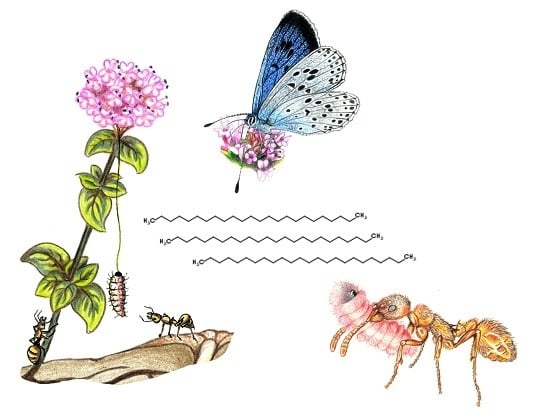
Cuticular Lipids as a Cross-Talk among Ants, Plants and Butterflies
Abstract
:1. Introduction and Outlines
Why Ants?
2. Myrmecophilous Interactions
3. Cuticular Lipids of Ants: Structure, Function, and Biosynthesis
4. Comparison between Plant and Ant Cuticular Lipids
5. Role of Plant Surface in Biotic Interactions as Sensing and Signaling Source
6. Lepidoptera Eavesdropping on Ant-Plant Cross-Talk: CHC Crypsis, Camouflage and Mimicry
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hadley, N.F. Cuticle: Ecological significance. In Biology of the Integument; Springer: Berlin, Germany, 1984; pp. 685–693. [Google Scholar]
- Lockey, K.H. Lipids of the insect cuticle—Origin, composition and function. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1988, 89, 595–645. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Jetter, R. Attachment to plant surface waxes by an insect predator. Integr. Comp. Biol. 2002, 42, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Coluccia, F.; Torres, M.; L’Haridon, F.; Metraux, J.P. The cuticle and plant defense to pathogens. Front. Plant Sci. 2014, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Kerstiens, G. Signalling across the divide: A wider perspective of cuticular structure-function relationships. Trends Plant Sci. 1996, 1, 125–129. [Google Scholar] [CrossRef]
- Riederer, M.; Schreiber, L. Protecting against water loss: Analysis of the barrier properties of plant cuticles. J. Exp. Bot. 2001, 52, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, A.G. Water-proofing properties of cuticular lipids. Am. Zool. 1998, 38, 471–482. [Google Scholar] [CrossRef]
- Lockey, K.H. Insect hydrocarbon classes—Implications for chemotaxonomy. Insect Biochem. 1991, 21, 91–97. [Google Scholar] [CrossRef]
- Edwards, D.; Abbott, G.; Raven, J. Cuticles of early land plants: A palaeoecophysiological evaluation. In Plant Cuticles: An Integrated Functional Approach; BIOS Scientific Publishers Ltd.: Oxford, UK, 1996; pp. 1–31. [Google Scholar]
- Hadley, N.F. Integumental lipids of plants and animals: Comparative function and biochemistry. Adv. Lipid Res. 1991, 303–320. [Google Scholar] [CrossRef]
- Domínguez, E.; Heredia-Guerrero, J.A.; Heredia, A. The biophysical design of plant cuticles: An overview. New Phytol. 2011, 189, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrode, S.D.; Espelie, K.E. Effects of plant epicuticular lipids on insect herbivores. Annu. Rev. Entomol. 1995, 40, 171–194. [Google Scholar] [CrossRef]
- Van Zweden, J.S.; d’Ettorre, P.; Blomquist, G.J.; Bagneres, A.G. Nestmate recognition in social insects and the role of hydrocarbons. In Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology; Cambridge University Press: Cambridge, UK, 2010; pp. 222–243. [Google Scholar]
- Maffei, M.; Badino, S.; Bossi, S. Chemotaxonomic significance of leaf wax n-alkanes in the Pinales (Coniferales). J. Biol. Res. 2004, 1, 3–19. [Google Scholar]
- Samuels, L.; Kunst, L.; Jetter, R. Sealing plant surfaces: Cuticular wax formation by epidermal cells. Annu. Rev. Plant Biol. 2008, 59, 683–707. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, M.D. Signalers and Receivers Mechanisms and Evolution of Arthropod Communication; Oxford University Press: New York, NY, USA, 2002. [Google Scholar]
- Hölldobler, B.; Wilson, E.O. The Ants; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Wilson, E.O. The sociogenesis of insect colonies. Science 1985, 228, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.E. Regulation of division of labor in insect societies. Annu. Rev. Entomol. 1992, 37, 637–665. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.M. The organization of work in social insect colonies. Nature 1996, 380, 121–124. [Google Scholar] [CrossRef]
- Mills, L.S.; Soulé, M.E.; Doak, D.F. The keystone-species concept in ecology and conservation. BioScience 1993, 43, 219–224. [Google Scholar] [CrossRef]
- Mueller, U.G.; Gerardo, N.M.; Aanen, D.K.; Six, D.L.; Schultz, T.R. The evolution of agriculture in insects. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 563–595. [Google Scholar] [CrossRef]
