Future Prospects for Scaffolding Methods and Biomaterials in Skin Tissue Engineering: A Review
Abstract
:1. Introduction
2. Regenerative Skin Tissue Engineering and Wound Healing Using Various Skin Substitutes
3. Scaffolding Approaches and Different Types of Scaffolds in Skin Tissue Engineering
3.1. Porous Scaffolds
3.2. Fibrous Scaffolds
3.3. Acellular Scaffolds
3.4. Scaffolds Based on Hydrogels
3.5. Microsphere Scaffolds
3.6. Polymer–Bioceramic Composite Scaffold
4. Biomaterials and Nanobiomaterials Used for Several Scaffolding Materials in Skin Tissue Engineering
4.1. Natural Biomaterials of Protein Nature
4.2. Polysaccharide Natural Biomaterials
4.3. Synthetic Biomaterials
4.4. Composite Biomaterials
5. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| CA | chitosan–alginate |
| HA | hyaluronic acid |
| ECM | extracellular matrix |
| PBT | Polybutylene terephthalate |
| PCL | Polycaprolactone |
| PDLLA | poly(d,l-lactic acid or d,l-lactide) |
| PEE | polyester elastomer |
| PEO | polyethylene oxide |
| PEG | Polyethylene glycol |
| PGA | polyglycolide |
| PLA | poly(lactic acid) |
| PLGA | poly(lactic-co-glycolic acid) |
| PTFE | polytetrafluoroethylene |
| PVP | poly(N-vinyl-2-pyrrolidone) |
| SF | Silk fibroin |
References
- Mogosanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, R.V.; James, S.L.; James, S.E. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J. R. Soc. Interface R. Soc. 2010, 7, 229–258. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.; Greenwood, J.; Cleland, H.; Woodruff, P.; Maddern, G. Bioengineered skin substitutes for the management of burns: A systematic review. Burns J. Int. Soc. Burn Inj. 2007, 33, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Alaribe, F.N.; Manoto, S.L.; Motaung, S. Scaffolds from biomaterials: Advantages and limitations in boneand tissue engineering. Biol. Sect. Cell Mol. Biol. 2016, 71, 353–366. [Google Scholar] [CrossRef]
- Brahatheeswaran Dhandayuthapani, Y.Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Debels, H.; Hamdi, M.; Abberton, K.; Morrison, W. Dermal matrices and bioengineered skin substitutes: A critical review of current options. Plast. Reconstr. Surg. Glob. Open 2015, 3, e284. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Boroujeni, S.M.; Omidvarkordshouli, N.; Soleimani, M. Advances in skin regeneration: Application of electrospun scaffolds. Adv. Healthc. Mater. 2015, 4, 1114–1133. [Google Scholar] [CrossRef] [PubMed]
- Nyame, T.T.; Chiang, H.A.; Leavitt, T.; Ozambela, M.; Orgill, D.P. Tissue-engineered skin substitutes. Plast. Reconstr. Surg. 2015, 136, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Fergal, O.B.J. Biomaterials and scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Cascone, M.G.; Barbani, N.; Cristallini, C.; Giusti, P.; Ciardelli, G.; Lazzeri, L. Bioartificial polymeric materials based on polysaccharides. J. Biomater. Sci. Polym. Ed. 2001, 12, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Wang, M. Production and evaluation of biodegradable composites based on phb-phv copolymer. Biomaterials 2002, 23, 2631–2639. [Google Scholar] [CrossRef]
- Ciardelli, G.; Chiono, V.; Vozzi, G.; Pracella, M.; Ahluwalia, A.; Barbani, N.; Cristallini, C.; Giusti, P. Blends of poly-(ε-caprolactone) and polysaccharides in tissue engineering applications. Biomacromolecules 2005, 6, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Gunatillake, P.; Mayadunne, R.; Adhikari, R. Recent developments in biodegradable synthetic polymers. Biotechnol. Annu. Rev. 2006, 12, 301–347. [Google Scholar] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Roether, J.A.; Boccaccini, A.R.; Hench, L.L.; Maquet, V.; Gautier, S.; Jerjme, R. Development and in vitro characterisation of novel bioresorbable and bioactive composite materials based on polylactide foams and bioglass for tissue engineering applications. Biomaterials 2002, 23, 3871–3878. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Yannas, I.V. Classes of Materials Used in Medicine: Natural Materials in Biomaterials Science. In An Introduction Tomaterials in Medicine; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2004; pp. 127–136. [Google Scholar]
- Akbarzadeh, R.; Yousefi, A.M. Effects of processing parameters in thermally induced phase separation technique on porous architecture of scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Braghirolli, D.I.; Steffens, D.; Pranke, P. Electrospinning for regenerative medicine: A review of the main topics. Drug Discov. Today 2014, 19, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Dalton, P.D.; Vaquete, C.; Farrugia, B.L.; Dargaville, T.R.; Brown, T.D.; Hutmacher, D.W. Electrospinning and additive manufacturing: Converging technologies. Biomater. Sci. 2013, 1, 171–185. [Google Scholar] [CrossRef]
- Giannitelli, S.M.; Accoto, D.; Trombetta, M.; Rainer, A. Current trends in the design of scaffolds for computer-aided tissue engineering. Acta Biomater. 2014, 10, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.D.; Kim, B.S.; Mooney, D.J. Open pore biodegradable matrices formed with gas foaming. J. Biomed. Mater. Res. 1998, 42, 396–402. [Google Scholar] [CrossRef]
- Ho, M.H.; Kuo, P.Y.; Hsieh, H.J.; Hsien, T.Y.; Hou, L.T.; Lai, J.Y.; Wang, D.M. Preparation of porous scaffolds by using freeze-extraction and freeze-gelation methods. Biomaterials 2004, 25, 129–138. [Google Scholar] [CrossRef]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Grijpma, D.W.; Feijen, J. Porous polymeric structures for tissue engineering prepared by a coagulation, compression moulding and salt leaching technique. Biomaterials 2003, 24, 1937–1947. [Google Scholar] [CrossRef]
- Liao, C.J.; Chen, C.F.; Chen, J.H.; Chiang, S.F.; Lin, Y.J.; Chang, K.Y. Fabrication of porous biodegradable polymer scaffolds using a solvent merging/particulate leaching method. J. Biomed. Mater. Res. 2002, 59, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Kotaki, M.; Inai, R.; Ramakrishna, S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005, 11, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.L.; Dennis, R.G.; Kileny, J.L.; Mooney, D.J. Salt fusion: An approach to improve pore interconnectivity within tissue engineering scaffolds. Tissue Eng. 2002, 8, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.S.; Park, T.G. Biodegradable polymeric microcellular foams by modified thermally induced phase separation method. Biomaterials 1999, 20, 1783–1790. [Google Scholar] [PubMed]
- Nam, Y.S.; Yoon, J.J.; Park, T.G. A novel fabrication method of macroporous biodegradable polymer scaffolds using gas foaming salt as a porogen additive. J. Biomed. Mater. Res. 2000, 53, 1–7. [Google Scholar] [CrossRef]
- Qian, L.Z. Controlled freezing and freeze drying: A versatile route for porous and micro-/nano-structured materials. J. Chem. Technol. Biotechnol. 2011, 86, 172–184. [Google Scholar] [CrossRef]
- Salerno, A.; Oliviero, M.; di Maio, E.; Iannace, S.; Netti, P.A. Design of porous polymeric scaffolds by gas foaming of heterogeneous blends. J. Mater. Sci. Mater. Med. 2009, 20, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Tamayol, A.; Akbari, M.; Annabi, N.; Paul, A.; Khademhosseini, A.; Juncker, D. Fiber-based tissue engineering: Progress, challenges, and opportunities. Biotechnol. Adv. 2013, 31, 669–687. [Google Scholar] [CrossRef] [PubMed]
