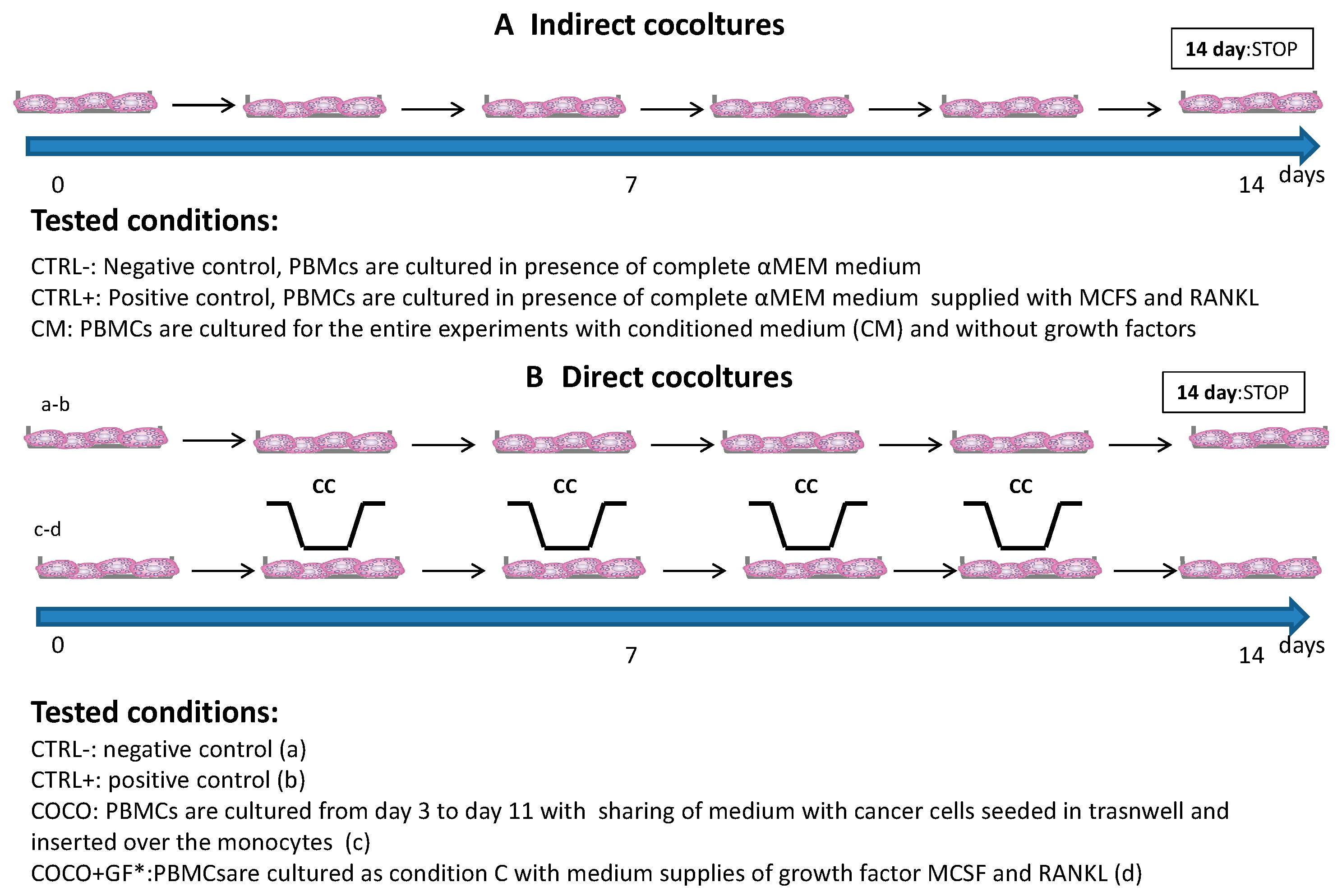
2.1.1. Indirect Co-Cultures
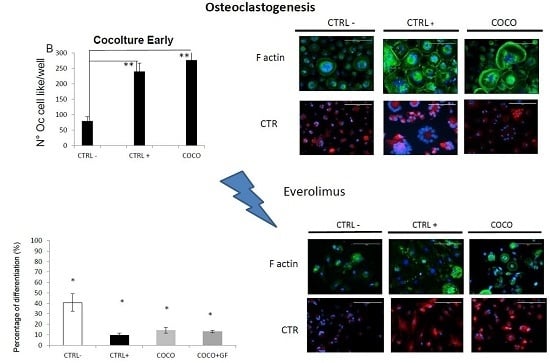
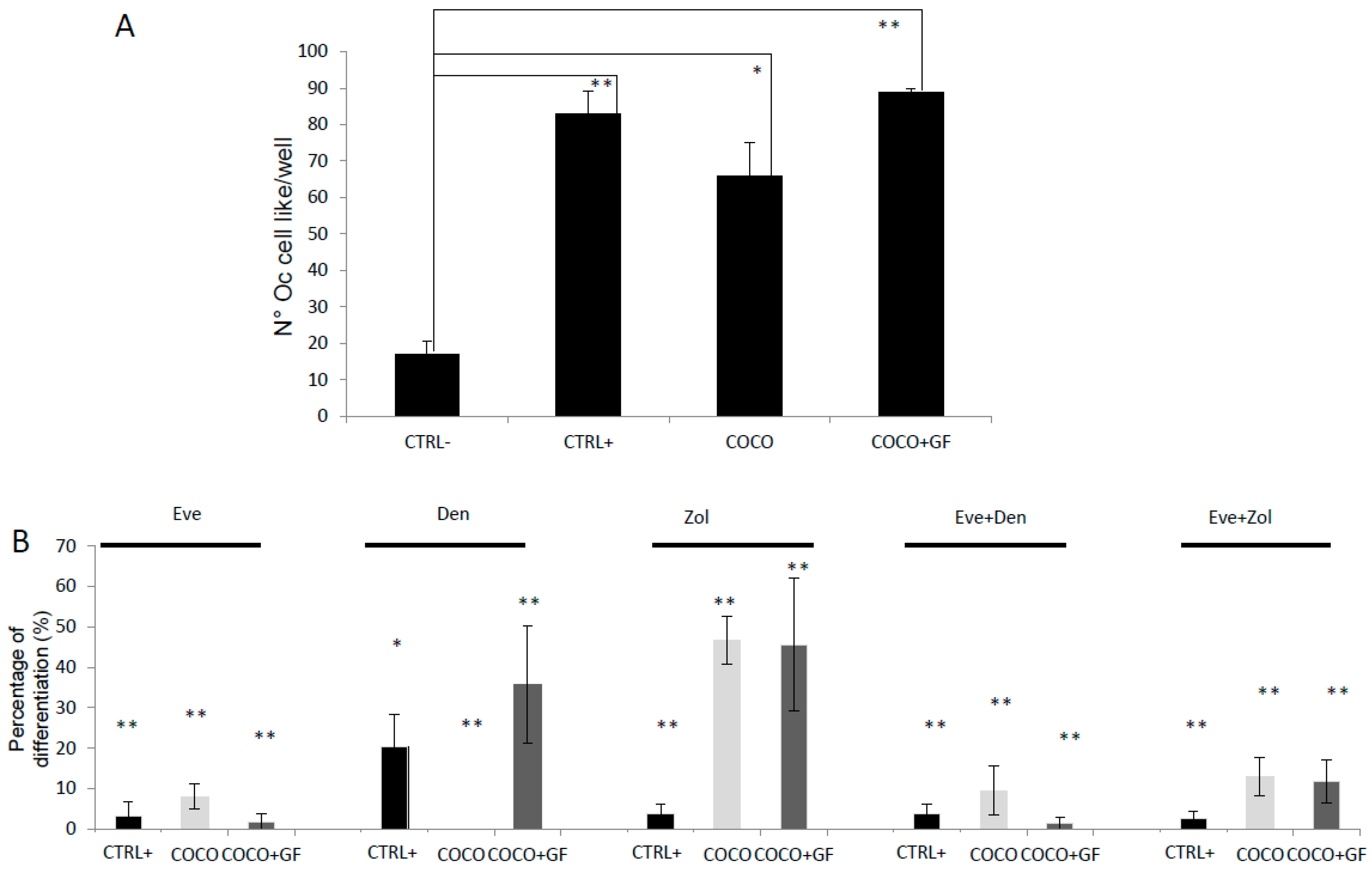
2.1.2. SCP2-PBMCs Direct Co-Culture (COCO)
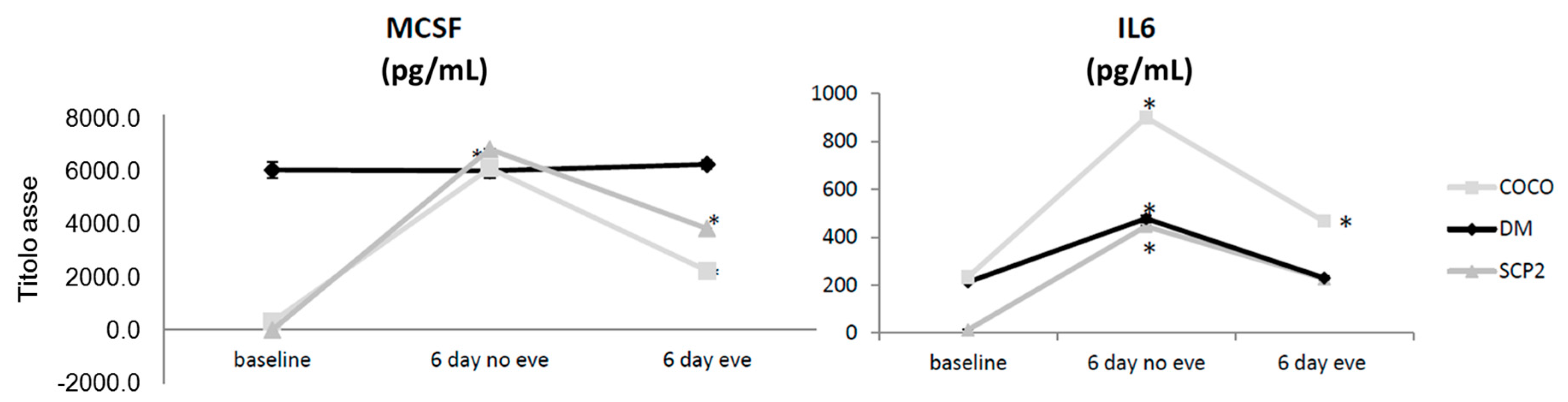
2.1.3. Soluble Mediator Profile during Osteoclastogenesis
2.1.4. Eve Effect on Early and Late Osteoclastogenesis in an Indirect Co-Culture Model System
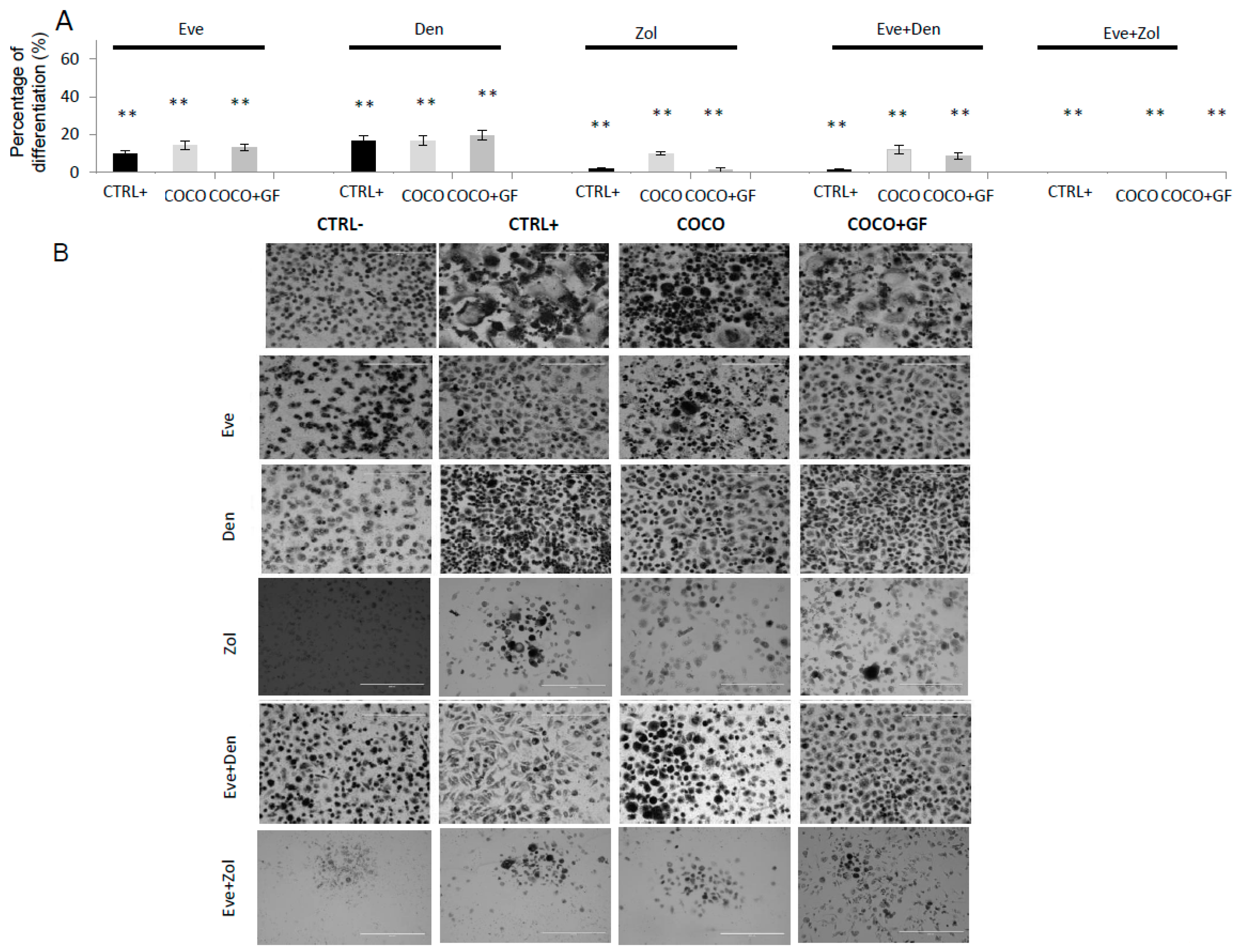
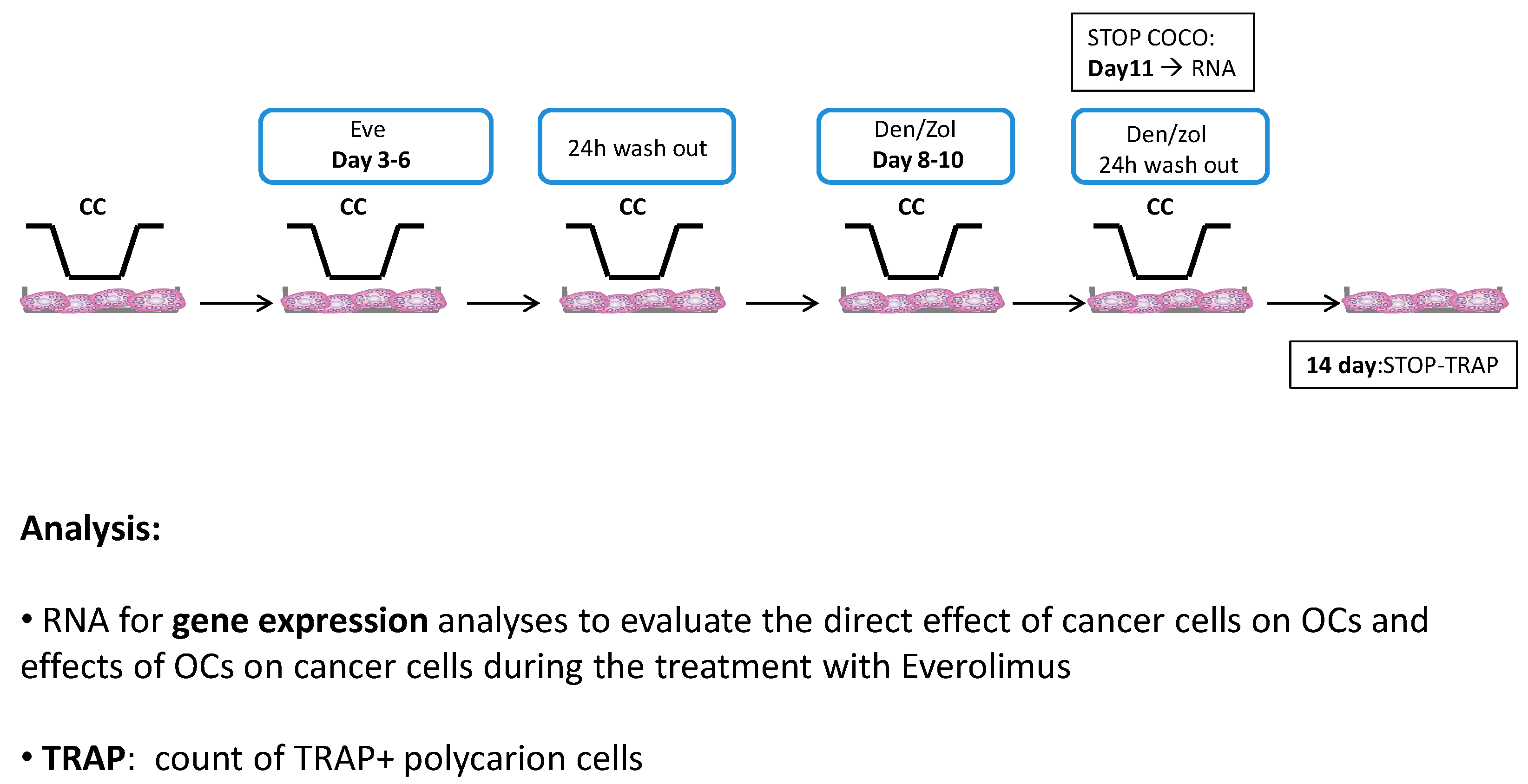
2.1.5. Eve Effect on Osteoclastogenesis in the Direct COCO System
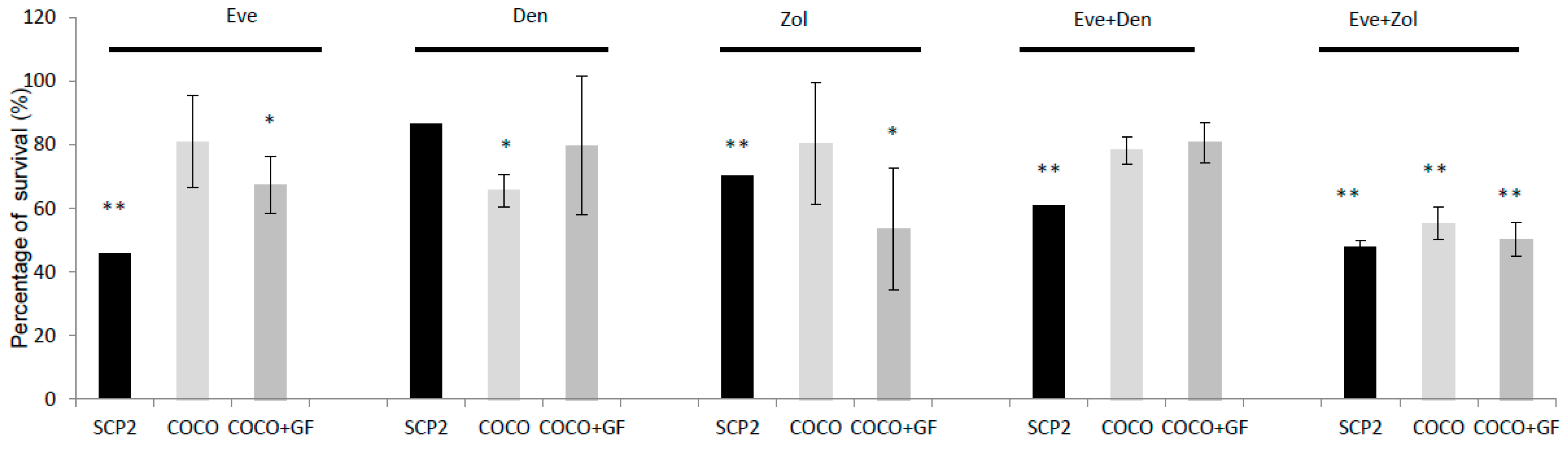
2.1.6. Inhibition of Osteoclastogenesis Induced by Bone-Targeted Therapy
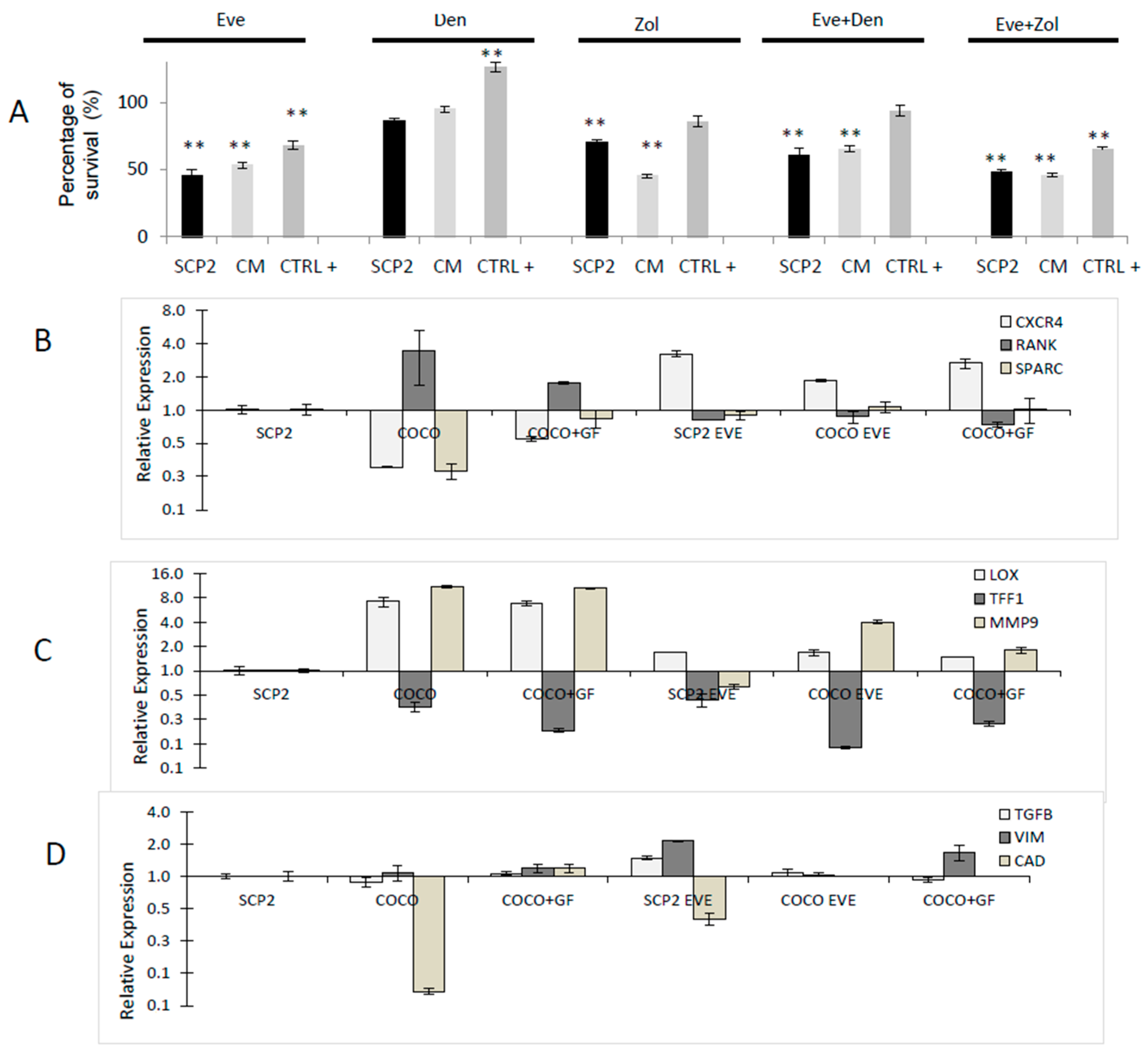
2.1.7. Drug Effect on CCs
2.1.8. Drug Effects on CC Gene Expression
2.1.9. Drug Effects on Soluble Mediator Profile
2.2. Osteoclastogenesis Model Induced by MCF7
2.2.1. Osteoclast Direct COCO
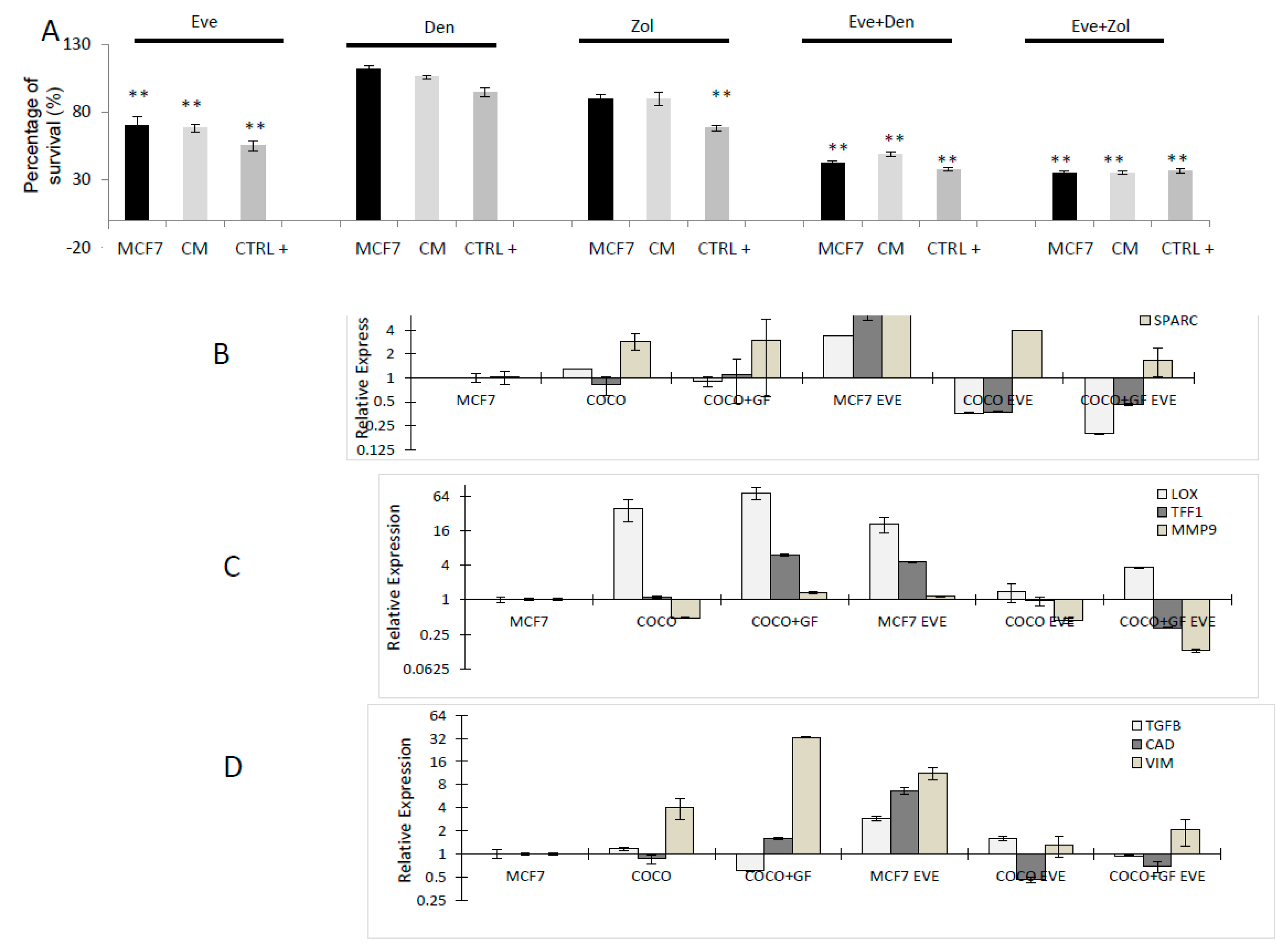
2.2.2. Drugs Effect on Osteoclastogenesis Induced by MCF7
2.2.3. Drug Effects on CC Survival
2.2.4. Drug Effects on CC Gene Expression
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Osteoclastogenesis Assay
4.3. Osteoclast Quantification
4.4. Phalloidin and CTR Staining
4.5. Drugs
4.6. Drug Exposure and Dose Selection
4.7. Inhibition of Osteoclastogenesis