- Davidson, D.W.; Cook, S.C.; Snelling, R.R.; Chua, T.H. Explaining the abundance of ants in lowland tropical rainforest canopies. Science 2003, 300, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.A.; Schönrogge, K.; Elmes, G.W. Specializations and Host Associations of Social Parasites of Ants. In Insect Evolutionary Ecology; Fellowes, M.D.E., Holloway, G.J., Rolff, J., Eds.; Royal Entomological Society: London, UK, 2005; pp. 475–514. [Google Scholar]
- Parker, J.; Grimaldi, D.A. Specialized myrmecophily at the ecological dawn of modern ants. Curr. Biol. 2014, 24, 2428–2434. [Google Scholar] [CrossRef] [PubMed]
- Wasmann, E. The ants and their guests. Smithonian Rep. 1913, 1912, 455–474. [Google Scholar]
- Akino, T. Chemical strategies to deal with ants: A review of mimicry, camouflage, propaganda, and phytomimesis by ants (Hymenoptera: Formicidae) and other arthropods. Myrmecol. News 2008, 11, 173–181. [Google Scholar]
- Witek, M.; Barbero, F.; Marko, B. Myrmica ants host highly diverse parasitic communities: From social parasites to microbes. Insect Soc. 2014, 61, 307–323. [Google Scholar] [CrossRef]
- Rico-Gray, V.; Oliveira, P.S. The Ecology and Evolution of Ant-Plant Interactions; University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Moreau, C.S.; Bell, C.D.; Vila, R.; Archibald, S.B.; Pierce, N.E. Phylogeny of the ants: Diversification in the age of angiosperms. Science 2006, 312, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.O.; Holldobler, B. The rise of the ants: A phylogenetic and ecological explanation. Proc. Natl. Acad. Sci. USA 2005, 102, 7411–7414. [Google Scholar] [CrossRef] [PubMed]
- Janzen, D.H. Coevolution of mutualism between ants and acacias in Central America. Evolution 1966, 20, 249–275. [Google Scholar] [CrossRef]
- Ricogray, V.; Thien, L.B. Effect of different ant species on reproductive fitness of Schomburgkia tibicinis (Orchidaceae). Oecologia 1989, 81, 487–489. [Google Scholar] [CrossRef]
- Frederickson, M.E.; Gordon, D.M. The devil to pay: A cost of mutualism with Myrmelachista schumanni ants in “devil’s gardens” is increased herbivory on Duroia hirsuta trees. Proc. R. Soc. Lond. B Biol. Sci. 2007, 274, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, M.E.; Greene, M.J.; Gordon, D.M. Ecology: “Devil’s gardens” bedevilled by ants. Nature 2005, 437, 495–496. [Google Scholar] [CrossRef] [PubMed]
- Delabie, J.H. Trophobiosis between Formicidae and Hemiptera (Sternorrhyncha and Auchenorrhyncha): An overview. Neotrop. Entomol. 2001, 30, 501–516. [Google Scholar] [CrossRef]
- Pierce, N.E.; Braby, M.F.; Heath, A.; Lohman, D.J.; Mathew, J.; Rand, D.B.; Travassos, M.A. The ecology and evolution of ant association in the Lycaenidae (Lepidoptera). Annu. Rev. Entomol. 2002, 47, 733–771. [Google Scholar] [CrossRef] [PubMed]
- DeVries, P.J. Evolutionary and Ecological Patterns in Myrmecophilous-Riodinid Butterflies. In Ant—Plant Interactions; Huxley, C.R., Cutler, D.F., Eds.; Oxford University Press: Oxford, UK, 1991; pp. 143–156. [Google Scholar]
- Hinton, H.E. Myrmecophilous Lycaenidae and other Lepidoptera—A summary. Proc. Trans. South Lond. Entomol. Nat. Hist. Soc. 1951, 1949–1950, 111–175. [Google Scholar]
- Fiedler, K. Systematic, Evolutionary, and Ecological Implications of Myrmecophily within the Lycaenidae (Insecta: Lepidoptera: Papilionoidea); Zoologisches Forschungsinstitut und Museum Alexander Koenig: Bonn, Germany, 1991. [Google Scholar]
- Heil, M.; McKey, D. Protective ant–plant interactions as model systems in ecological and evolutionary research. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 425–453. [Google Scholar] [CrossRef]
- Donisthorpe, H.S.J.K. The Guests of British Ants; Routledge: London, UK, 1927. [Google Scholar]
- Maschwitz, U.; Schroth, M.; Hänel, H.; Pong, T.Y. Lycaenids parasitizing symbiotic plant-ant partnerships. Oecologia 1984, 64, 78–80. [Google Scholar] [CrossRef]
- Malicky, H. New aspects of the association between lycaenid larvae (Lycaenidae) and ants (Formicidae, Hymenoptera). J. Lepid. Soc. 1970, 24, 190–202. [Google Scholar]
