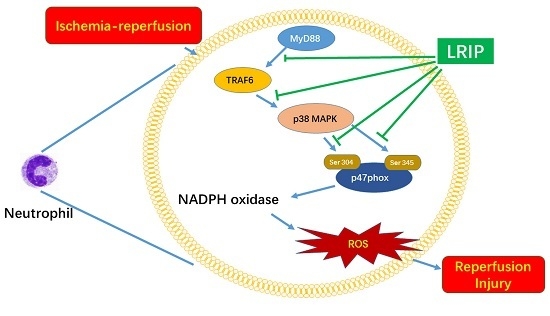
Limb Remote Ischemic Postconditioning Reduces Ischemia-Reperfusion Injury by Inhibiting NADPH Oxidase Activation and MyD88-TRAF6-P38MAP-Kinase Pathway of Neutrophils
Abstract
:1. Introduction
2. Results
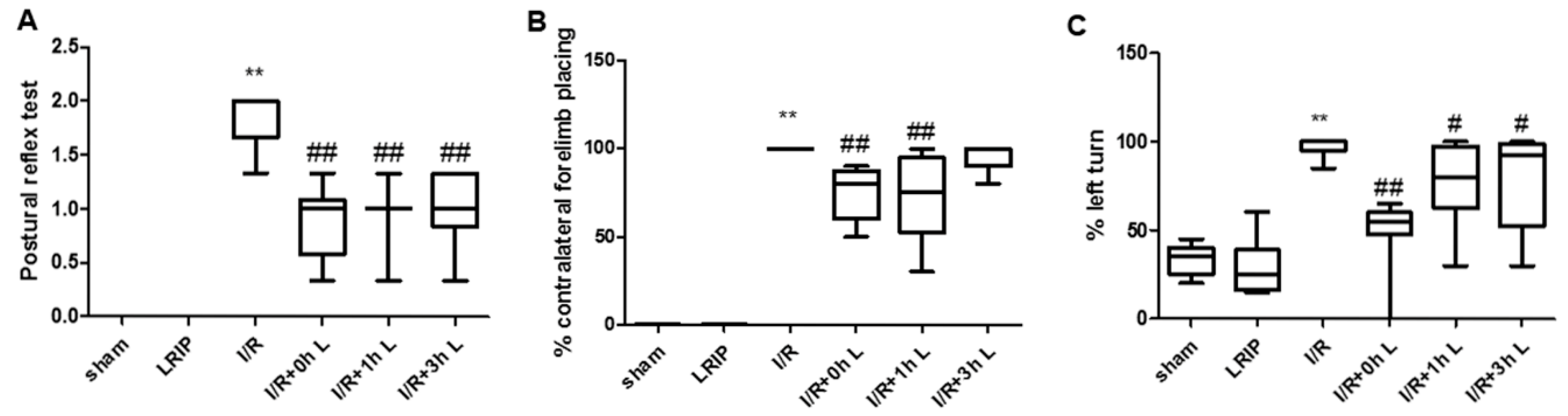
2.1. Effect of Limb Remote Ischemic Postconditioning (LRIP) on the Neurobehavioral Deficits in Middle Cerebral Artery Occlusion (MCAO) Rats
2.2. Effect of LRIP on Neutrophil Infiltration in the Brain and Left Hind Limb Gastrocnemius Muscle in MCAO Rats
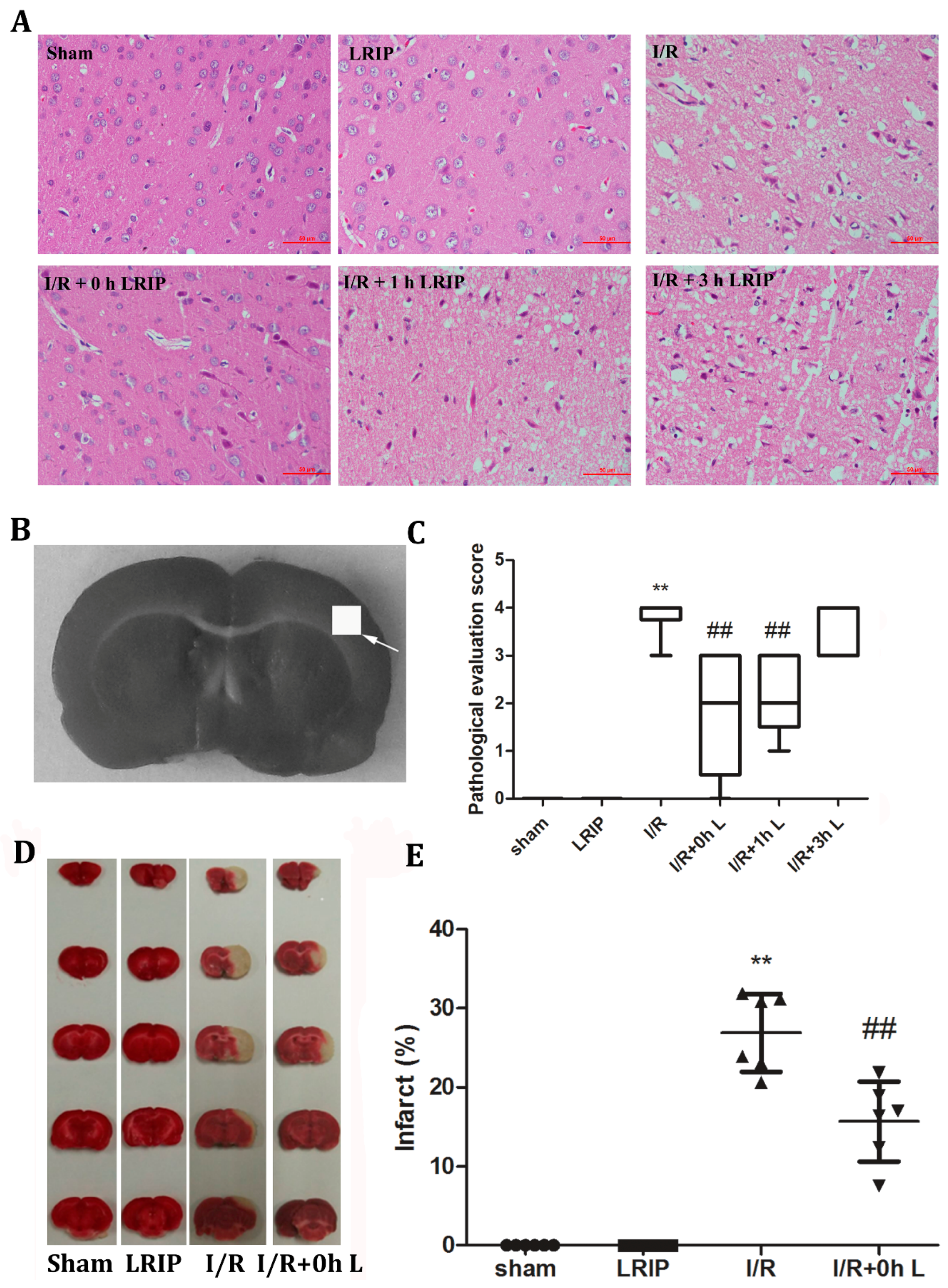
2.3. Effects of LRIP on the Histopathological Changes and Infarct Volume of the Brain in MCAO Rats
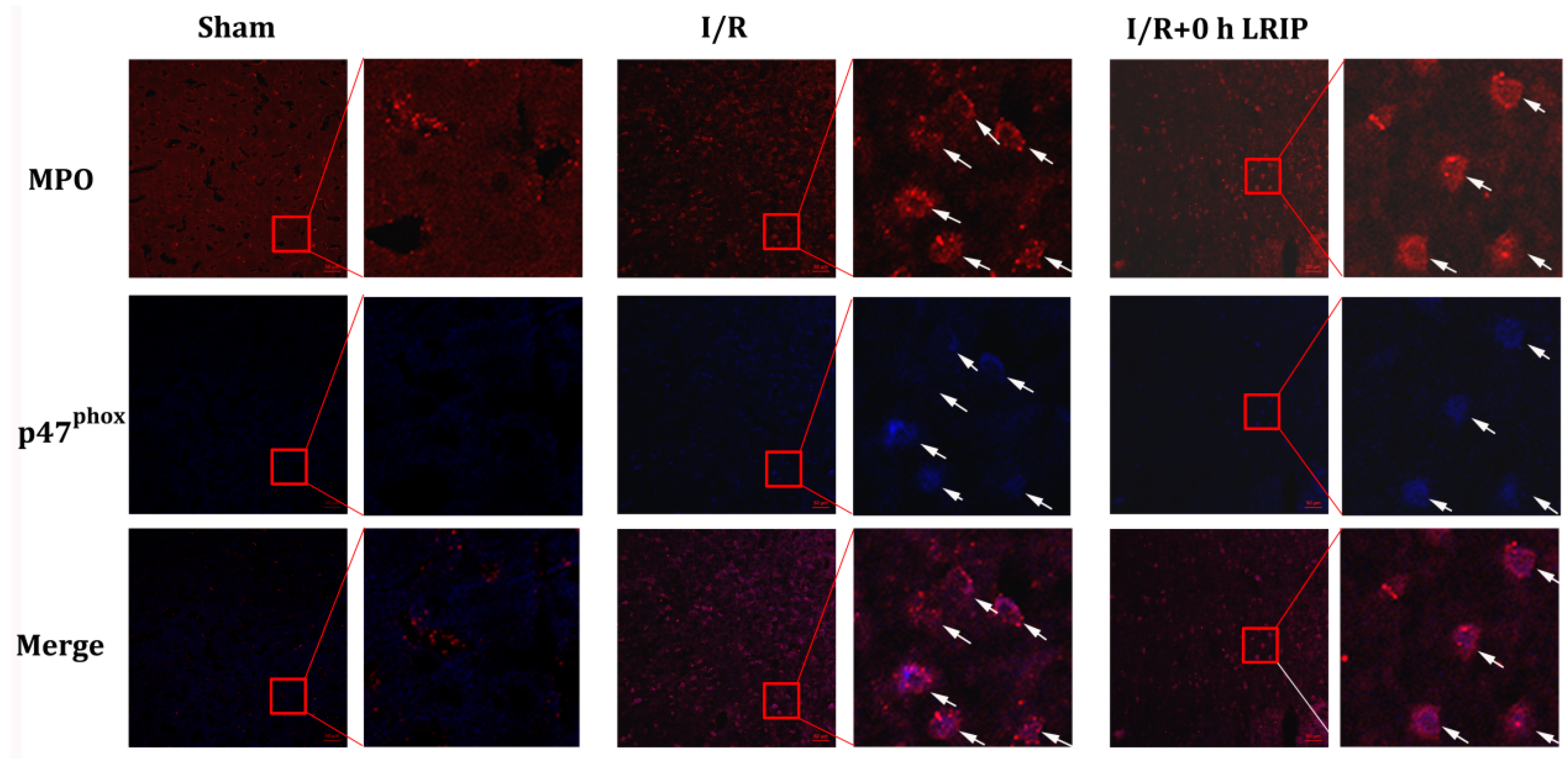
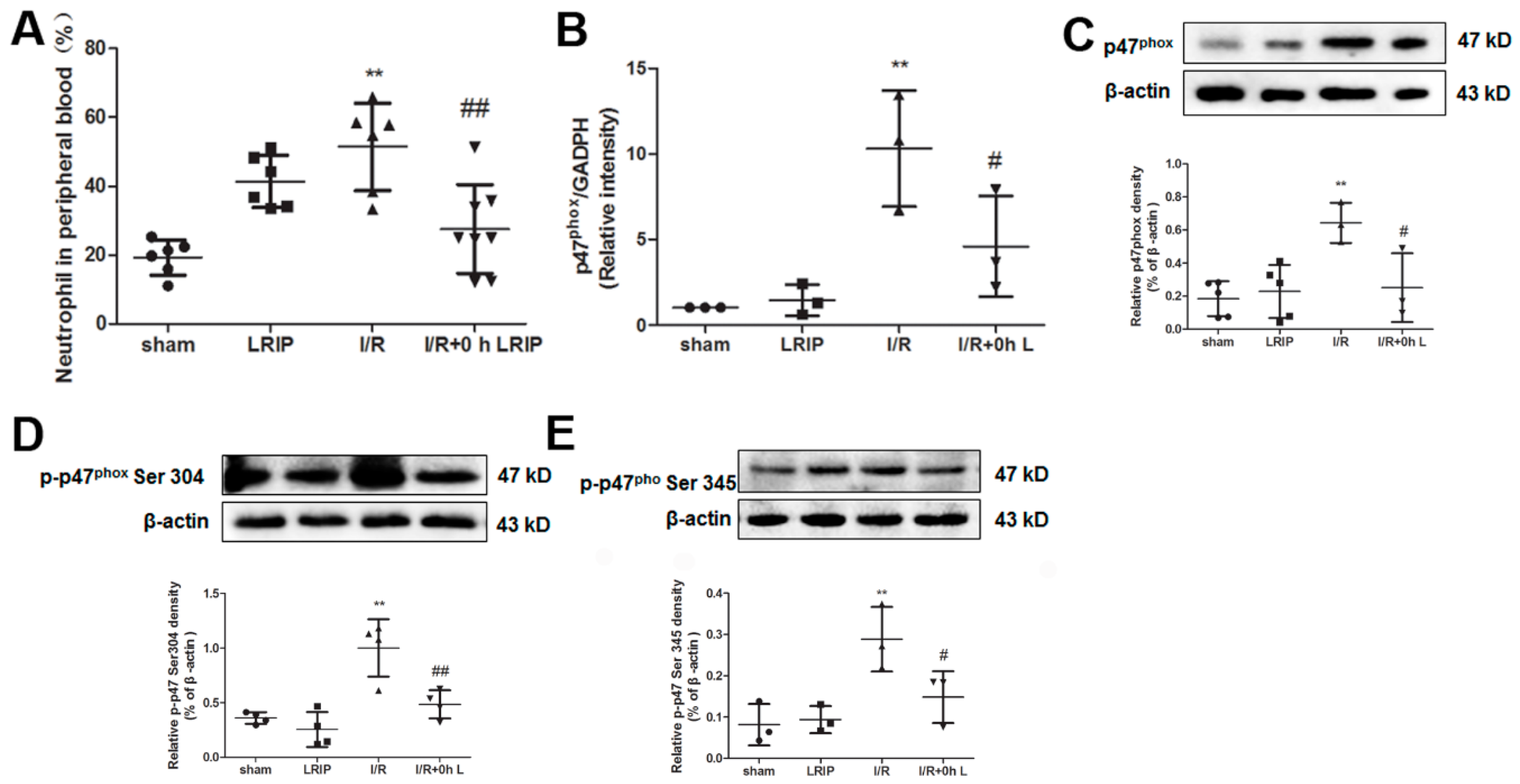
2.4. Effect of LRIP on the Infiltration and the Expression of Nicotinamide-Adenine Dinucleotide Phosphate (NADPH) Oxidase p47phox of Neutrophils in MCAO Rats
2.5. Effect of LRIP on the Number of Neutrophil and NADPH Oxidase Activation from the Peripheral Blood in MCAO Rats
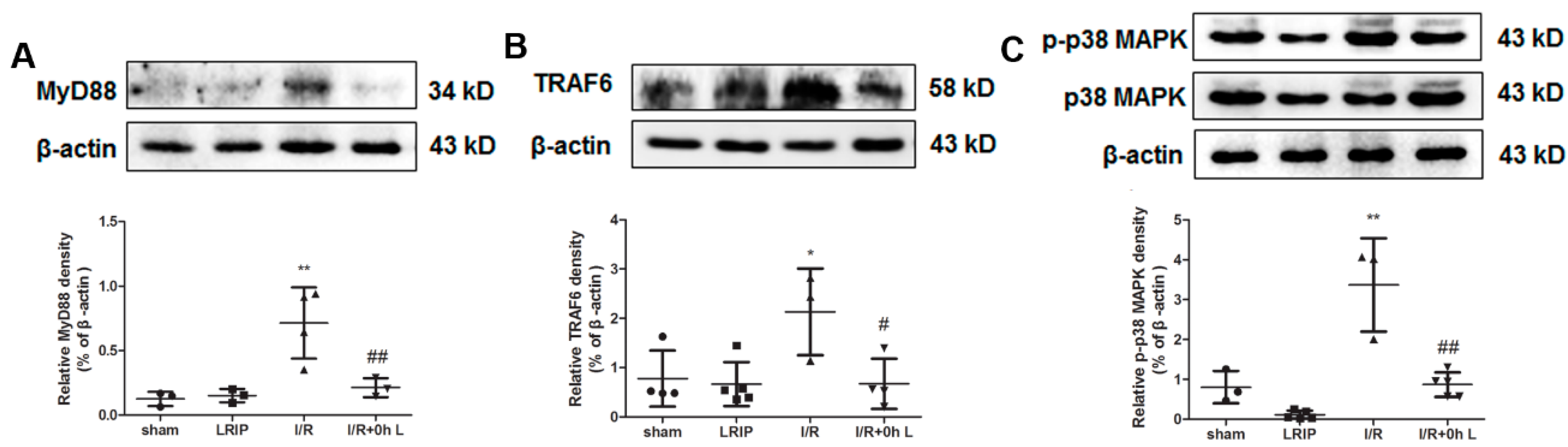
2.6. Effect of LRIP on Myeloid Differentiation Factor 88 (MyD88)-Tumor Necrosis Factor (TNF) Receptor-Associated Factor 6 (TRAF6)-p38 Mitogen-Activated Protein Kinase (p38-MAPK) Signaling Pathways in MCAO Rats
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Transient Focal Ischemia and Postconditioning in Rats
4.3. Behavioral Testing
4.4. Determination of Myeloperoxidase (MPO) Activity
4.5. Hematoxylin and Eosin Staining and Infarct Size Measurement
4.6. Immunofluorescence Staining
4.7. Peripheral Blood Count
4.8. Neutrophil Isolation
4.9. Real-Time PCR
4.10. Western Blotting
4.11. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barber, P.A.; Krishnamurthi, R.; Parag, V.; Anderson, N.E.; Ranta, A.; Kilfoyle, D.; Wong, E.; Green, G.; Arroll, B.; Bennett, D.A.; et al. Incidence of Transient Ischemic Attack in Auckland, New Zealand, in 2011 to 2012. Stroke 2016, 47, 2183–2188. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Hookway, C.; Weekes, C.E.; Royal College of Physicians Intercollegiate Stroke Working Party. Royal College of Physicians Intercollegiate Stroke Working Party evidence-based guidelines for the nutritional support of patients who have had a stroke. J. Hum. Nutr. Diet. 2014, 27, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Myint, P.K.; Hellkamp, A.S.; Fonarow, G.C.; Reeves, M.J.; Schwamm, L.H.; Schulte, P.J.; Xian, Y.; Suter, R.E.; Bhatt, D.L.; Saver, J.L.; et al. Prior Antithrombotic Use Is Associated With Favorable Mortality and Functional Outcomes in Acute Ischemic Stroke. Stroke 2016, 47, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C. Thrombolysis and Thrombectomy for Acute Ischemic Stroke: Strengths and Synergies. Semin. Thromb. Hemost. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kerendi, F.; Kin, H.; Halkos, M.E.; Jiang, R.; Zatta, A.J.; Zhao, Z.Q.; Guyton, R.A.; Vinten-Johansen, J. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res. Cardiol. 2005, 100, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Deftereos, S.; Giannopoulos, G.; Tzalamouras, V.; Raisakis, K.; Kossyvakis, C.; Kaoukis, A.; Panagopoulou, V.; Karageorgiou, S.; Avramides, D.; Toutouzas, K.; et al. Renoprotective effect of remote ischemic post-conditioning by intermittent balloon inflations in patients undergoing percutaneous coronary intervention. J. Am. Coll. Cardiol. 2013, 61, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Andreka, G.; Vertesaljai, M.; Szantho, G.; Font, G.; Piroth, Z.; Fontos, G.; Juhasz, E.D.; Szekely, L.; Szelid, Z.; Turner, M.S.; et al. Remote ischaemicpostconditioning protects the heart during acute myocardial infarction in pigs. Heart 2007, 93, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Dezfulian, C.; Garrett, M.; Gonzalez, N.R. Clinical application of preconditioning and postconditioning to achieve neuroprotection. Transl. Stroke Res. 2013, 4, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yang, J.; Lu, G.; Guo, J.; Dou, Y. Limb remote ischemic post-conditioning reduces brain reperfusion injury by reversing eNOS uncoupling. Indian J. Exp. Biol. 2014, 52, 597–605. [Google Scholar] [PubMed]