4.8. Drug Effects Evaluation on CCs
4.9. Gene Expression Analyses
4.10. MCSF, RANKL, IL-6 and ICAM1 Evaluation
4.11. Western Blot
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Ibrahim, T.; Mercatali, L.; Amadori, D. A new emergency in oncology: Bone metastases in breast cancer patients. Oncol. Lett. 2013, 6, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Rubens, R.D. The clinical course of bone metastases from breast cancer. Br. J. Cancer 1987, 55, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–647. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M.A.; Lida, N.; Roberts, E.W.; Sangaletti, S.; Wong, M.H.; Yull, F.E.; Coussens, L.M.; DeClerck, Y.A. Tumor microenvironment complexity: Emerging roles in cancer therapy. Cancer Res. 2012, 72, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Roodman, G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Patel, L.R.; Camacho, D.F.; Shiozawa, Y.; Pienta, K.J.; Taichman, R.S. Mechanisms of cancer cell metastasis to the bone: A multistep process. Future Oncol. 2011, 7, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, L.A.; Fournier, P.G.; Chirgwin, J.M.; Guise, T.A. Molecular biology of bone metastasis. Mol. Cancer Ther. 2007, 6, 2609–2617. [Google Scholar] [CrossRef] [PubMed]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Sosnoski, D.M.; Mastro, A.M. Breast cancer metastasis to the bone: Mechanisms of bone loss. Breast Cancer Res. 2010, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Easton, J.B.; Houghton, P.J. mTOR and cancer therapy. Oncogene 2006, 25, 6436–6446. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; di Martino, M.T.; Morelli, E.; Leotta, M.; Rizzo, A.; Grimaldi, A.; Misso, G.; Tassone, P.; Caraglia, M. Molecular targets for the treatment of multiple myeloma. Curr. Cancer Drug Targets 2012, 12, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Faggiano, A.; Ramundo, V.; Dicitore, A.; Castiglioni, S.; Borghi, M.O.; Severino, R.; Ferolla, P.; Crinò, L.; Abbruzzese, A.; Sperlongano, P.; et al. Eve is an active agent in medullary thyroid cancer: A clinical and in vitro study. J. Cell. Mol. Med. 2012, 7, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Campone, M.; Piccart, M.; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, KI.; Lebrun, F.; Beck, J.; et al. Eve in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Gnant, M.; Baselga, J.; Rugo, H.S.; Noguchi, S.; Burris, H.A.; Piccart, M.; Hortobagyi, G.N.; Eakle, J.; Mukai, H.; Iwata, H.; et al. Effect of eve on bone marker levels and progressive disease