- Schönrogge, K.; Wardlaw, J.C.; Peters, A.J.; Everett, S.; Thomas, J.A.; Elmes, G.W. Changes in chemical signature and host specificity from larval retrieval to full social integration in the myrmecophilous butterfly Maculinea rebeli. J. Chem. Ecol. 2004, 30, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.A.; Elmes, G.W.; Sielezniew, M.; Stankiewicz-Fiedurek, A.; Simcox, D.J.; Settele, J.; Schonrogge, K. Mimetic host shifts in an endangered social parasite of ants. Proc. R. Soc. B Biol. Sci. 2013, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbero, F.; Bonelli, S.; Thomas, J.A.; Balletto, E.; Schonrogge, K. Acoustical mimicry in a predatory social parasite of ants. J. Exp. Biol. 2009, 212, 4084–4090. [Google Scholar] [CrossRef] [PubMed]
- Barbero, F.; Patricelli, D.; Witek, M.; Balletto, E.; Casacci, L.P.; Sala, M.; Bonelli, S. Myrmica ants and their butterfly parasites with special focus on the acoustic communication. Psyche 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Lenoir, A.; D’Ettorre, P.; Errard, C.; Hefetz, A. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 2001, 46, 573–599. [Google Scholar] [CrossRef] [PubMed]
- Holldobler, B. The chemistry of social regulation—Multicomponent signals in ant societies. Proc. Natl. Acad. Sci. USA 1995, 92, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Hölldobler, B. Multimodal signals in ant communication. J. Comp. Physiol. A 1999, 184, 129–141. [Google Scholar] [CrossRef]
- Barbero, F.; Casacci, L.P. Butterflies that trick ants with sound. Phys. Today 2015, 68, 64–65. [Google Scholar] [CrossRef]
- Sala, M.; Casacci, L.P.; Balletto, E.; Bonelli, S.; Barbero, F. Variation in Butterfly Larval Acoustics as a Strategy to Infiltrate and Exploit Host Ant Colony Resources. PLoS ONE 2014, 9, e94341. [Google Scholar] [CrossRef] [PubMed]
- Barbero, F.; Thomas, J.A.; Bonelli, S.; Balletto, E.; Schönrogge, K. Queen ants make distinctive sounds that are mimicked by a butterfly social parasite. Science 2009, 323, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Von Thienen, W.; Metzler, D.; Choe, D.-H.; Witte, V. Pheromone communication in ants: A detailed analysis of concentration-dependent decisions in three species. Behav. Ecol. Sociobiol. 2014, 68, 1611–1627. [Google Scholar] [CrossRef]
- Smith, A.A.; Hölldober, B.; Liebig, J. Cuticular hydrocarbons reliably identify cheaters and allow enforcement of altruism in a social insect. Curr. Biol. 2009, 19, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.W.; Blomquist, G.J. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. In Annual Review of Entomology; Annual Reviews: Palo Alto, CA, USA, 2005; Volume 50, pp. 371–393. [Google Scholar]
- Martin, S.; Drijfhout, F. A Review of Ant Cuticular Hydrocarbons. J. Chem. Ecol. 2009, 35, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, A. Physical properties of insect cuticular hydrocarbons: Model mixtures and lipid interactions. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 112, 667–672. [Google Scholar] [CrossRef]
- Wagner, D.; Brown, M.J.; Broun, P.; Cuevas, W.; Moses, L.E.; Chao, D.L.; Gordon, D.M. Task-related differences in the cuticular hydrocarbon composition of harvester ants, Pogonomyrmex barbatus. J. Chem. Ecol. 1998, 24, 2021–2037. [Google Scholar] [CrossRef]
- Nelson, D.R.; Tissot, M.; Nelson, L.J.; Fatland, C.L.; Gordon, D.M. Novel wax esters and hydrocarbons in the cuticular surface lipids of the red harvester ant, Pogonomyrmex barbatus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 128, 575–595. [Google Scholar] [CrossRef]
- Wagner, D.; Tissot, M.; Cuevas, W.; Gordon, D.M. Harvester ants utilize cuticular hydrocarbons in nestmate recognition. J. Chem. Ecol. 2000, 26, 2245–2257. [Google Scholar] [CrossRef]
- Greene, M.J.; Pinter-Wollman, N.; Gordon, D.M. Interactions with combined chemical cues inform harvester ant foragers’ decisions to leave the nest in search of food. PLoS ONE 2013, 8, e52219. [Google Scholar] [CrossRef] [PubMed]
- Cheesbrough, T.; Kolattukudy, P. Alkane biosynthesis by decarbonylation of aldehydes catalyzed by a particulate preparation from Pisum sativum. Proc. Natl. Acad. Sci. USA 1984, 81, 6613–6617. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.R.; Quilici, D.R.; Blomquist, G.J.; Reitz, R.C. Proposed mechanism for the cytochrome P 450-catalyzed conversion of aldehydes to hydrocarbons in the house fly, Musca domestica. Biochemistry 1995, 34, 16221–16227. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.R.; Vanderwel, D.; Choi, S.; Pomonis, J.G.; Reitz, R.C.; Blomquist, G.J. Unusual mechanism of hydrocarbon formation in the housefly: Cytochrome P450 converts aldehyde to the sex pheromone component (Z)-9-tricosene and CO2. Proc. Natl. Acad. Sci. USA 1994, 91, 10000–10004. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Tittiger, C.; Wicker-Thomas, C.; Le Goff, G.; Young, S.; Wajnberg, E.; Fricaux, T.; Taquet, N.; Blomquist, G.J.; Feyereisen, R. An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 14858–14863. [Google Scholar] [CrossRef] [PubMed]