- Whang, K.; Thomas, C.H.; Healy, K.E.; Nuber, G. A novel method to fabricate bioabsorbable scaffolds. Polymer 1995, 36, 837–842. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; Okuhara, M.; Sakai, H.; Sakurai, Y. Mechanism of cell detachment from temperature-modulated, hydrophilic-hydrophobic polymer surfaces. Biomaterials 1995, 16, 297–303. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; Sakai, H.; Sakurai, Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J. Biomed. Mater. Res. 1993, 27, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Takezawa, T.; Mori, Y.; Yoshizato, K. Cell culture on a thermo-responsive polymer surface. Biotechnology 1990, 8, 854–856. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Blaker, J.J. Bioactive composite materials for tissue engineering scaffolds. Expert Rev. Med. Devices 2005, 2, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Hui, T.Y.; Chan, O.C.; So, K.F.; Lu, W.; Cheung, K.M.; Salomatina, E.; Yaroslavsky, A. Photochemical cross-linking for collagen-based scaffolds: A study on optical properties, mechanical properties, stability, and hematocompatibility. Tissue Eng. 2007, 13, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; So, K.F. Photochemical crosslinking improves the physicochemical properties of collagen scaffolds. J. Biomed. Mater. Res. Part A 2005, 75, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, E.; Chulia, D.; Pouget, C.; Viana, M. Fabrication of porous substrates: A review of processes using pore forming agents in the biomaterial field. J. Pharm. Sci. 2008, 97, 1135–1154. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Baez, C.E.; Atala, A. Biomaterials for tissue engineering. World J. Urol. 2000, 18, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.L.; Wilcox, H.E.; Korossis, S.A.; Fisher, J.; Ingham, E. The use of acellular matrices for the tissue engineering of cardiac valves. Proc. Inst. Mech. Eng. 2008, 22, 129–143. [Google Scholar] [CrossRef]
- Borschel, G.H.; Huang, Y.C.; Calve, S.; Arruda, E.M.; Lynch, J.B.; Dow, D.E.; Kuzon, W.M.; Dennis, R.G.; Brown, D.L. Tissue engineering of recellularized small-diameter vascular grafts. Tissue Eng. 2005, 11, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Hall, S. Axonal regeneration through acellular muscle grafts. J. Anat. 1997, 190, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.H.; Korossis, S.; Howling, G.; Fisher, J.; Ingham, E. The use of ultrasonication to aid recellularization of acellular natural tissue scaffolds for use in anterior cruciate ligament reconstruction. Tissue Eng. 2007, 13, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Lanza, R.P.; Hayes, J.L.; Chick, W.L. Encapsulated cell technology. Nat. Biotechnol. 1996, 14, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Orive, G.; Hernandez, R.M.; Gascon, A.R.; Calafiore, R.; Chang, T.M.; de Vos, P.; Hortelano, G.; Hunkeler, D.; Lacik, I.; Shapiro, A.M.; et al. Cell encapsulation: Promise and progress. Nat. Med. 2003, 9, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Orive, G.; Hernandez, R.M.; Rodriguez Gascon, A.; Calafiore, R.; Chang, T.M.; de Vos, P.; Hortelano, G.; Hunkeler, D.; Lacik, I.; Pedraz, J.L. History, challenges and perspectives of cell microencapsulation. Trends Biotechnol. 2004, 22, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Uludag, H.; de Vos, P.; Tresco, P.A. Technology of mammalian cell encapsulation. Adv. Drug Deliv. Rev. 2000, 42, 29–64. [Google Scholar] [CrossRef]
- Dhakshinamoorthy Sundaramurthi, U.M.K. Swaminathan Sethuraman. Electrospun nanofibers as scaffolds for skin tissue engineering. Polym. Rev. 2014, 54, 348–376. [Google Scholar] [CrossRef]
- Liu, X.; Lin, T.; Fang, J.; Yao, G.; Zhao, H.; Dodson, M.; Wang, X. In vivo wound healing and antibacterial performances of electrospun nanofibre membranes. J. Biomed. Mater. Res. Part A 2010, 94, 499–508. [Google Scholar]
- Zhang, R.; Ma, P.X. Porous poly(l-lactic acid)/apatite composites created by biomimetic process. J. Biomed. Mater. Res. 1999, 45, 285–293. [Google Scholar] [CrossRef]
- Ouriemchi, E.M.; Vergnaud, J.M. Processes of drug transfer with three different polymeric systems with transdermal drug delivery. Comput. Theor. Polym. Sci. 2000, 10, 391–401. [Google Scholar]
- Wei, G.; Ma, P.X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Grijpma, D.W.; Feijen, J. Preparation of porous poly(ε-caprolactone) structures. Macromol. Rapid Commun. 2002, 23, 247–252. [Google Scholar] [CrossRef]
- Kumbar, S.G.; Nukavarapu, S.P.; James, R.; Nair, L.S.; Laurencin, C.T. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials 2008, 29, 4100–4107. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Algan, C.; Jacobs, V.; John, M.; Oksman, K.; Mathew, A.P. Electrospun chitosan-based nanocomposite mats reinforced with chitin nanocrystals for wound dressing. Carbohydr. Polym. 2014, 109, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Venugopal, J.R.; Sundarrajan, S.; Mukherjee, S.; Sridhar, R.; Ramakrishna, S. Composite poly-l-lactic acid/poly-(α,β)-dl-aspartic acid/collagen nanofibrous scaffolds for dermal tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.-Y.; Wang, Z.-M.; Rena, J.; Zhang, C.-Y. Electrospinning of gelatin and gelatin/poly(l-lactide) blend and its characteristics for wound dressing. Mater. Sci. Eng. C 2009, 29, 1822–1828. [Google Scholar] [CrossRef]
- Zhong, W.; Xing, M.M.; Maibach, H.I. Nanofibrous materials for wound care. Cutan. Ocul. Toxicol. 2010, 29, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Still, J.; Glat, P.; Silverstein, P.; Griswold, J.; Mozingo, D. The use of a collagen sponge/living cell composite material to treat donor sites in burn patients. Burns J. Int. Soc. Burn Inj. 2003, 29, 837–841. [Google Scholar] [CrossRef]
- Boyce, S.T.; Kagan, R.J.; Greenhalgh, D.G.; Warner, P.; Yakuboff, K.P.; Palmieri, T.; Warden, G.D. Cultured skin substitutes reduce requirements for harvesting of skin autograft for closure of excised, full-thickness burns. J. Trauma 2006, 60, 821–829. [Google Scholar] [PubMed]
- Supp, D.M.; Boyce, S.T. Engineered skin substitutes: Practices and potentials. Clin. Dermatol. 2005, 23, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Yi, S.B.; Hwang, J.W.; Yang, W.S.; Lee, K.K. Treatment of bone and tendon-exposed wounds using terudermis. J. Korean Soc. Plast. Reconstr. Surg. 1999, 26, 491–497. [Google Scholar]
- Lee, J.W.; Jang, Y.C.; Oh, S.J. Use of the artificial dermis for free radial forearm flap donor site. Ann. Plast. Surg. 2005, 55, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Yurugi, S.; Hatoko, M.; Kuwahara, M.; Tanaka, A.; Iioka, H.; Niitsuma, K. Usefulness and limitations of artificial dermis implantation for posttraumatic deformity. Aesthet. Plast. Surg. 2002, 26, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Akita, S.; Tanaka, K.; Hirano, A. Lower extremity reconstruction after necrotising fasciitis and necrotic skin lesions using a porcine-derived skin substitute. J. Plast. Reconstr. Aesthet. Surg. 2006, 59, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.O.; Lee, J.W.; Koh, J.H.; Seo, D.K.; Oh, S.J.; Jang, Y.C. Burn management and reconstruction using artificial dermis pelnac. J. Korean Burn Soc. 2006, 9, 115–120. [Google Scholar]
- Suzuki, S.; Kawai, K.; Ashoori, F.; Morimoto, N.; Nishimura, Y.; Ikada, Y. Long-term follow-up study of artificial dermis composed of outer silicone layer and inner collagen sponge. Br. J. Plast. Surg. 2000, 53, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Dong, W.R.; Xiao, Y.Q.; Zhao, B.L.; Hu, G.D.; An, L.B. Preparation and bioactivity of human hair keratin-collagen sponge, a new type of dermal analogue. Nan Fang Yi Ke Da Xue Xue Bao J. South. Med. Univ. 2006, 26, 131–138. [Google Scholar]
- Yeo, J.H.; Lee, K.G.; Kim, H.C.; Oh, H.Y.L.; Kim, A.J.; Kim, S.Y. The effects of Pva/chitosan/fibroin (PCF)-blended spongy sheets on wound healing in rats. Biol. Pharm. Bull. 2000, 23, 1220–1223. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lim, J.; Teoh, S.H. Review: Development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol. Adv. 2013, 31, 688–705. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Soleimani, M.; Shabani, I.; Atyabi, F.; Ahvaz, H.H.; Rashidi, A. Protein encapsulated in electrospun nanofibrous scaffolds for tissue engineering applications. Polym. Int. 2013, 62, 1250–1256. [Google Scholar] [CrossRef]
- Bhattarai, S.R.; Bhattarai, N.; Yi, H.K.; Hwang, P.H.; Cha, D.I.; Kim, H.Y. Novel biodegradable electrospun membrane: Scaffold for tissue engineering. Biomaterials 2004, 25, 2595–2602. [Google Scholar] [CrossRef] [PubMed]
- Vasita, R.; Katti, D.S. Nanofibers and their applications in tissue engineering. Int. J. Nanomed. 2006, 1, 15–30. [Google Scholar] [CrossRef]
- Geng, X.; Kwon, O.H.; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005, 26, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Chen, J.; Karageorgiou, V.; Altman, G.H.; Kaplan, D.L. Human bone marrow stromal cell responses on electrospun silk fibroin mats. Biomaterials 2004, 25, 1039–1047. [Google Scholar] [CrossRef]