in bone in BOLERO-2. J. Natl. Cancer. Inst. 2013, 105, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Bertoldo, F.; Silvestris, F.; Ibrahim, T.; Cognetti, F.; Generali, D.; Ripamonti, C.I.; Amadori, D.; Colleoni, M.A.; Conte, P.; del Mastro, L.; et al. Targeting bone metastatic cancer: Role of the mTOR pathway. Biochim. Biophys. Acta 2014, 1845, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.C.; Novik, Y.; Stein, S.; Volm, M.; Meyers, M.; Smith, J.; Omene, C.; Speyer, J.; Schneider, R.; Jhaveri, K.; et al. Phase 2 trial of eve and carboplatin combination in patients with triple negative metastatic breast cancer. Breast Cancer Res. 2014, 16, R32. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed]
- Liverani, C.; Mercatali, L.; Spadazzi, C.; La Manna, F.; de Vita, A.; Riva, N.; Calpona, S.; Ricci, M.; Bongiovanni, A.; Gunelli, E.; et al. CSF-1 blockade impairs breast cancer osteoclastogenic potential in co-culture systems. Bone 2014, 66, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Siegel, P.M.; Shu, W.; Drobnjak, M.; Kakonen, S.M.; Cordón-Cardo, C.; Guise, T.A.; Massagué, J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003, 3, 537–549. [Google Scholar] [CrossRef]