- Kather, R.; Martin, S.J. Evolution of Cuticular Hydrocarbons in the Hymenoptera: A Meta-Analysis. J. Chem. Ecol. 2015, 41, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Kunst, L.; Samuels, A.L. Biosynthesis and secretion of plant cuticular wax. Prog. Lipid. Res. 2003, 42, 51–80. [Google Scholar] [CrossRef]
- Schal, C.; Sevala, V.L.; Young, H.P.; Bachmann, J.A. Sites of synthesis and transport pathways of insect hydrocarbons: Cuticle and ovary as target tissues. Am. Zool. 1998, 38, 382–393. [Google Scholar] [CrossRef]
- Chung, H.; Carroll, S.B. Wax, sex and the origin of species: Dual roles of insect cuticular hydrocarbons in adaptation and mating. Bioessays 2015, 37, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Vander Meer, R.K.; Morel, L. Nestmate Recognition in Ants. In Pheromone Communication in Social Insects; vander Meer, R.K., Breed, M.D., Winston, M.L., Espelie, K.E., Eds.; Westview Press: Oxford, UK, 1998; pp. 79–103. [Google Scholar]
- Bagneres, A.G.; Riviere, G.; Clement, J.L. Artificial neural network modeling of caste odor discrimination based on cuticular hydrocarbons in termites. Chemoecology 1998, 8, 201–209. [Google Scholar] [CrossRef]
- Singer, T.L. Roles of hydrocarbons in the recognition systems of insects. Am. Zool. 1998, 38, 394–405. [Google Scholar] [CrossRef]
- Greene, M.J.; Gordon, D.M. Social insects: Cuticular hydrocarbons inform task decisions. Nature 2003, 423, 32. [Google Scholar] [CrossRef] [PubMed]
- Akino, T.; Yamamura, K.; Wakamura, S.; Yamaoka, R. Direct behavioral evidence for hydrocarbons as nestmate recognition cues in Formica japonica (Hymenoptera: Formicidae). Appl. Entomol. Zool. 2004, 39, 381–387. [Google Scholar] [CrossRef]
- Martin, S.J.; Vitikainen, E.; Helantera, H.; Drijfhout, F.P. Chemical basis of nest-mate discrimination in the ant Formica exsecta. Proc. R. Soc. B Biol. Sci. 2008, 275, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; Drijfhout, F.P. Nestmate and task cues are influenced and encoded differently within ant cuticular hydrocarbon profiles. J. Chem. Ecol. 2009, 35, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Hora, R.R.; Ionescu-Hirsh, A.; Simon, T.; Delabie, J.; Robert, J.; Fresneau, D.; Hefetz, A. Postmating changes in cuticular chemistry and visual appearance in Ectatomma tuberculatum queens (Formicidae: Ectatomminae). Naturwissenschaften 2008, 95, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, F.J.; Nehring, V.; Jørgensen, C.G.; Nielsen, J.; Galizia, C.G.; d’Ettorre, P. Ants recognize foes and not friends. Proc. R. Soc. Lond. B Biol. Sci. 2009, 276, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Greene, M.J.; Gordon, D.M. Structural complexity of chemical recognition cues affects the perception of group membership in the ants Linephithema humile and Aphaenogaster cockerelli. J. Exp. Biol. 2007, 210, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, M.; Wada-Katsumata, A.; Fujikawa, K.; Iwasaki, M.; Yokohari, F.; Satoji, Y.; Nisimura, T.; Yamaoka, R. Ant nestmate and non-nestmate discrimination by a chemosensory sensillum. Science 2005, 309, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Van Zweden, J.S.; Dreier, S.; d’Ettorre, P. Disentangling environmental and heritable nestmate recognition cues in a carpenter ant. J. Insect Physiol. 2009, 55, 158–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obin, M.S.; Vander Meer, R.K. Mechanism of template-label matching in fire ant, Solenopsis invicta Buren, nestmate recognition. Anim. Behav. 1989, 38, 430–435. [Google Scholar] [CrossRef]
- Stuart, R.J. Differences in aggression among sympatric, facultatively polygynous Leptothorax ant species. Anim. Behav. 1993, 46, 809–812. [Google Scholar] [CrossRef]
- Menzel, F.; Schmitt, T.; Bluthgen, N. Intraspecific nestmate recognition in two parabiotic ant species: Acquired recognition cues and low inter-colony discrimination. Insect Soc. 2009, 56, 251–260. [Google Scholar] [CrossRef]