- Ren, C.; Yan, Z.; Wei, D.; Gao, X.; Chen, X.; Zhao, H. Limb remote ischemic postconditioning protects against focal ischemia in rats. Brain Res. 2009, 1288, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, S.; Liu, F.; Kang, J.; Xiao, A.; Li, F.; Zhang, C.; Yan, F.; Zhao, H.; Luo, M.; et al. Remote ischemic postconditioning alleviates cerebral ischemic injury by attenuating endoplasmic reticulum stress-mediated apoptosis. Transl. Stroke Res. 2014, 5, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.L.; Teixeira, R.K.; Yamaki, V.N.; Valente, A.L.; Silva, A.M.; Brito, M.V.; Percário, S. Remote ischemic conditioning temporarily improves antioxidant defense. J. Surg. Res. 2016, 200, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, S.; Wu, L.; Liu, K.; Zhang, Y.; Ma, G.; Wang, L. The role of p38MAPK signal pathway in the neuroprotective mechanism of limb postconditioning against rat cerebral ischemia/reperfusion injury. J. Neurol. Sci. 2015, 357, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Su, L.; Li, X.; Di, W.; Zhang, X.; Zhang, C.; He, T.; Zhu, X.; Zhang, Y.; Li, Y. Remote limb ischemic postconditioning protects mouse brain against cerebral ischemia/reperfusion injury via upregulating expression of Nrf2, HO-1 and NQO-1 in mice. Int. J. Neurosci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, X.; Zhang, M.; Zhou, F.; Lin, N.; Xia, Q.; Zhou, Y.; Qi, W.; Zong, Y.; Yang, H.; et al. Remote ischemic post-conditioning improves neurological function by AQP4 down-regulation in astrocytes. Behav. Brain Res. 2015, 289, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Jiang, L.; Zhang, M.; Zhou, F.; Qi, W.; Li, S.; Yang, H.; Zou, Y.; Xia, Q.; Zhou, X.; et al. Limb remote ischemic postconditioning protects cerebral ischemia from injury associated with expression of HIF-1α in rats. BMC Neurosci. 2015, 16, 97. [Google Scholar] [CrossRef] [PubMed]
- Fellman, V.; Raivio, K.O. Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatr. Res. 1997, 41, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Dar, N.J.; Bhat, Z.S.; Hussain, A.; Shah, A.; Liu, H.; Graham, S.H. Inflammation in ischemic stroke: Mechanisms, consequences and possible drug targets. CNS Neurol. Disord. Drug Targets 2014, 13, 1378–1396. [Google Scholar] [CrossRef] [PubMed]