- Maggiani, F.; Forsyth, R.; Hogendoorn, P.C.; Krenacs, T.; Athanasou, N.A. The immunophenotype of osteoclasts and macrophage polykaryons. J. Clin. Pathol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.J. Regulation of the differentiation and function of osteoclasts. J. Pathol. 2000, 192, 4–13. [Google Scholar] [CrossRef]
- Faust, J.; Lacey, D.L.; Hunt, P.; Burgess, T.L.; Scully, S.; Van, G.; Eli, A.; Qian, Y.X.; Shalhoub, V. Osteoclast markers accumulate on cells developing from human peripheral blood mononuclear precursors. J. Cell. Biochem. 1999, 72, 67–80. [Google Scholar] [CrossRef]
- Sethi, N.; Dai, X.; Winter, C.G.; Kang, Y. Tumor-derived Jagged1 Promotes Osteolytic Bone Metastasis of Breast Cancer by Engaging Notch Signaling in Bone Cells. Cancer Cell 2011, 19, 192–205. [Google Scholar] [CrossRef] [PubMed]
- De la Mata, J.I.; Uy, H.L.; Guise, T.A.; Story, B.; Boyce, B.F.; Mundy, G.R.; Roodman, G.D. Interleukin-6 enhances hypercalcemia and bone resorption mediated by parathyroid hormone-related protein in vivo. J. Clin. Investig. 1995, 95, 2846–2852. [Google Scholar] [CrossRef] [PubMed]
- Hussein, O.; Tiedemann, K.; Murshed, M.; Komarova, S.V. Rapamycin inhibits osteolysis and improves survival in a model of experimental bone metastases. Cancer Lett. 2012, 314, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Glantschnig, H.; Fisher, J.E.; Wesolowski, G.; Rodan, G.A.; Reszka, A.A. M-CSF, TNF-α and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003, 10, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Sugatani, T.; Hruska, K.A. Akt1/Akt2 and mammalian target of rapamycin/Bim play critical roles in osteoclast differentiation and survival, respectively, whereas Akt is dispensable for cell survival in isolated osteoclast precursors. J. Biol. Chem. 2005, 280, 3583–3589. [Google Scholar] [CrossRef] [PubMed]
- Simone, V.; Ciavarella, S.; Brunetti, O.; Savonarola, A.; Cives, M.; Tucci, M.; Opinto, G.; Maiorano, E.; Silvestris, F. Everolimus restrains the paracrine pro-osteoclast activity of breast cancer cells. BMC Cancer 2015, 15, 692. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.; Liverani, C.; Mercatali, L.; Sacanna, E.; Zanoni, M.; Fabbri, F.; Zoli, W.; Amadori, D. Cisplatin in combination with zoledronic acid: A synergistic effect in triple-negative breast cancer cell lines. Int. J. Oncol. 2013, 42, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.; Mercatali, L.; Sacanna, E.; Tesei, A.; Carloni, S.; Ulivi, P.; Liverani, C.; Fabbri, F.; Zanoni, M.; Zoli, W.; et al. Inhibition of breast cancer cell proliferation in repeated and non-repeated