- Kenawy el, R.; Bowlin, G.L.; Mansfield, K.; Layman, J.; Simpson, D.G.; Sanders, E.H.; Wnek, G.E. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. J. Control. Release Off. J. Control. Release Soc. 2002, 81, 57–64. [Google Scholar] [CrossRef]
- Li, W.J.; Danielson, K.G.; Alexander, P.G.; Tuan, R.S. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly(ε-caprolactone) scaffolds. J. Biomed. Mater. Res. Part A 2003, 67, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of collagen nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.M.; Xu, C.Y.; Kotaki, M.; Ramakrishna, S. Electrospun P(LLA-CL) nanofiber: A biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials 2004, 25, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Riboldi, S.A.; Sampaolesi, M.; Neuenschwander, P.; Cossu, G.; Mantero, S. Electrospun degradable polyesterurethane membranes: Potential scaffolds for skeletal muscle tissue engineering. Biomaterials 2005, 26, 4606–4615. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, K.; Hattori, K.; Ishimoto, Y.; Yamauchi, J.; Habata, T.; Takakura, Y.; Ohgushi, H.; Fukuchi, T.; Sato, M. Cartilage regeneration using mesenchymal stem cells and a three-dimensional poly-lactic-glycolic acid (PLGA) scaffold. Biomaterials 2005, 26, 4273–4279. [Google Scholar] [CrossRef] [PubMed]
- Um, I.C.; Fang, D.; Hsiao, B.S.; Okamoto, A.; Chu, B. Electro-spinning and electro-blowing of hyaluronic acid. Biomacromolecules 2004, 5, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of nano/micro scale poly(l-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ouyang, H.; Lim, C.T.; Ramakrishna, S.; Huang, Z.M. Electrospinning of gelatin fibers and gelatin/pcl composite fibrous scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 72, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Verreck, G.; Chun, I.; Rosenblatt, J.; Peeters, J.; Dijck, A.V.; Mensch, J.; Noppe, M.; Brewster, M.E. Incorporation of drugs in an amorphous state into electrospun nanofibers composed of a water-insoluble, nonbiodegradable polymer. J. Control. Release Off. J. Control. Release Soc. 2003, 92, 349–360. [Google Scholar] [CrossRef]
- Rieger, K.A.; Birch, N.P.; Schiffman, J.D. Schiffman Designing electrospun nanofiber mats to promote wound healing—A review. J. Mater. Chem. B 2013, 1, 4531–4541. [Google Scholar] [CrossRef]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Duan, H.; Fu, W.; Cao, Y.; Jie Zhang, W.; Zhang, Y. Effect of inhomogeneity of the electrospun fibrous scaffolds of gelatin/polycaprolactone hybrid on cell proliferation. J. Biomed. Mater. Res. Part A 2015, 103, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Lim, T.-C.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientifi Publishing: Singapore, 2005. [Google Scholar]
- Hong, Y.; Chen, X.; Jing, X.; Fan, H.; Gu, Z.; Zhang, X. Fabrication and drug delivery of ultrathin mesoporous bioactive glass hollow fibers. Adv. Funct. Mater. 2010, 20, 1503–1510. [Google Scholar] [CrossRef]
- Chen, F.; Yoo, J.J.; Atala, A. Acellular collagen matrix as a possible “off the shelf” biomaterial for urethral repair. Urology 1999, 54, 407–410. [Google Scholar] [CrossRef]
- Dahms, S.E.; Piechota, H.J.; Dahiya, R.; Lue, T.F.; Tanagho, E.A. Composition and biomechanical properties of the bladder acellular matrix graft: Comparative analysis in rat, pig and human. Br. J. Urol. 1998, 82, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Meng, J.; Oberpenning, F.; Atala, A. Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology 1998, 51, 221–225. [Google Scholar] [CrossRef]
- Dahms, S.E.; Piechota, H.J.; Nunes, L.; Dahiya, R.; Lue, T.F.; Tanagho, E.A. Free ureteral replacement in rats: Regeneration of ureteral wall components in the acellular matrix graft. Urology 1997, 50, 818–825. [Google Scholar] [CrossRef]
- Wilson, G.J.; Courtman, D.W.; Klement, P.; Lee, J.M.; Yeger, H. Acellular matrix: A biomaterials approach for coronary artery bypass and heart valve replacement. Ann. Thorac. Surg. 1995, 60, S353–S358. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Shores, J.T.; Gabriel, A.; Gupta, S. Skin substitutes and alternatives: A review. Adv. Skin Wound Care 2007, 20, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.F.; Dubin, D.; Lavigne, L.; Logan, B.; Dvorak, H.F.; van de Water, L. Macrophages and fibroblasts express embryonic fibronectins during cutaneous wound healing. Am. J. Pathol. 1993, 142, 793–801. [Google Scholar] [PubMed]
- Caravaggi, C.; de Giglio, R.; Pritelli, C.; Sommaria, M.; Dalla Noce, S.; Faglia, E.; Mantero, M.; Clerici, G.; Fratino, P.; Dalla Paola, L.; et al. Hyaff 11-based autologous dermal and epidermal grafts in the treatment of noninfected diabetic plantar and dorsal foot ulcers: A prospective, multicenter, controlled, randomized clinical trial. Diabetes Care 2003, 26, 2853–2859. [Google Scholar] [CrossRef] [PubMed]
- Daamen, W.F.; van Moerkerk, H.T.; Hafmans, T.; Buttafoco, L.; Poot, A.A.; Veerkamp, J.H.; van Kuppevelt, T.H. Preparation and evaluation of molecularly-defined collagen-elastin-glycosaminoglycan scaffolds for tissue engineering. Biomaterials 2003, 24, 4001–4009. [Google Scholar] [CrossRef]
- Debelle, L.; Tamburro, A.M. Elastin: Molecular description and function. Int. J. Biochem. Cell Biol. 1999, 31, 261–272. [Google Scholar] [CrossRef]
- Hodde, J.P.; Ernst, D.M.; Hiles, M.C. An investigation of the long-term bioactivity of endogenous growth factor in oasis wound matrix. J. Wound Care 2005, 14, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Lamberg, S.I.; Stoolmiller, A.C. Glycosaminoglycans. A biochemical and clinical review. J. Investig. Dermatol. 1974, 63, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Ma, L.; Gao, C.; Mao, Z.; Zhou, J.; Shen, J.; Hu, X.; Han, C. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef]
- Mast, B.A.; Haynes, J.H.; Krummel, T.M.; Diegelmann, R.F.; Cohen, I.K. In vivo degradation of fetal wound hyaluronic acid results in increased fibroplasia, collagen deposition, and neovascularization. Plast. Reconstr. Surg. 1992, 89, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Price, R.D.; Berry, M.G.; Navsaria, H.A. Hyaluronic acid: The scientific and clinical evidence. J. Plast. Reconstr. Aesthet. Surg. 2007, 60, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Vats, A.; Tolley, N.S.; Polak, J.M.; Gough, J.E. Scaffolds and biomaterials for tissue engineering: A review of clinical applications. Clin. Otolaryngol. Allied Sci. 2003, 28, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, H.J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials 2006, 27, 6064–6082. [Google Scholar] [CrossRef] [PubMed]
- West, D.C.; Shaw, D.M.; Lorenz, P.; Adzick, N.S.; Longaker, M.T. Fibrotic healing of adult and late gestation fetal wounds correlates with increased hyaluronidase activity and removal of hyaluronan. Int. J. Biochem. Cell Biol. 1997, 29, 201–210. [Google Scholar] [CrossRef]
- Zavan, B.; Cortivo, R.; Tonello, C.; Abatangelo, G. Gland cell cultures into 3D hyaluronan-based scaffolds. J. Mater. Sci. Mater. Med. 2003, 14, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.P.; Zhang, Y.Z.; Lim, C.T. Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 510–525. [Google Scholar] [CrossRef] [PubMed]
- Ono, I.; Tateshita, T.; Inoue, M. Effects of a collagen matrix containing basic fibroblast growth factor on wound contraction. J. Biomed. Mater. Res. 1999, 48, 621–630. [Google Scholar] [CrossRef]
- Dai, N.T.; Williamson, M.R.; Khammo, N.; Adams, E.F.; Coombes, A.G. Composite cell support membranes based on collagen and polycaprolactone for tissue engineering of skin. Biomaterials 2004, 25, 4263–4271. [Google Scholar] [CrossRef] [PubMed]