- Tuma, R.F.; Steffens, S. Targeting the endocannabinod system to limit myocardial and cerebral ischemic and reperfusion injury. Curr. Pharm. Biotechnol. 2012, 13, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Grace, P.A. Ischaemia-reperfusion injury. Br. J. Surg. 1994, 81, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yoshioka, H.; Kim, G.S.; Jung, J.E.; Okami, N.; Sakata, H.; Maier, C.M.; Narasimhan, P.; Goeders, C.E.; Chan, P.H. Oxidative stress in ischemic brain damage: Mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid. Redox Signal. 2011, 14, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.L.; Bayraktutan, U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int. J. Stroke 2009, 4, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Vinten-Johansen, J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc. Res. 2004, 61, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Ritzel, R.M.; Pan, S.J.; Verma, R.; Wizeman, J.; Crapser, J.; Patel, A.R.; Lieberman, R.; Mohan, R.; McCullough, L.D. Early retinal inflammatory biomarkers in the middle cerebral artery occlusion model of ischemic stroke. Mol. Vis. 2016, 22, 575–588. [Google Scholar] [PubMed]
- Chen, H.; Song, Y.S.; Chan, P.H. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J. Cereb. Blood Flow Metab. 2009, 29, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Mulloy, D.P.; Le, L.T.; Laubach, V.E. NADPH oxidase mediates synergistic effects of IL-17 and TNF-α on CXCL1 expression by epithelial cells after lung ischemia-reperfusion. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L69–L79. [Google Scholar] [CrossRef] [PubMed]
- Braunersreuther, V.; Montecucco, F.; Asrih, M.; Ashri, M.; Pelli, G.; Galan, K.; Frias, M.; Burger, F.; Quinderé, A.L.; Montessuit, C.; et al. Role of NADPH oxidase isoforms NOX1, NOX2 and NOX4 in myocardial ischemia/reperfusion injury. J. Mol. Cell. Cardiol. 2013, 64, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Pamenter, M.E.; Ali, S.S.; Tang, Q.; Finley, J.C.; Gu, X.Q.; Dugan, L.L.; Haddad, G.G. An in vitro ischemic penumbral mimic perfusate increases NADPH oxidase-mediated superoxide production in cultured hippocampal neurons. Brain Res. 2012, 1452, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Li, Y.; Levy, R.M.; Fan, J.J.; Hackam, D.J.; Vodovotz, Y.; Yang, H.; Tracey, K.J.; Billiar, T.R.; Wilson, M.A. Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: Role of HMGB1-TLR4 signaling. J. Immunol. 