- Brandstaetter, A.S.; Rössler, W.; Kleineidam, C.J. Friends and foes from an ant brain’s point of view–neuronal correlates of colony odors in a social insect. PLoS ONE 2011, 6, e21383. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.R.; Enzmann, B.L.; Schmidt, Y.; Moore, D.; Jones, G.R.; Parker, J.; Berger, S.L.; Reinberg, D.; Zwiebel, L.J.; Breit, B. Cuticular hydrocarbon pheromones for social behavior and their coding in the ant antenna. Cell Rep. 2015, 12, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Brandstaetter, A.S.; Kleineidam, C.J. Distributed representation of social odors indicates parallel processing in the antennal lobe of ants. J. Neurophysiol. 2011, 106, 2437–2449. [Google Scholar] [CrossRef] [PubMed]
- Heinze, J.; Stengl, B.; Sledge, M.F. Worker rank, reproductive status and cuticular hydrocarbon signature in the ant, Pachycondyla cf. inversa. Behav. Ecol. Sociobiol. 2002, 52, 59–65. [Google Scholar] [CrossRef]
- Nielsen, J.; Boomsma, J.J.; Oldham, N.; Petersen, H.; Morgan, E. Colony-level and season-specific variation in cuticular hydrocarbon profiles of individual workers in the ant Formica truncorum. Insect Soc. 1999, 46, 58–65. [Google Scholar] [CrossRef]
- Dani, F.R.; Jones, G.R.; Corsi, S.; Beard, R.; Pradella, D.; Turillazzi, S. Nestmate recognition cues in the honey bee: Differential importance of cuticular alkanes and alkenes. Chem. Sens. 2005, 30, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Châline, N.; Sandoz, J.-C.; Martin, S.J.; Ratnieks, F.L.; Jones, G.R. Learning and discrimination of individual cuticular hydrocarbons by honeybees (Apis mellifera). Chem. Sens. 2005, 30, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Fürst, M.A.; Durey, M.; Nash, D.R. Testing the adjustable threshold model for intruder recognition on Myrmica ants in the context of a social parasite. Proc. Biol. Sci. R. Soc. 2012, 279, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Fernández, V.; Guzmán-Delgado, P.; Graça, J.; Santos, S.; Gil, L. Cuticle Structure in Relation to Chemical Composition: Re-assessing the Prevailing Model. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffree, C.E. Structure and ontogeny of plant cuticles. In Plant Cuticles: An Integrated Functional Approach; Kerstiens, G., Ed.; BIOS Scientific: Oxford, UK, 1996; pp. 33–85. [Google Scholar]
- Jetter, R.; Kunst, L.; Samuels, A.L. Composition of plant cuticular waxes. Annu. Plant Rev. Biol. Plant Cuticle 2008, 23. [Google Scholar] [CrossRef]
- Kolattukudy, P.; Espelie, K. Chemistry, biochemistry, and function of suberin and associated waxes. In Natural Products of Woody Plants; Springer: Berlin, Germany, 1989; pp. 304–367. [Google Scholar]
- Khayet, M.; Fernandez, V. Estimation of the solubility parameters of model plant surfaces and agrochemicals: A valuable tool for understanding plant surface interactions. Theor. Biol. Med. Model. 2012, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jetter, R.; Schaffer, S. Chemical composition of the Prunus laurocerasus leaf surface: Dynamic changes of the epicuticular wax film during leaf development. Plant Physiol. 2001, 126, 1725–1737. [Google Scholar] [CrossRef] [PubMed]
- Knoche, M.; Beyer, M.; Peschel, S.; Oparlakov, B.; Bukovac, M.J. Changes in strain and deposition of cuticle in developing sweet cherry fruit. Physiol. Plant. 2004, 120, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Delgado, P.; Graca, J.; Cabral, V.; Gil, L.; Fernandez, V. The presence of cutan limits the interpretation of cuticular chemistry and structure: Ficus elastica leaf as an example. Physiol. Plant. 2016, 157, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Aitzetmuller, K.; Tsevegsuren, N. Seed fatty-acids, front-end-desaturases and chemotaxonomy—A case-study in the ranunculaceae. J. Plant Physiol. 1994, 143, 538–543. [Google Scholar] [CrossRef]
- Maffei, M. Chemotaxonomic significance of leaf wax alkanes in the Compositae. In Compositae: Systematics. Proceedings of the International Compositae Conference; Hind, D.J.N., Beentje, H.J., Eds.; Royal Botanic Gardens: Kew, UK, 1996; pp. 141–158. [Google Scholar]
- Maffei, M. Chemotaxonomic significance of leaf wax alkanes in the Gramineae. Biochem. Syst. Ecol. 1996, 24, 53–64. [Google Scholar] [CrossRef]