- Melman, L.; Jenkins, E.D.; Hamilton, N.A.; Bender, L.C.; Brodt, M.D.; Deeken, C.R.; Greco, S.C.; Frisella, M.M.; Matthews, B.D. Early biocompatibility of crosslinked and non-crosslinked biologic meshes in a porcine model of ventral hernia repair. Hernia J. Hernias Abdom. Wall Surg. 2011, 15, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Almany, L.; Seliktar, D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3d cell cultures. Biomaterials 2005, 26, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Bano, F.; Barrington, J.W.; Dyer, R. Comparison between porcine dermal implant (permacol) and silicone injection (macroplastique) for urodynamic stress incontinence. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2005, 16, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Bello, Y.M.; Falabella, A.F.; Eaglstein, W.H. Tissue-engineered skin. Current status in wound healing. Am. J. Clin. Dermatol. 2001, 2, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.V.; Silverio, L.; Sperling, J.W. Strategies in biologic augmentation of rotator cuff repair: A review. Clin. Orthop. Relat. Res. 2010, 468, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.; Burd, A. “Xenograft” dressing in the treatment of burns. Clin. Dermatol. 2005, 23, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Healy, C.M.; Boorman, J.G. Comparison of e-z derm and jelonet dressings for partial skin thickness burns. Burns Incl. Therm. Inj. 1989, 15, 52–54. [Google Scholar] [CrossRef]
- Hsu, P.W.; Salgado, C.J.; Kent, K.; Finnegan, M.; Pello, M.; Simons, R.; Atabek, U.; Kann, B. Evaluation of porcine dermal collagen (permacol) used in abdominal wall reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, T.M.; Cambrey, A.; Williams, G.; Sanders, R.; Green, C.J. Evaluation of permacol as a cultured skin equivalent. Burns J. Int. Soc. Burn Inj. 2008, 34, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Pitkin, L.; Rimmer, J.; Lo, S.; Hosni, A. Aesthetic augmentation rhinoplasty with permacol: How we do it. Clin. Otolaryngol. 2008, 33, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Saray, A. Porcine dermal collagen (permacol) for facial contour augmentation: Preliminary report. Aesthet. Plast. Surg. 2003, 27, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, J.; Bailoor, D.; Widdison, A.L. Treatment of anastomotic-vaginal fistula complicating colorectal resection using permacol interposition in lieu of omentum. Int. J. Colorectal Dis. 2007, 22, 845–846. [Google Scholar] [CrossRef] [PubMed]
- Troy, J.; Karlnoski, R.; Downes, K.; Brown, K.S.; Cruse, C.W.; Smith, D.J.; Payne, W.G. The use of EZ Derm(R) in partial-thickness burns: An institutional review of 157 patients. Eplasty 2013, 13, e14. [Google Scholar] [PubMed]
- Vanstraelen, P. Comparison of calcium sodium alginate (KALTOSTAT) and porcine xenograft (E-Z DERM) in the healing of split-thickness skin graft donor sites. Burns J. Int. Soc. Burn Inj. 1992, 18, 145–148. [Google Scholar] [CrossRef]
- Elliott, W.H.; Bonani, W.; Maniglio, D.; Motta, A.; Tan, W.; Migliaresi, C. Silk hydrogels of tunable structure and viscoelastic properties using different chronological orders of genipin and physical cross-linking. ACS Appl. Mater. Interfaces 2015, 7, 12099–12108. [Google Scholar] [CrossRef] [PubMed]
- Eyrich, D.; Brandl, F.; Appel, B.; Wiese, H.; Maier, G.; Wenzel, M.; Staudenmaier, R.; Goepferich, A.; Blunk, T. Long-term stable fibrin gels for cartilage engineering. Biomaterials 2007, 28, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.J.; Park, J.; Li, C.; Jin, H.J.; Valluzzi, R.; Kaplan, D.L. Structure and properties of silk hydrogels. Biomacromolecules 2004, 5, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.J.; Smith, M.K.; Mooney, D.J. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials 2003, 24, 4023–4029. [Google Scholar] [CrossRef]
- Solchaga, L.A.; Gao, J.; Dennis, J.E.; Awadallah, A.; Lundberg, M.; Caplan, A.I.; Goldberg, V.M. Treatment of osteochondral defects with autologous bone marrow in a hyaluronan-based delivery vehicle. Tissue Eng. 2002, 8, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.G.; Rosenblatt, J. Collagen gel systems for sustained delivery and tissue engineering. Adv. Drug Deliv. Rev. 2003, 55, 1631–1649. [Google Scholar] [CrossRef] [PubMed]
- Behravesh, E.; Mikos, A.G. Three-dimensional culture of differentiating marrow stromal osteoblasts in biomimetic poly(propylene fumarate-co-ethylene glycol)-based macroporous hydrogels. J. Biomed. Mater. Res. Part A 2003, 66, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Schmedlen, R.H.; Masters, K.S.; West, J.L. Photocrosslinkable polyvinyl alcohol hydrogels that can be modified with cell adhesion peptides for use in tissue engineering. Biomaterials 2002, 23, 4325–4332. [Google Scholar] [CrossRef]
- Cabodi, M.; Choi, N.W.; Gleghorn, J.P.; Lee, C.S.; Bonassar, L.J.; Stroock, A.D. A microfluidic biomaterial. J. Am. Chem. Soc. 2005, 127, 13788–13789. [Google Scholar] [CrossRef] [PubMed]
- Jhon, M.S.; Andrade, J.D. Water and hydrogels. J. Biomed. Mater. Res. 1973, 7, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Ann. N. Y. Acad. Sci. 2001, 944, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.A. Bioactive biomaterials. Curr. Opin. Biotechnol. 1999, 10, 123–129. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Anseth, K.S. The effects of scaffold thickness on tissue engineered cartilage in photocrosslinked poly(ethylene oxide) hydrogels. Biomaterials 2001, 22, 619–626. [Google Scholar] [CrossRef]
- Tabata, Y. Tissue regeneration based on growth factor release. Tissue Eng. 2003, 9, S5–S15. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.A.; Nguyen, T.H.; Lee, B.T. Preparation and characterization of electrospun PCL/PLGA membranes and chitosan/gelatin hydrogels for skin bioengineering applications. J. Mater. Sci. Mater. Med. 2011, 22, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Gugerell, A.; Neumann, A.; Kober, J.; Tammaro, L.; Hoch, E.; Schnabelrauch, M.; Kamolz, L.; Kasper, C.; Keck, M. Adipose-derived stem cells cultivated on electrospun l-lactide/glycolide copolymer fleece and gelatin hydrogels under flow conditions—Aiming physiological reality in hypodermis tissue engineering. Burns J. Int. Soc. Burn Inj. 2015, 41, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhang, X.; Shen, Y.I.; Sebastian, R.; Dickinson, L.E.; Fox-Talbot, K.; Reinblatt, M.; Steenbergen, C.; Harmon, J.W.; Gerecht, S. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc. Natl. Acad. Sci. USA 2011, 108, 20976–20981. [Google Scholar] [CrossRef] [PubMed]
- Hartwell, R.; Poormasjedi-Meibod, M.S.; Chavez-Munoz, C.; Jalili, R.B.; Hossenini-Tabatabaei, A.; Ghahary, A. An in-situ forming skin substitute improves healing outcome in a hypertrophic scar model. Tissue Eng. Part A 2015, 21, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Peng, C.W.; Wu, W.W. Fibrous hydrogel scaffolds with cells embedded in the fibers as a potential tissue scaffold for skin repair. J. Mater. Sci. Mater. Med. 2014, 25, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.T.; Leung, M.; Chang, J.Y.; Zhang, M. A simple material model to generate epidermal and dermal layers in vitro for skin regeneration. J. Mater. Chem. B Mater. Biol. Med. 2014, 2, 5256–5264. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable gelatin hydrogel for epidermal tissue engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Gupta, A.; Agrawal, A.K.; Jassal, M.; Dinda, A.K.; Koul, V. Bi-layer composite dressing of gelatin nanofibrous mat and poly vinyl alcohol hydrogel for drug delivery and wound healing application: In Vitro and in vivo studies. J. Biomed. Nanotechnol. 2013, 9, 1495–1508. [Google Scholar] [CrossRef] [PubMed]
- Natesan, S.; Zamora, D.O.; Wrice, N.L.; Baer, D.G.; Christy, R.J. Bilayer hydrogel with autologous stem cells derived from debrided human burn skin for improved skin regeneration. J. Burn Care Res. Off. Publ. Am. Burn Assoc. 2013, 34, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, M.; Ho, D.; Fear, M.W.; Gelain, F.; Wood, F.M.; Iyer, K.S. Designer self-assembling hydrogel scaffolds can impact skin cell proliferation and migration. Sci. Rep. 2014, 4, 6903. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.; Wong, Y.C.; Cai, E.Z.; Ang, C.H.; Raju, A.; Lakshmanan, A.; Koh, A.G.; Zhou, H.J.; Lim, T.C.; Moochhala, S.M.; et al. Ultrashort peptide nanofibrous hydrogels for the acceleration of healing of burn wounds. Biomaterials 2014, 35, 4805–4814. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, C.T.; Attawia, M.A.; Elgendy, H.E.; Herbert, K.M. Tissue engineered bone-regeneration using degradable polymers: The formation of mineralized matrices. Bone 1996, 19, 93S–99S. [Google Scholar] [CrossRef]