2007, 178, 6573–6580. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Z.; Wu, C.C.; Huang, Y.C.; Huang, C.Y.; Yang, C.Y.; Lee, T.C.; Chen, C.F.; Yu, L.C. Neutrophil priming by hypoxic preconditioning protects against epithelial barrier damage and enteric bacterial translocation in intestinal ischemia/reperfusion. Lab. Investig. 2012, 92, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Hirooka, K.; Nakamura, T.; Itano, T.; Nishiyama, A.; Nagai, Y.; Shiraga, F. Neuroprotective effects of angiotensin II type 1 receptor (AT1-R) blocker via modulating AT1-R signaling and decreased extracellular glutamate levels. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4099–4110. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Yoon, D.W.; Kim, J.H.; Lee, J.H.; Lim, C.H. Effect of remote ischemic post-conditioning on systemic inflammatory response and survival rate in lipopolysaccharide-induced systemic inflammation model. J. Inflamm. (Lond.) 2014, 11, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.P.; Pang, X.F.; Zhang, L.H.; Tootle, S.; Harmouche, S.; Zhao, Z.Q. Attenuation of inflammatory response and reduction in infarct size by postconditioning are associated with downregulation of early growth response 1 during reperfusion in rat heart. Shock 2014, 41, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, H.; Marui, A.; Esaki, J.; Bir, S.C.; Ikeda, T.; Sakata, R. Remote postconditioning may attenuate ischaemia-reperfusion injury in the murine hindlimb through adenosine receptor activation. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Li, C.M.; Zhang, X.H.; Ma, X.J.; Luo, M. Limb ischemic postconditioning protects myocardium from ischemia-reperfusion injury. Scand. Cardiovasc. J. 2006, 40, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, M.; Bainbridge, A.; Broad, K.D.; Kawano, G.; Oliver-Taylor, A.; Rocha-Ferreira, E.; Alonso-Alconada, D.; Fierens, I.; Rostami, J.; Jane, H.K.; et al. Immediate remote ischemic postconditioning after hypoxia ischemia in piglets protects cerebral white matter but not grey matter. J. Cereb. Blood Flow Metab. 2016, 36, 1396–1411. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Lee, J.H.; Ko, J.S.; Min, J.J.; Gwak, M.S.; Kim, G.S.; Lee, S.K. Effect of remote ischemic postconditioning on patients undergoing living donor liver transplantation. Liver Transpl. 2014, 20, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Pecoraro, R.; di Raimondo, D.; di Sciacca, R.; Canino, B.; Arnao, V.; Buttà, C.; della Corte, V.; Maida, C.; Licata, G.; et al. Immune-inflammatory markers and arterial stiffness indexes in subjects with acute ischemic stroke with and without metabolic syndrome. Diabetol. Metab. Syndr. 2014, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Argano, C.; Colomba, D.; Cammà, C.; di Marco, V.; Cabibi, D.; Tuttolomondo, A.; Marchesini, G.; Pinto, A.; Licata, G.; et al. Epicardial fat, cardiac geometry and cardiac function in patients with non-alcoholic fatty liver disease: Association with the severity of liver disease. J. Hepatol. 2015, 62, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.F.; Luo, Y.M.; Liu, X.R.; Wang, R.L.; Zhao, H.P.; Yan, F.; Song, Z.J.; Luo, M.; Ji, X.M. AKT/GSK3β-dependent autophagy contributes to the neuroprotection of limb remote ischemic postconditioning in the transient cerebral ischemic rat model. CNS Neurosci. Ther. 2012, 18, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Liang, Y.; Huang, Z.; Jones, D.W.; Pritchard, K.A.; Zhang, H. Inhibition of myeloperoxidase oxidant production by N-acetyl lysyltyrosylcysteine amide reduces brain damage in a murine model of stroke. J. Neuroinflamm. 2016, 13, 119. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Cho, G.S.; Ryu, S.; Kim, H.J.; Song, H.Y.; Yune, T.Y.; Ju, C.; Kim, W.K. Post-ischemic treatment of WIB801C, standardized Cordyceps extract, reduces cerebral ischemic injury via inhibition of inflammatory cell migration. J. Ethnopharmacol. 2016, 186, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Dong, W.; Xie, P.; Cheng, P.; Bai, S.; Ren, Y.; Wang, G.; Chen, X.; Cui, C.; Zhuang, Y.; et al. The Effect of Pre-Condition Cerebella Fastigial Nucleus Electrical Stimulation within and beyond the Time Window of Thrombolytic on Ischemic Stroke in the Rats. PLoS ONE 2015, 10, e0128447. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhou, Y.T.; Chen, X.N.; Zhu, A.X.; Wu, B.H. Remote ischemic postconditioning protects against gastric mucosal lesions in rats. World J. Gastroenterol. 2014, 20, 9519–9527. [Google Scholar] [PubMed]