treatment with zoledronic acid. Cancer Cell Int. 2012, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Tanino, H.; Horiguchi, J.; Miura, D.; Ishikawa, T.; Hayashi, M.; Takao, S.; Kim, S.J.; Yamagami, K.; Miyashita, M.; et al. JONIE Study Group. Randomized controlled trial of zoledronic acid plus chemotherapy versus chemotherapy alone as neoadjuvant treatment of HER2-negative primary breast cancer (JONIE Study). PLoS ONE 2015, 10, e0143643. [Google Scholar] [CrossRef] [PubMed]
- Stopeck, A.T.; Lipton, A.; Body, J.J.; Steger, G.G.; Tonkin, K.; de Boer, R.H.; Lichinitser, M.; Fujiwara, Y.; Yardley, D.A.; Viniegra, M.; et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J. Clin. Oncol. 2010, 28, 5132–5139. [Google Scholar] [CrossRef] [PubMed]
- Block, G.A.; Bone, H.G.; Fang, L.; Lee, E.; Padhi, D. A single-dose study of denosumab in patients with various degrees of renal impairment. J. Bone Miner. Res. 2012, 27, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Berenson, J.; Vescio, R.; Swift, R.; Gilchick, A.; Goodin, S.; Lo Russo, P.; Ma, P.; Ravera, C.; Deckert, F.; et al. Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J. Clin. Pharmacol. 2002, 42, 1228–1236. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercatali, L.; Spadazzi, C.; Miserocchi, G.; Liverani, C.; De Vita, A.; Bongiovanni, A.; Recine, F.; Amadori, D.; Ibrahim, T. The Effect of Everolimus in an In Vitro Model of Triple Negative Breast Cancer and Osteoclasts. Int. J. Mol. Sci. 2016, 17, 1827. https://doi.org/10.3390/ijms17111827
Mercatali L, Spadazzi C, Miserocchi G, Liverani C, De Vita A, Bongiovanni A, Recine F, Amadori D, Ibrahim T. The Effect of Everolimus in an In Vitro Model of Triple Negative Breast Cancer and Osteoclasts. International Journal of Molecular Sciences. 2016; 17(11):1827. https://doi.org/10.3390/ijms17111827
Chicago/Turabian StyleMercatali, Laura, Chiara Spadazzi, Giacomo Miserocchi, Chiara Liverani, Alessandro De Vita, Alberto Bongiovanni, Federica Recine, Dino Amadori, and Toni Ibrahim. 2016. "The Effect of Everolimus in an In Vitro Model of Triple Negative Breast Cancer and Osteoclasts" International Journal of Molecular Sciences 17, no. 11: 1827. https://doi.org/10.3390/ijms17111827
APA StyleMercatali, L., Spadazzi, C., Miserocchi, G., Liverani, C., De Vita, A., Bongiovanni, A., Recine, F., Amadori, D., & Ibrahim, T. (2016). The Effect of Everolimus in an In Vitro Model of Triple Negative Breast Cancer and Osteoclasts. International Journal of Molecular Sciences, 17(11), 1827. https://doi.org/10.3390/ijms17111827