- Singh, M.; Morris, C.P.; Ellis, R.J.; Detamore, M.S.; Berkland, C. Microsphere-based seamless scaffolds containing macroscopic gradients of encapsulated factors for tissue engineering. Tissue Eng. Part C Methods 2008, 14, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.; Li, L.; Robinson, D.; Chen, S.; Chang, H.; Liu, R.M.; Tian, Y.; Ginsburg, E.J.; Gao, X.; Stultz, T. Investigation of the in vitro release of gentamicin from a polyanhydride matrix. J. Control. Release Off. J. Control. Release Soc. 2000, 63, 305–317. [Google Scholar] [CrossRef]
- Berkland, C.; King, M.; Cox, A.; Kim, K.; Pack, D.W. Precise control of PLG microsphere size provides enhanced control of drug release rate. J. Control. Release Off. J. Control. Release Soc. 2002, 82, 137–147. [Google Scholar] [CrossRef]
- Jain, R.A.; Rhodes, C.T.; Railkar, A.M.; Malick, A.W.; Shah, N.H. Controlled delivery of drugs from a novel injectable in situ formed biodegradable PLGA microsphere system. J. Microencapsul. 2000, 17, 343–362. [Google Scholar] [PubMed]
- Ravivarapu, H.B.; Burton, K.; DeLuca, P.P. Polymer and microsphere blending to alter the release of a peptide from PLGA microspheres. Eur. J. Pharm. Biopharm. 2000, 50, 263–270. [Google Scholar] [CrossRef]
- Berkland, C.; Kim, K.; Pack, D.W. Plg microsphere size controls drug release rate through several competing factors. Pharm. Res. 2003, 20, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Borden, M.; Attawia, M.; Khan, Y.; El-Amin, S.F.; Laurencin, C.T. Tissue-engineered bone formation in vivo using a novel sintered polymeric microsphere matrix. J. Bone Jt. Surg. Br. Vol. 2004, 86, 1200–1208. [Google Scholar] [CrossRef]
- Yao, J.; Radin, S.; P, S.L.; Ducheyne, P. The effect of bioactive glass content on synthesis and bioactivity of composite poly (lactic-co-glycolic acid)/bioactive glass substrate for tissue engineering. Biomaterials 2005, 26, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Jaklenec, A.; Hinckfuss, A.; Bilgen, B.; Ciombor, D.M.; Aaron, R.; Mathiowitz, E. Sequential release of bioactive IGF-I AND TGF-β 1 from PLGA microsphere-based scaffolds. Biomaterials 2008, 29, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Jaklenec, A.; Wan, E.; Murray, M.E.; Mathiowitz, E. Novel scaffolds fabricated from protein-loaded microspheres for tissue engineering. Biomaterials 2008, 29, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Nair, L.S.; Laurencin, C.T. Laurencin. Solvent/nonsolvent sintering: A novel route to create porous microsphere scaffolds for tissue regeneration. J. Biomed. Mater. Res. B 2008, 86, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Nukavarapu, S.P.; Kumbar, S.G.; Brown, J.L.; Krogman, N.R.; Weikel, A.L.; Hindenlang, M.D.; Nair, L.S.; Allcock, H.R.; Laurencin, C.T. Polyphosphazene/nano-hydroxyapatite composite microsphere scaffolds for bone tissue engineering. Biomacromolecules 2008, 9, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Borden, M.; Attawia, M.; Laurencin, C.T. The sintered microsphere matrix for bone tissue engineering: In vitro osteoconductivity studies. J. Biomed. Mater. Res. 2002, 61, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Borden, M.; El-Amin, S.F.; Attawia, M.; Laurencin, C.T. Structural and human cellular assessment of a novel microsphere-based tissue engineered scaffold for bone repair. Biomaterials 2003, 24, 597–609. [Google Scholar] [CrossRef]
- PP, B.M.; Pedro, A.J.; Peterbauer, A.; Gabriel, C.; Redl, H.; Reis, R.L. Chitosan particles agglomerated scaffolds for cartilage and osteochondral tissue engineering approaches with adipose tissue derived stem cells. J. Mater. Sci. Mater. Med. 2005, 16, 1077–1085. [Google Scholar]
- Cao, H.; Chen, M.M.; Liu, Y.; Liu, Y.Y.; Huang, Y.Q.; Wang, J.H.; Chen, J.D.; Zhang, Q.Q. Fish collagen-based scaffold containing PLGA microspheres for controlled growth factor delivery in skin tissue engineering. Colloids Surf. B Biointerfaces 2015, 136, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Gwak, S.J.; Choi, C.Y.; Kim, B.S. Skin regeneration using keratinocytes and dermal fibroblasts cultured on biodegradable microspherical polymer scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 75, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lan, Y.; Guo, R.; Zhang, Y.; Xue, W. In vitro and in vivo evaluation of a novel collagen/cellulose nanocrystals scaffold for achieving the sustained release of basic fibroblast growth factor. J. Biomater. Appl. 2015, 29, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fan, H.; Cui, Y.; Chen, Y.; Yao, K.; Goh, J.C. Effects of the controlled-released basic fibroblast growth factor from chitosan-gelatin microspheres on human fibroblasts cultured on a chitosan-gelatin scaffold. Biomacromolecules 2007, 8, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Mirdailami, O.; Soleimani, M.; Dinarvand, R.; Khoshayand, M.R.; Norouzi, M.; Hajarizadeh, A.; Dodel, M.; Atyabi, F. Controlled release of rhEGF and rhbFGF from electrospun scaffolds for skin regeneration. J. Biomed. Mater. Res. Part A 2015, 103, 3374–3385. [Google Scholar] [CrossRef] [PubMed]
- Seland, H.; Gustafson, C.J.; Johnson, H.; Junker, J.P.; Kratz, G. Transplantation of acellular dermis and keratinocytes cultured on porous biodegradable microcarriers into full-thickness skin injuries on athymic rats. Burns J. Int. Soc. Burn Inj. 2011, 37, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, M.; She, Z.; Fan, K.; Xu, C.; Chu, B.; Chen, C.; Shi, S.; Tan, R. Collagen/chitosan based two-compartment and bi-functional dermal scaffolds for skin regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 52, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Lu, G.; Wu, Y.; Jirigala, E.; Xu, Y.; Ma, K.; Fu, X. Mesenchymal stem cells delivered in a microsphere-based engineered skin contribute to cutaneous wound healing and sweat gland repair. J. Dermatol. Sci. 2012, 66, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, Z.; Zhang, H.; Lu, W.; Liu, S.; Huang, X.; Luo, H.; Jin, Y. Expansion and delivery of adipose-derived mesenchymal stem cells on three microcarriers for soft tissue regeneration. Tissue Eng. Part A 2011, 17, 2981–2997. [Google Scholar] [CrossRef] [PubMed]
- Gilmartin, D.J.; Alexaline, M.M.; Thrasivoulou, C.; Phillips, A.R.; Jayasinghe, S.N.; Becker, D.L. Integration of scaffolds into full-thickness skin wounds: The connexin response. Adv. Healthc. Mater. 2013, 2, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioceramics: From concept to clinic. Am. Ceram. Soc. Bull. 1993, 72, 93–98. [Google Scholar] [CrossRef]
- Hentrich, R.L.; Graves, G.A.; Stein, H.G.; Bajpai, P.K. An evaluation of inert and resorbable ceramics for future clinical orthopedic applications. J. Biomed. Mater. Res. 1971, 5, 25–51. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lakes, R.S. Biomaterials—An Introduction, 2nd ed.; Plenum Press: New York, NY, USA, 1992. [Google Scholar]
- Blaker, J.J.; Gough, J.E.; Maquet, V.; Notingher, I.; Boccaccini, A.R. In vitro evaluation of novel bioactive composites based on bioglass-filled polylactide foams for bone tissue engineering scaffolds. J. Biomed. Mater. Res. Part A 2003, 67, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Lee, E.J.; Jun, I.K.; Kim, H.E.; Knowles, J.C. Degradation and drug release of phosphate glass/polycaprolactone biological composites for hard-tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 75, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and scaffolds: A winning combination for tissue engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef] [PubMed]
- Day, R.M.; Boccaccini, A.R.; Shurey, S.; Roether, J.A.; Forbes, A.; Hench, L.L.; Gabe, S.M. Assessment of polyglycolic acid mesh and bioactive glass for soft-tissue engineering scaffolds. Biomaterials 2004, 25, 5857–5866. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Boccaccini, A.R.; Knowles, J.C.; Locke, I.C.; Gordge, M.P.; McCormick, A.; Salih, V. Fabrication of a Novel Poly(3-hydroxyoctanoate)/Nanoscale Bioactive Glass Composite Film with Potential as a Multifunctional Wound Dressing. In Proceedings of the AIP Conference Procedings, Ischia, Italy, 20–23 June 2010; pp. 126–128.