- Zhao, H.; Ren, C.; Chen, X.; Shen, J. From rapid to delayed and remote postconditioning: The evolving concept of ischemic postconditioning in brain ischemia. Curr. Drug Targets 2012, 13, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Bonheur, J.A.; Albadawi, H.; Patton, G.M.; Watkins, M.T. A noninvasive murine model of hind limb ischemia-reperfusion injury. J. Surg. Res. 2004, 116, 55–63. [Google Scholar] [CrossRef]
- Woodruff, T.M.; Arumugam, T.V.; Shiels, I.A.; Reid, R.C.; Fairlie, D.P.; Taylor, S.M. Protective effects of a potent C5a receptor antagonist on experimental acute limb ischemia-reperfusion in rats. J. Surg. Res. 2004, 116, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Couturier, J.Y.; Ding, Z.L.; Croci, N.; Plotkine, M.; Margaill, I. 3-Aminobenzamide reduces brain infarction and neutrophil infiltration after transient focal cerebral ischemia in mice. Exp. Neurol. 2003, 184, 973–980. [Google Scholar] [CrossRef]
- Athens, J.W.; Haab, O.P.; Raab, S.O.; Mauer, A.M.; Ashenbrucker, H.; Cartwright, G.E.; Wintrobe, M.M. Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J. Clin. Investig. 1961, 40, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Summers, C.; Rankin, S.M.; Condliffe, A.M.; Singh, N.; Peters, A.M.; Chilvers, E.R. Neutrophil kinetics in health and disease. Trends Immunol. 2010, 31, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Makni-Maalej, K.; Marzaioli, V.; Boussetta, T.; Belambri, S.A.; Gougerot-Pocidalo, M.A.; Hurtado-Nedelec, M.; Dang, P.M.; El-Benna, J. TLR8, but not TLR7, induces the priming of the NADPH oxidase activation in human neutrophils. J. Leukoc. Biol. 2015, 97, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Laing, R.J.; Jakubowski, J.; Laing, R.W. Middle cerebral artery occlusion without craniectomy in rats. Which method works best? Stroke 1993, 24, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Bederson, J.B.; Pitts, L.H.; Tsuji, M.; Nishimura, M.C.; Davis, R.L.; Bartkowski, H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke 1986, 17, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Schallert, T.; Fleming, S.M.; Leasure, J.L.; Tillerson, J.L.; Bland, S.T. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 2000, 39, 777–787. [Google Scholar] [CrossRef]