- Maffei, M. Chemotaxonomic significance of leaf wax n-Alkanes in the Umbelliferae, Cruciferae and Leguminosae (subf papilionoideae). Biochem. Syst. Ecol. 1996, 24, 531–545. [Google Scholar] [CrossRef]
- Maffei, M.; Meregalli, M.; Scannerini, S. Chemotaxonomic significance of surface wax n-alkanes in the Cactaceae. Biochem. Syst. Ecol. 1997, 25, 241–253. [Google Scholar] [CrossRef]
- Espelie, K.E.; Bernays, E.A.; Brown, J.J. Plant and insect cuticular lipids serve as behavioral cues for insects. Arch. Insect Biochem. Physiol. 1991, 17, 223–233. [Google Scholar] [CrossRef]
- De Jong, R.; Städler, E. The influence of odour on the oviposition behaviour of the cabbage root fly. Chemoecology 1999, 9, 151–154. [Google Scholar] [CrossRef]
- Spencer, J.L. Waxes enhance Plutella xylostella oviposition in response to sinigrin and cabbage homogenates. Entomol. Exp. Appl. 1996, 81, 165–173. [Google Scholar] [CrossRef]
- Marazzi, C.; Patrian, B.; Städler, E. Secondary metabolites of the leaf surface affected by sulphur fertilisation and perceived by the diamondback moth. Chemoecology 2004, 14, 81–86. [Google Scholar] [CrossRef]
- Braccini, C.L.; Vega, A.S.; Araoz, M.V.C.; Teal, P.E.; Cerrillo, T.; Zavala, J.A.; Fernandez, P.C. Both Volatiles and Cuticular Plant Compounds Determine Oviposition of the Willow Sawfly Nematus oligospilus on Leaves of Salix spp. (Salicaceae). J. Chem. Ecol. 2015, 41, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Adati, T.; Matsuda, K. Feeding stimulants for various leaf beetles (Coleoptera, Chrysomelidae) in the leaf surface wax of their host plants. Appl. Entomol. Zool. 1993, 28, 319–324. [Google Scholar]
- Powell, G.; Maniar, S.P.; Pickett, J.A.; Hardie, J. Aphid responses to non-host epicuticular lipids. Entomol. Exp. Appl. 1999, 91, 115–123. [Google Scholar] [CrossRef]
- Morris, B.D.; Foster, S.P.; Harris, M.O. Identification of 1-octacosanal and 6-methoxy-2-benzoxazolinone from wheat as ovipositional stimulants for Hessian fly, Mayetiola destructor. J. Chem. Ecol. 2000, 26, 859–873. [Google Scholar] [CrossRef]
- Li, G.Q.; Ishikawa, Y. Leaf epicuticular wax chemicals of the Japanese knotweed Fallopia japonica as oviposition stimulants for Ostrinia latipennis. J. Chem. Ecol. 2006, 32, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Hagley, E.A.; Bronskill, J.; Ford, E.J. Effect of the physical nature of leaf and fruit surfaces on oviposition by the codling moth, Cydia pomonella (Lepidoptera: Tortricidae). Can. Entomol. 1980, 112, 503–510. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Stoner, K.A.; Shelton, A.M.; Kain, W.C. Characteristics of glossy leaf waxes associated with resistance to diamondback moth (Lepidoptera: Plutellidae) in Brassica oleracea. J. Econ. Entomol. 1991, 84, 1609–1618. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Pillai, S.K. Neonate Plutella xylostella responses to surface wax components of a resistant cabbage (Brassica oleracea). J. Chem. Ecol. 1998, 24, 1611–1627. [Google Scholar] [CrossRef]
- Espelie, K. Integrated case study: Effects of maize leaf epicuticular lipids on insect pests. In Plant Cuticles: An Integrated Functional Approach; BIOS Scientific Publishers Ltd.: Oxford, UK, 1996; pp. 223–230. [Google Scholar]
- Yang, G.; Isenhour, D.J.; Espelie, K.E. Activity of maize leaf cuticular lipids in resistance to leaf-feeding by the fall armyworm. Florida Entomol. 1991, 2, 229–236. [Google Scholar] [CrossRef]
- Yang, G.; Wiseman, B.R.; Espelie, K.E. Cuticular lipids from silks of seven corn genotypes and their effect on development of corn earworm larvae (Helicoverpa zea (Boddie)). J. Agric. Food Chem. 1992, 40, 1058–1061. [Google Scholar] [CrossRef]
- Bernays, E.; Blaney, W.; Chapman, R.; Cook, A. The ability of Locusta migratoria L. to perceive plant surface waxes. In The Host-Plant in Relation to Insect Behaviour and Reproduction; Springer: Berlin, Germany, 1976; pp. 35–40. [Google Scholar]
- Steinbauer, M.J.; Schiestl, F.P.; Davies, N.W. Monoterpenes and epicuticular waxes help female autumn gum moth differentiate between waxy and glossy Eucalyptus and leaves of different ages. J. Chem. Ecol. 2004, 30, 1117–1142. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Riederer, M. Plant surface properties in chemical ecology. J. Chem. Ecol. 2005, 31, 2621–2651. [Google Scholar] [CrossRef] [PubMed]