- Jia, T.B.; Chen, J.Y.; Feng, X.X.; Chang, J. Fabrication and characterization of chitosan/mesoporous bioactive glasses porous films. J. Clin. Rehabil. Tissue Eng. Res. 2011, 15, 7877–7880. [Google Scholar]
- Huang, S.; Fu, X. Naturally derived materials-based cell and drug delivery systems in skin regeneration. J. Control. Release Off. J. Control. Release Soc. 2010, 142, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Peter, S.J.; Lyman, M.D.; Lai, H.L.; Leite, S.M.; Tamada, J.A.; Uyama, S.; Vacanti, J.P.; Langer, R.; Mikos, A.G. In vitro and in vivo degradation of porous poly(dl-lactic-co-glycolic acid) foams. Biomaterials 2000, 21, 1837–1845. [Google Scholar] [CrossRef]
- Oh, S.H.; Kang, S.G.; Kim, E.S.; Cho, S.H.; Lee, J.H. Fabrication and characterization of hydrophilic poly(lactic-co-glycolic acid)/poly(vinyl alcohol) blend cell scaffolds by melt-molding particulate-leaching method. Biomaterials 2003, 24, 4011–4021. [Google Scholar] [CrossRef]
- Rowlands, A.S.; Lim, S.A.; Martin, D.; Cooper-White, J.J. Polyurethane/poly(lactic-co-glycolic) acid composite scaffolds fabricated by thermally induced phase separation. Biomaterials 2007, 28, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, S.K.; Bretscher, L.E.; Taylor, K.M.; Raines, R.T. A hyperstable collagen mimic. Chem. Biol. 1999, 6, 63–70. [Google Scholar] [CrossRef]
- Rho, K.S.; Jeong, L.; Lee, G.; Seo, B.M.; Park, Y.J.; Hong, S.D.; Roh, S.; Cho, J.J.; Park, W.H.; Min, B.M. Electrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials 2006, 27, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Burck, J.; Heissler, S.; Geckle, U.; Ardakani, M.F.; Schneider, R.; Ulrich, A.S.; Kazanci, M. Resemblance of electrospun collagen nanofibers to their native structure. Langmuir ACS J. Surf. Colloids 2013, 29, 1562–1572. [Google Scholar] [CrossRef] [PubMed]
- Shabani, I.; Haddadi-Asl, V.; Soleimani, M.; Seyedjafari, E.; Babaeijandaghi, F.; Ahmadbeigi, N. Enhanced infiltration and biomineralization of stem cells on collagen-grafted three-dimensional nanofibers. Tissue Eng. Part A 2011, 17, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Ruszczak, Z. Effect of collagen matrices on dermal wound healing. Adv. Drug Deliv. Rev. 2003, 55, 1595–1611. [Google Scholar] [CrossRef] [PubMed]
- Sedlarik, K.M.; Schoots, C.; Fidler, V.; Oosterbaan, J.A.; Klopper, J.P. [comparative animal experiment studies of the effect of exogenous collagen on healing of a deep skin wound]. Unfallchirurgie 1991, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sedlarik, K.M.; Schoots, C.; Oosterbaan, J.A.; Klopper, J.P. [healing of a deep skin wound using a collagen sponge as dressing in the animal experiment]. Aktuelle Traumatol. 1992, 22, 219–228. [Google Scholar] [PubMed]
- Patino, M.G.; Neiders, M.E.; Andreana, S.; Noble, B.; Cohen, R.E. Collagen as an implantable material in medicine and dentistry. J. Oral Implantol. 2002, 28, 220–225. [Google Scholar] [CrossRef]
- Shen, X.; Nagai, N.; Murata, M.; Nishimura, D.; Sugi, M.; Munekata, M. Development of salmon milt DNA/salmon collagen composite for wound dressing. J. Mater. Sci. Mater. Med. 2008, 19, 3473–3479. [Google Scholar] [CrossRef] [PubMed]
- Mitra, T.; Sailakshmi, G.; Gnanamani, A.; Raja, S.T.; Thiruselvi, T.; Gowri, V.M.; Selvaraj, N.V.; Ramesh, G.; Mandal, A.B. Preparation and characterization of a thermostable and biodegradable biopolymers using natural cross-linker. Int. J. Biol. Macromol. 2011, 48, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Sailakshmi, G.; Mitra, T.; Gnanamani, A.; Kumara Raja, S.T.; Thiruselvi, T.; Selvaraj, N.V.; Ramesh, G.; Mandal, A.B. Bonding interactions and stability assessment of biopolymer material prepared using type III collagen of avian intestine and anionic polysaccharides. J. Mater. Sci. Mater. Med. 2011, 22, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Ghica, M.V.; Albu, M.G.; Leca, M.; Popa, L.; Moisescu, S.T. Design and optimization of some collagen-minocycline based hydrogels potentially applicable for the treatment of cutaneous wound infections. Die Pharm. 2011, 66, 853–861. [Google Scholar]
- Kempf, M.; Miyamura, Y.; Liu, P.Y.; Chen, A.C.; Nakamura, H.; Shimizu, H.; Tabata, Y.; Kimble, R.M.; McMillan, J.R. A denatured collagen microfiber scaffold seeded with human fibroblasts and keratinocytes for skin grafting. Biomaterials 2011, 32, 4782–4792. [Google Scholar] [CrossRef] [PubMed]
- Fullana, M.J.; Wnek, G.E. Electrospun collagen and its applications in regenerative medicine. Drug Deliv. Transl. Res. 2012, 2, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun nanofibers for regenerative medicine. Adv. Healthc. Mater. 2012, 1, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Zeugolis, D.I.; Khew, S.T.; Yew, E.S.; Ekaputra, A.K.; Tong, Y.W.; Yung, L.Y.; Hutmacher, D.W.; Sheppard, C.; Raghunath, M. Electro-spinning of pure collagen nano-fibres—just an expensive way to make gelatin? Biomaterials 2008, 29, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.J.; Phan, T.T.; Lim, I.J.; Zhang, Y.Z.; Bay, B.H.; Ramakrishna, S.; Lim, C.T. Evaluation of electrospun pcl/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007, 3, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Tae, G.; Kim, Y.H.; Park, I.S.; Kim, S.H. The effect of gelatin incorporation into electrospun poly(l-lactide-co-epsilon-caprolactone) fibers on mechanical properties and cytocompatibility. Biomaterials 2008, 29, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.; Sahebghadam Lotfi, A.; Barzin, J.; Hatam, M.; Adibi, B.; Khalaj, Z.; Massumi, M. Human bone marrow mesenchymal stem cell behaviors on PCL/gelatin nanofibrous scaffolds modified with a collagen IV-derived RGD-containing peptide. Cell J. 2014, 16, 1–10. [Google Scholar] [PubMed]
- Choi, Y.S.; Lee, S.B.; Hong, S.R.; Lee, Y.M.; Song, K.W.; Park, M.H. Studies on gelatin-based sponges. Part III: A comparative study of cross-linked gelatin/alginate, gelatin/hyaluronate and chitosan/hyaluronate sponges and their application as a wound dressing in full-thickness skin defect of rat. J. Mater. Sci. Mater. Med. 2001, 12, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Ulubayram, K.; Nur Cakar, A.; Korkusuz, P.; Ertan, C.; Hasirci, N. Egf containing gelatin-based wound dressings. Biomaterials 2001, 22, 1345–1356. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Min, B.M.; Lee, G.; Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials 2004, 25, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Mottaghitalab, F.; Farokhi, M.; Zaminy, A.; Kokabi, M.; Soleimani, M.; Mirahmadi, F.; Shokrgozar, M.A.; Sadeghizadeh, M. A biosynthetic nerve guide conduit based on silk/swnt/fibronectin nanocomposite for peripheral nerve regeneration. PLoS ONE 2013, 8, e74417. [Google Scholar] [CrossRef]
- Min, B.M.; Jeong, L.; Nam, Y.S.; Kim, J.M.; Kim, J.Y.; Park, W.H. Formation of silk fibroin matrices with different texture and its cellular response to normal human keratinocytes. Int. J. Biol. Macromol. 2004, 34, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.L.; Miao, J.C.; Sheng, W.H.; Xie, Y.F.; Huang, Q.; Shan, Y.B.; Yang, J.C. Cytocompatibility of regenerated silk fibroin film: A medical biomaterial applicable to wound healing. J. Zhejiang Univ. Sci. B 2010, 11, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Sell, S.A.; Francis, M.P.; Garg, K.; McClure, M.J.; Simpson, D.G.; Bowlin, G.L. Cross-linking methods of electrospun fibrinogen scaffolds for tissue engineering applications. Biomed. Mater. 2008, 3, 045001. [Google Scholar] [CrossRef] [PubMed]
- McManus, M.C.; Boland, E.D.; Koo, H.P.; Barnes, C.P.; Pawlowski, K.J.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Mechanical properties of electrospun fibrinogen structures. Acta Biomater. 2006, 2, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Nehrer, S.; Chiari, C.; Domayer, S.; Barkay, H.; Yayon, A. Results of chondrocyte implantation with a fibrin-hyaluronan matrix: A preliminary study. Clin. Orthop. Relat. Res. 2008, 466, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, S.W.; Sawyer, E.; Dorsey, J.; Flournoy, W.S.; Settle, T.; Simpson, D.; Cadd, G.; Janmey, P.; White, C.; Szabo, K.A. Wound healing and the immune response in swine treated with a hemostatic bandage composed of salmon thrombin and fibrinogen. J. Mater. Sci. Mater. Med. 2009, 20, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.; Cavaco-Paulo, A. Wound dressings for a proteolytic-rich environment. Appl. Microbiol. Biotechnol. 2011, 90, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Bragulla, H.H.; Homberger, D.G. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J. Anat. 2009, 214, 516–559. [Google Scholar] [CrossRef] [PubMed]
- Dror, Y.; Ziv, T.; Makarov, V.; Wolf, H.; Admon, A.; Zussman, E. Nanofibers made of globular proteins. Biomacromolecules 2008, 9, 2749–2754. [Google Scholar] [CrossRef] [PubMed]