- Borlongan, C.V.; Tajima, Y.; Trojanowski, J.Q.; Lee, V.M.; Sanberg, P.R. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp. Neurol. 1998, 149, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Mullane, K.M.; Kraemer, R.; Smith, B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J. Pharmacol. Methods 1985, 14, 157–167. [Google Scholar] [CrossRef]
- Cao, G.; Ye, X.; Xu, Y.; Yin, M.; Chen, H.; Kou, J.; Yu, B. YiQiFuMai powder injection ameliorates blood-brain barrier dysfunction and brain edema after focal cerebral ischemia-reperfusion injury in mice. Drug Des. Dev. Ther. 2016, 10, 315–325. [Google Scholar]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Ye, X.; Zhang, J.; Tang, T.; Li, L.; Lu, P.; Wu, Q.; Yu, B.; Kou, J. Limb Remote Ischemic Postconditioning Reduces Ischemia-Reperfusion Injury by Inhibiting NADPH Oxidase Activation and MyD88-TRAF6-P38MAP-Kinase Pathway of Neutrophils. Int. J. Mol. Sci. 2016, 17, 1971. https://doi.org/10.3390/ijms17121971
Chen G, Ye X, Zhang J, Tang T, Li L, Lu P, Wu Q, Yu B, Kou J. Limb Remote Ischemic Postconditioning Reduces Ischemia-Reperfusion Injury by Inhibiting NADPH Oxidase Activation and MyD88-TRAF6-P38MAP-Kinase Pathway of Neutrophils. International Journal of Molecular Sciences. 2016; 17(12):1971. https://doi.org/10.3390/ijms17121971
Chicago/Turabian StyleChen, Gangling, Xinyi Ye, Jiangwei Zhang, Tingli Tang, Lin Li, Peirong Lu, Qi Wu, Boyang Yu, and Junping Kou. 2016. "Limb Remote Ischemic Postconditioning Reduces Ischemia-Reperfusion Injury by Inhibiting NADPH Oxidase Activation and MyD88-TRAF6-P38MAP-Kinase Pathway of Neutrophils" International Journal of Molecular Sciences 17, no. 12: 1971. https://doi.org/10.3390/ijms17121971
APA StyleChen, G., Ye, X., Zhang, J., Tang, T., Li, L., Lu, P., Wu, Q., Yu, B., & Kou, J. (2016). Limb Remote Ischemic Postconditioning Reduces Ischemia-Reperfusion Injury by Inhibiting NADPH Oxidase Activation and MyD88-TRAF6-P38MAP-Kinase Pathway of Neutrophils. International Journal of Molecular Sciences, 17(12), 1971. https://doi.org/10.3390/ijms17121971