- Sugayama, R.L.; Salatino, A. Influence of leaf epicuticular waxes from cerrado species on substrate selection by atta Sexdens rubropilosa. Entomol. Exp. Appl. 1995, 74, 63–69. [Google Scholar] [CrossRef]
- Salatino, A.; Sugayama, R.L.; Negri, G.; Vilegas, W. Effect of constituents of the foliar wax of Didymopanax vinosum on the foraging activity of the leaf-cutting ant Atta sexdens rubropilosa. Entomol. Exp. Appl. 1998, 86, 261–266. [Google Scholar] [CrossRef]
- Hubbell, S.P.; Howard, J.J.; Wiemer, D.F. Chemical leaf repellency to an attine ant: Seasonal distribution among potential host plant species. Ecology 1984, 65, 1067–1076. [Google Scholar] [CrossRef]
- Federle, W.; Maschwitz, U.; Fiala, B.; Riederer, M.; Hölldobler, B. Slippery ant-plants and skilful climbers: Selection and protection of specific ant partners by epicuticular wax blooms in Macaranga (Euphorbiaceae). Oecologia 1997, 112, 217–224. [Google Scholar] [CrossRef]
- Markstadter, C.; Federle, W.; Jetter, R.; Riederer, M.; Holldobler, B. Chemical composition of the slippery epicuticular wax blooms on Macaranga (Euphorbiaceae) ant–plants. Chemoecology 2000, 10, 33–40. [Google Scholar] [CrossRef]
- Riedel, M.; Eichner, A.; Jetter, R. Slippery surfaces of carnivorous plants: Composition of epicuticular wax crystals in Nepenthes alata Blanco pitchers. Planta 2003, 218, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Blenn, B.; Bandoly, M.; Kuffner, A.; Otte, T.; Geiselhardt, S.; Fatouros, N.E.; Hilker, M. Insect Egg Deposition Induces Indirect Defense and Epicuticular Wax Changes in Arabidopsis thaliana. J. Chem. Ecol. 2012, 38, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, G.D. Non-visual crypsis: A review of the empirical evidence for camouflage to senses other than vision. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Jeral, J.M.; Breed, M.D.; Hibbard, B.E. Thief ants have reduced quantities of cuticular compounds in a ponerine ant, Ectatomma ruidum. Physiol. Entomol. 1997, 22, 207–211. [Google Scholar] [CrossRef]
- Lorenzi, M.C.; Bagneres, A.G. Concealing identity and mimicking hosts: A dual chemical strategy for a single social parasite? (Polistes atrimandibularis, Hymenoptera: Vespidae). Parasitology 2002, 125, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.W.; McDaniel, C.; Blomquist, G.J. Chemical mimicry as an integrating mechanism: Cuticular hydrocarbons of a termitophile and its host. Science 1980, 210, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Dettner, K.; Liepert, C. Chemical mimicry and camouflage. Annu. Rev. Entomol. 1994, 39, 129–154. [Google Scholar] [CrossRef]
- Espelie, K.E.; Brown, J.J. Cuticular hydrocarbons of species which interact on four trophic levels: Apple, Malus pumila Mill.; codling moth, Cydia pomonella L.; a hymenopteran parasitoid, Ascogaster quadridentata Wesmael; and a hyperparasite, Perilampus fulvicornis Ashmead. Comp. Biochem. Physiol. Part B Comp. Biochem. 1990, 95, 131–136. [Google Scholar] [CrossRef]
- Akino, T.; Nakamura, K.-I.; Wakamura, S. Diet-induced chemical phytomimesis by twig-like caterpillars of Biston robustum Butler (Lepidoptera: Geometridae). Chemoecology 2004, 14, 165–174. [Google Scholar] [CrossRef]
- Portugal, A.H.A.; Trigo, J.R. Similarity of cuticular lipids between a caterpillar and its host plant: A way to make prey undetectable for predatory ants? J. Chem. Ecol. 2005, 31, 2551–2561. [Google Scholar] [PubMed]
- Whitehead, S.R.; Reid, E.; Sapp, J.; Poveda, K.; Royer, A.M.; Posto, A.L.; Kessler, A. A Specialist Herbivore Uses Chemical Camouflage to Overcome the Defenses of an Ant–Plant Mutualism. PLoS ONE 2014, 9, e102604. [Google Scholar] [CrossRef] [PubMed]
- Inui, Y.; Shimizu-kaya, U.; Okubo, T.; Yamsaki, E.; Itioka, T. Various Chemical Strategies to Deceive Ants in Three Arhopala Species (Lepidoptera: Lycaenidae) Exploiting Macaranga Myrmecophytes. PLoS ONE 2015, 10, e0120652. [Google Scholar] [CrossRef] [PubMed]
- Inui, Y.; Itioka, T. Species-specific leaf volatile compounds of obligate Macaranga myrmecophytes and host-specific aggressiveness of symbiotic Crematogaster ants. J. Chem. Ecol. 2007, 33, 2054–2063. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, H. Life history of Niphanda fusca Bremer et Grey. Insect Ecol. 1950, 3, 9–18. [Google Scholar]