- Ohto-Fujita, E.; Konno, T.; Shimizu, M.; Ishihara, K.; Sugitate, T.; Miyake, J.; Yoshimura, K.; Taniwaki, K.; Sakurai, T.; Hasebe, Y.; et al. Hydrolyzed eggshell membrane immobilized on phosphorylcholine polymer supplies extracellular matrix environment for human dermal fibroblasts. Cell Tissue Res. 2011, 345, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Mikus, D.; Sikiric, P.; Seiwerth, S.; Petricevic, A.; Aralica, G.; Druzijancic, N.; Rucman, R.; Petek, M.; Pigac, B.; Perovic, D.; et al. Pentadecapeptide BPC 157 cream improves burn-wound healing and attenuates burn-gastric lesions in mice. Burns J. Int. Soc. Burn Inj. 2001, 27, 817–827. [Google Scholar] [CrossRef]
- Miao, J.; Pangule, R.C.; Paskaleva, E.E.; Hwang, E.E.; Kane, R.S.; Linhardt, R.J.; Dordick, J.S. Lysostaphin-functionalized cellulose fibers with antistaphylococcal activity for wound healing applications. Biomaterials 2011, 32, 9557–9567. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.A.; Vaz, C.M.; Coutinho, O.P.; Cunha, A.M.; Reis, R.L. In vitro degradation and cytocompatibility evaluation of novel soy and sodium caseinate-based membrane biomaterials. J. Mater. Sci. Mater. Med. 2003, 14, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, J.; Hu, X.; Liang, J.; Fan, Y.; Zhang, X. Superabsorbent polysaccharide hydrogels based on pullulan derivate as antibacterial release wound dressing. J. Biomed. Mater. Res. Part A 2011, 98, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Lehtovaara, B.C.; Gu, F.X. Pharmacological, structural, and drug delivery properties and applications of 1,3-beta-glucans. J. Agric. Food Chem. 2011, 59, 6813–6828. [Google Scholar] [CrossRef] [PubMed]
- Logeart-Avramoglou, D.; Jozefonvicz, J. Carboxymethyl benzylamide sulfonate dextrans (CMDBS), a family of biospecific polymers endowed with numerous biological properties: A review. J. Biomed. Mater. Res. 1999, 48, 578–590. [Google Scholar] [CrossRef]
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown, R.M., Jr. Microbial cellulose—The natural power to heal wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhang, J.; Yang, G. Present status and applications of bacterial cellulose-based materials for skin tissue repair. Carbohydr. Polym. 2013, 92, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Muangman, P.; Opasanon, S.; Suwanchot, S.; Thangthed, O. Efficiency of microbial cellulose dressing in partial-thickness burn wounds. J. Am. Coll. Certif. Wound Spec. 2011, 3, 16–19. [Google Scholar]
- Fu, L.; Zhou, P.; Zhang, S.; Yang, G. Evaluation of bacterial nanocellulose-based uniform wound dressing for large area skin transplantation. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2995–3000. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.K.; Park, S.J.; Kim, M.S.; Kang, C.M.; Kim, J.I.; Kim, C.H. Fabrication of sonicated chitosan nanofiber mat with enlarged porosity for use as hemostatic materials. Carbohydr. Polym. 2013, 97, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, J.D.; Schauer, C.L. One-step electrospinning of cross-linked chitosan fibers. Biomacromolecules 2007, 8, 2665–2667. [Google Scholar] [CrossRef] [PubMed]
- Tchemtchoua, V.T.; Atanasova, G.; Aqil, A.; Filee, P.; Garbacki, N.; Vanhooteghem, O.; Deroanne, C.; Noel, A.; Jerome, C.; Nusgens, B.; et al. Development of a chitosan nanofibrillar scaffold for skin repair and regeneration. Biomacromolecules 2011, 12, 3194–3204. [Google Scholar] [CrossRef] [PubMed]
- Sugamori, T.; Iwase, H.; Maeda, M.; Inoue, Y.; Kurosawa, H. Local hemostatic effects of microcrystalline partially deacetylated chitin hydrochloride. J. Biomed. Mater. Res. 2000, 49, 225–232. [Google Scholar] [CrossRef]
- Madhumathi, K.; Sudheesh Kumar, P.T.; Abhilash, S.; Sreeja, V.; Tamura, H.; Manzoor, K.; Nair, S.V.; Jayakumar, R. Development of novel chitin/nanosilver composite scaffolds for wound dressing applications. J. Mater. Sci. Mater. Med. 2010, 21, 807–813. [Google Scholar] [CrossRef] [PubMed]
- P T, S.K.; Lakshmanan, V.K.; Raj, M.; Biswas, R.; Hiroshi, T.; Nair, S.V.; Jayakumar, R. Evaluation of wound healing potential of β-chitin hydrogel/nano zinc oxide composite bandage. Pharm. Res. 2013, 30, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Sermsintham, N.; Chandrkrachang, S.; Stevens, W.F. Chitosan membrane as a wound-healing dressing: Characterization and clinical application. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 69, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Altiok, D.; Altiok, E.; Tihminlioglu, F. Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. J. Mater. Sci. Mater. Med. 2010, 21, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.S.; Popa, E.G.; Gomes, M.E.; Cerqueira, M.; Marques, A.P.; Caridade, S.G.; Teixeira, P.; Sousa, C.; Mano, J.F.; Reis, R.L. An investigation of the potential application of chitosan/aloe-based membranes for regenerative medicine. Acta Biomater. 2013, 9, 6790–6797. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Avci, E.N. Radiation synthesis of poly(N-vinyl-2-pyrrolidone)-κ-carrageenan hydrogels and their use in wound dressing applications. I. Preliminary laboratory tests. J. Biomed. Mater. Res. Part A 2005, 74, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Shalumon, K.T.; Anulekha, K.H.; Nair, S.V.; Chennazhi, K.P.; Jayakumar, R. Sodium alginate/poly(vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int. J. Biol. Macromol. 2011, 49, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Thu, H.E.; Zulfakar, M.H.; Ng, S.F. Alginate based bilayer hydrocolloid films as potential slow-release modern wound dressing. Int. J. Pharm. 2012, 434, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.S.; Maan, Z.N.; Wu, J.C.; Rennert, R.C.; Hong, W.X.; Lai, T.S.; Cheung, A.T.; Walmsley, G.G.; Chung, M.T.; McArdle, A.; et al. Tissue engineering and regenerative repair in wound healing. Ann. Biomed. Eng. 2014, 42, 1494–1507. [Google Scholar] [CrossRef] [PubMed]
- Salbach, J.; Rachner, T.D.; Rauner, M.; Hempel, U.; Anderegg, U.; Franz, S.; Simon, J.C.; Hofbauer, L.C. Regenerative potential of glycosaminoglycans for skin and bone. J. Mol. Med. 2012, 90, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.Y.; Hung, Y.S.; Liou, H.M.; Shen, C.H. Electrospun hyaluronate-collagen nanofibrous matrix and the effects of varying the concentration of hyaluronate on the characteristics of foreskin fibroblast cells. Acta Biomater. 2010, 6, 2140–2147. [Google Scholar] [CrossRef] [PubMed]
- Uppal, R.; Ramaswamy, G.N.; Arnold, C.; Goodband, R.; Wang, Y. Hyaluronic acid nanofiber wound dressing—Production, characterization, and in vivo behavior. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Kogan, G.; Soltes, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kurpinski, K.T.; Stephenson, J.T.; Janairo, R.R.; Lee, H.; Li, S. The effect of fiber alignment and heparin coating on cell infiltration into nanofibrous plla scaffolds. Biomaterials 2010, 31, 3536–3542. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Tuk, B.; Hekking, I.M.; Vermeij, M.; Barritault, D.; van Neck, J.W. Stimulated neovascularization, inflammation resolution and collagen maturation in healing rat cutaneous wounds by a heparan sulfate glycosaminoglycan mimetic, OTR4120. Wound Repair Regen. 2009, 17, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Dinarvand, P.; Hashemi, S.M.; Seyedjafari, E.; Shabani, I.; Mohammadi-Sangcheshmeh, A.; Farhadian, S.; Soleimani, M. Function of poly (lactic-co-glycolic acid) nanofiber in reduction of adhesion bands. J. Surg. Res. 2012, 172, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Khang, D.; Carpenter, J.; Chun, Y.W.; Pareta, R.; Webster, T.J. Nanotechnology for regenerative medicine. Biomed. Microdevices 2010, 12, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Y.; Nie, W.; Wang, Y.C.; Shen, Y.; Li, Y.; Gan, S.J. Electrospun emodin polyvinylpyrrolidone blended nanofibrous membrane: A novel medicated biomaterial for drug delivery and accelerated wound healing. J. Mater. Sci. Mater. Med. 2012, 23, 2709–2716. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, G.; Atalay-Oral, C.; Erkal, S.; Sahin, F.; Karastova, D.; Tantekin-Ersolmaz, S.B.; Guner, F.S. Fatty acid-based polyurethane films for wound dressing applications. J. Mater. Sci. Mater. Med. 2009, 20, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Losi, P.; Briganti, E.; Costa, M.; Sanguinetti, E.; Soldani, G. Silicone-coated non-woven polyester dressing enhances reepithelialisation in a sheep model of dermal wounds. J. Mater. Sci. Mater. Med. 2012, 23, 2235–2243. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhao, M.; Zhao, Y.; Mou, Y. A fibrin gel loaded with chitosan nanoparticles for local delivery of rhegf: Preparation and in vitro release studies. J. Mater. Sci. Mater. Med. 2011, 22, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Strukova, S.M.; Dugina, T.N.; Chistov, I.V.; Lange, M.; Markvicheva, E.A.; Kuptsova, S.; Zubov, V.P.; Glusa, E. Immobilized thrombin receptor agonist peptide accelerates wound healing in mice. Clin. Appl. Thromb. Hemost. 2001, 7, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Zisch, A.H.; Lutolf, M.P.; Hubbell, J.A. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc. Pathol. 2003, 12, 295–310. [Google Scholar] [CrossRef]