- Hojo, M.K.; Wada-Katsumata, A.; Akino, T.; Yamaguchi, S.; Ozaki, M.; Yamaoka, R. Chemical disguise as particular caste of host ants in the ant inquiline parasite Niphanda fusca (Lepidoptera: Lycaenidae). Proc. R. Soc. B Biol. Sci. 2009, 276, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Akino, T.; Knapp, J.J.; Thomas, J.A.; Elmes, G.W. Chemical mimicry and host specificity in the butterfly Maculinea rebeli, a social parasite of Myrmica ant colonies. Proc. R. Soc. B Biol. Sci. 1999, 266, 1419–1426. [Google Scholar]
- Nash, D.R.; Als, T.D.; Maile, R.; Jones, G.R.; Boomsma, J.J. A mosaic of chemical coevolution in a large blue butterfly. Science 2008, 319, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Steiner, F.M.; Schlick-Steiner, B.C.; Höttinger, H.; Nikiforov, A.; Moder, K.; Christian, E. Maculinea alcon and M. rebeli (InsectaL Lepidoptera: Lycaenidae)—One or two Alcon Blues? Larval cuticular compounds and egg morphology of East Austrian populations. Annalen des Naturhistorischen Museums in Wien 2006, 107, 165–180. [Google Scholar]
- Witek, M.; Casacci, L.P.; Barbero, F.; Patricelli, D.; Sala, M.; Bossi, S.; Maffei, M.; Woyciechowski, M.; Balletto, E.; Bonelli, S. Interspecific relationships in co-occurring populations of social parasites and their host ants. Biol. J. Linn. Soc. 2013, 109, 699–709. [Google Scholar] [CrossRef]
- Solazzo, G.; Seidelmann, K.; Moritz, R.F.A.; Settele, J. Tetracosane on the cuticle of the parasitic butterfly Phengaris (Maculinea) nausithoustriggers the first contact in the adoption process by Myrmica rubra foragers. Physiol. Entomol. 2014, 60, 57–64. [Google Scholar]
- Dicke, M. Chemical ecology of host-plant selection by herbivorous arthropods: A multitrophic perspective. Biochem. Syst. Ecol. 2000, 28, 601–617. [Google Scholar] [CrossRef]
- Takabayashi, J.; Dicke, M. Plant-carnivore mutualism through herbivore-induced carnivore attractants. Trends Plant Sci. 1996, 1, 109–113. [Google Scholar] [CrossRef]
- Dicke, M.; Vet, L.E.M. Plant-Carnivore Interactions: Evolutionary and Ecological Consequences for Plant, Herbivore and Carnivore; Blackwell Science Publisher: Oxford, UK, 1999; pp. 483–520. [Google Scholar]
- Patricelli, D.; Barbero, F.; Occhipinti, A.; Bertea, C.M.; Bonelli, S.; Casacci, L.P.; Zebelo, S.A.; Crocoll, C.; Gershenzon, J.; Maffei, M.E.; et al. Plant defences against ants provide a pathway to social parasitism in butterflies. Proc. R. Soc. B Biol. Sci. 2015, 282. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.N. Variation in interspecific interactions. Annu. Rev. Ecol. Syst. 1988, 19, 65–87. [Google Scholar] [CrossRef]


| Compound Classes | Chemical Structure | Occurrence in Ant Species (%) |
|---|---|---|
| n-Alkanes |  |  |
| Monomethylalkanes |  |  |
| Dimethylalkanes |  |  |
| Alkenes |  |  |
| Dienes |  |  |
| Trimethylalkanes |  |  |
| Methylalkenes |  |  |
| Tetramethylalkanes |  |  |
| Trienes |  |  |
| Dimethylalkenes |  |  |
| – | – |  |
| Compound Classes | Chemical Structure | Chain Lenght |
|---|---|---|
| n-Alkanes | CH3(CH2)nCH3 | [C21–C35] |
| Secondary alcohols | CH3(CH2)nCHOH(CH2)mCH3 | [C21–C35] |
| Ketones | CH3(CH2)nCO(CH2)mCH3 | [C21–C35] |
| Fatty alcohols | CH3(CH2)nCH2OH | [C22–C34] |
| Fatty acids | CH3(CH2)nCO2H | [C16–C34] |
| Aldehydes | CH3(CH2)nCHO | [C21–C35] |
| Wax esters | CH3(CH2)nCO2(CH2)mCH3 | [C32–C64] |
| Diketones | CH3(CH2)nCOCH2CO(CH2)mCH3 | [C27–C33] |
| Triterpenoids | C30H50O | See [97] |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbero, F. Cuticular Lipids as a Cross-Talk among Ants, Plants and Butterflies. Int. J. Mol. Sci. 2016, 17, 1966. https://doi.org/10.3390/ijms17121966
Barbero F. Cuticular Lipids as a Cross-Talk among Ants, Plants and Butterflies. International Journal of Molecular Sciences. 2016; 17(12):1966. https://doi.org/10.3390/ijms17121966
Chicago/Turabian StyleBarbero, Francesca. 2016. "Cuticular Lipids as a Cross-Talk among Ants, Plants and Butterflies" International Journal of Molecular Sciences 17, no. 12: 1966. https://doi.org/10.3390/ijms17121966
APA StyleBarbero, F. (2016). Cuticular Lipids as a Cross-Talk among Ants, Plants and Butterflies. International Journal of Molecular Sciences, 17(12), 1966. https://doi.org/10.3390/ijms17121966