- Degim, Z. Use of microparticulate systems to accelerate skin wound healing. J. Drug Target. 2008, 16, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Tian, F.; Yang, J.; He, C.N.; Xing, N.; Li, F. Chitosan and alginate polyelectrolyte complex membranes and their properties for wound dressing application. J. Mater. Sci. Mater. Med. 2010, 21, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Alemdaroglu, C.; Degim, Z.; Celebi, N.; Zor, F.; Ozturk, S.; Erdogan, D. An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns J. Int. Soc. Burn Inj. 2006, 32, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, L.; Stegemann, J.P. Phase-separated chitosan-fibrin microbeads for cell delivery. J. Microencapsul. 2011, 28, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Lee, J.W.; Lee, J.S.; Lee, J.H.; Yoon, T.R.; Kuroyanagi, Y.; Park, M.H.; Pyun, D.G.; Kim, H.J. Hyaluronic acid and silver sulfadiazine-impregnated polyurethane foams for wound dressing application. J. Mater. Sci. Mater. Med. 2002, 13, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Chen, W. Preparation of hyaluronan-DNA matrices and films. Cold Spring Harb. Protoc. 2012, 2012, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Eng, D.; Caplan, M.; Preul, M.; Panitch, A. Hyaluronan scaffolds: A balance between backbone functionalization and bioactivity. Acta Biomater. 2010, 6, 2407–2414. [Google Scholar] [CrossRef] [PubMed]
- Galili, U.; Wigglesworth, K.; Abdel-Motal, U.M. Accelerated healing of skin burns by anti-gal/alpha-gal liposomes interaction. Burns J. Int. Soc. Burn Inj. 2010, 36, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Weyers, A.; Linhardt, R.J. Neoproteoglycans in tissue engineering. FEBS J. 2013, 280, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Unnithan, A.R.; Barakat, N.A.; Pichiah, P.B.; Gnanasekaran, G.; Nirmala, R.; Cha, Y.S.; Jung, C.H.; El-Newehy, M.; Kim, H.Y. Wound-dressing materials with antibacterial activity from electrospun polyurethane-dextran nanofiber mats containing ciprofloxacin hcl. Carbohydr. Polym. 2012, 90, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Park, S.N.; Jang, H.J.; Choi, Y.S.; Cha, J.M.; Son, S.Y.; Han, S.H.; Kim, J.H.; Lee, W.J.; Suh, H. Preparation and characterization of biodegradable anti-adhesive membrane for peritoneal wound healing. J. Mater. Sci. Mater. Med. 2007, 18, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Shirokova, L.N.; Aleksandrova, V.A.; Egorova, E.M.; Vikhoreva, G.A. macromolecular systems and bactericidal films based on chitin derivatives and silver nanoparticles. Prikl. Biokhimiia Mikrobiol. 2009, 45, 422–426. [Google Scholar] [CrossRef]
- Casper, C.L.; Yamaguchi, N.; Kiick, K.L.; Rabolt, J.F. Functionalizing electrospun fibers with biologically relevant macromolecules. Biomacromolecules 2005, 6, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Shingel, K.I.; di Stabile, L.; Marty, J.P.; Faure, M.P. Inflammatory inert poly(ethylene glycol)—Protein wound dressing improves healing responses in partial- and full-thickness wounds. Int. Wound J. 2006, 3, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.A.; Herzog, K.T.; Kao, W.J. A study of diffusion in poly(ethyleneglycol)-gelatin based semi-interpenetrating networks for use in wound healing. Polym. Bull. 2009, 62, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Ulubayram, K.; Aksu, E.; Gurhan, S.I.; Serbetci, K.; Hasirci, N. Cytotoxicity evaluation of gelatin sponges prepared with different cross-linking agents. J. Biomater. Sci. Polym. Ed. 2002, 13, 1203–1219. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Lee, J.H.; Piao, M.G.; Lee, M.K.; Oh, D.H.; Hwang du, H.; Quan, Q.Z.; Yong, C.S.; Choi, H.G. Effect of sodium carboxymethylcellulose and fucidic acid on the gel characterization of polyvinylalcohol-based wound dressing. Arch. Pharm. Res. 2010, 33, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Biomedical evaluation of polyvinyl alcohol-gelatin esterified hydrogel for wound dressing. J. Mater. Sci. Mater. Med. 2007, 18, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Kennedy, J.E.; Higginbotham, C.L. Development of a novel porous cryo-foam for potential wound healing applications. J. Mater. Sci. Mater. Med. 2009, 20, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Yari, A.; Yeganeh, H.; Bakhshi, H. Synthesis and evaluation of novel absorptive and antibacterial polyurethane membranes as wound dressing. J. Mater. Sci. Mater. Med. 2012, 23, 2187–2202. [Google Scholar] [CrossRef] [PubMed]
- Atac, B.; Wagner, I.; Horland, R.; Lauster, R.; Marx, U.; Tonevitsky, A.G.; Azar, R.P.; Lindner, G. Skin and hair on-a-chip: In vitro skin models versus ex vivo tissue maintenance with dynamic perfusion. Lab Chip 2013, 13, 3555–3561. [Google Scholar] [CrossRef] [PubMed]




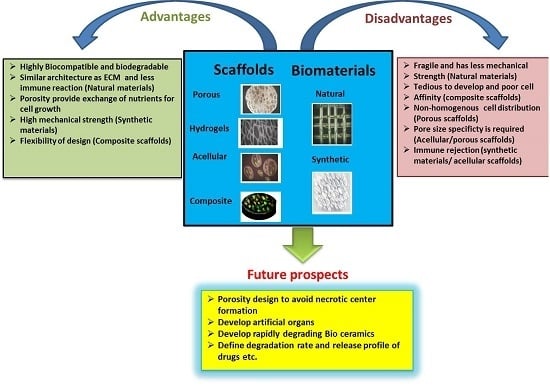
| Scaffold Types | Advantages | Disadvantages | Future Prospects |
|---|---|---|---|
| Porous scaffolds | High porosity provides a suitable environment for extracellular matrix (ECM) secretion and nutrient supplies to the cells. Pore sizes specific to the cell types prevent clustering of the cells, thus avoiding necrotic center formation. | Porous nature limits the homogenous distribution of the cells. Different pore sizes are required for the specific cell types and are therefore time consuming. | Improvement in the connectivity of pores and thereby the structure of the scaffolds is required. |
| Fibrous scaffolds | Highly microporous structure is best suitable for cell adhesion, proliferation and differentiation. Low inflammatory response upon implantation. | Surface functionalization is required to create the nanofibers of these scaffolds. | Drugs and biological molecules such as proteins, genes, growth factors, etc., can be incorporated in fibrous scaffolds for release applications. |
| Hydrogel scaffolds | Highly biocompatible and controlled biodegradation rate. | Limited mechanical strength due to soft structures. | Degradation behavior of the hydrogels and tenability should be well-defined. Hydrogels incorporating growth factors to facilitate cell differentiation. |
| Microsphere scaffolds | Easily fabricated with controlled physical characteristics suitable for slow or fast drug delivery. Provides enhanced cell attachment and migration properties. | Microsphere sintering methods are sometimes not compatible to the cells and reduces the cell viability. | These scaffolds can be used as a target specific delivery vehicle for the drugs such as antibiotics, anti-cancer, etc. |
| Composite scaffolds | Highly biodegradable and offer mechanical strength. Greater absorbability. | Acidic byproducts are generated upon degradation. Poor cell affinity. Require tedious efforts to develop composite scaffolds. | Nano-bioceramic and polymer composites with faster degradation are currently being developed. |
| Acellular scaffolds | Native ECM is retained and thus normal anatomical features are maintained. Less inflammatory and immune response with higher mechanical strength. | Incomplete decellularization is required to avoid immune responses. | Such scaffolds hold promise towards developing artificial organs. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future Prospects for Scaffolding Methods and Biomaterials in Skin Tissue Engineering: A Review. Int. J. Mol. Sci. 2016, 17, 1974. https://doi.org/10.3390/ijms17121974
Chaudhari AA, Vig K, Baganizi DR, Sahu R, Dixit S, Dennis V, Singh SR, Pillai SR. Future Prospects for Scaffolding Methods and Biomaterials in Skin Tissue Engineering: A Review. International Journal of Molecular Sciences. 2016; 17(12):1974. https://doi.org/10.3390/ijms17121974
Chicago/Turabian StyleChaudhari, Atul A., Komal Vig, Dieudonné Radé Baganizi, Rajnish Sahu, Saurabh Dixit, Vida Dennis, Shree Ram Singh, and Shreekumar R. Pillai. 2016. "Future Prospects for Scaffolding Methods and Biomaterials in Skin Tissue Engineering: A Review" International Journal of Molecular Sciences 17, no. 12: 1974. https://doi.org/10.3390/ijms17121974
APA StyleChaudhari, A. A., Vig, K., Baganizi, D. R., Sahu, R., Dixit, S., Dennis, V., Singh, S. R., & Pillai, S. R. (2016). Future Prospects for Scaffolding Methods and Biomaterials in Skin Tissue Engineering: A Review. International Journal of Molecular Sciences, 17(12), 1974. https://doi.org/10.3390/ijms17121974