Skin Involvement and Pulmonary Hypertension Are Associated with Vitamin D Insufficiency in Scleroderma
Abstract
:1. Introduction
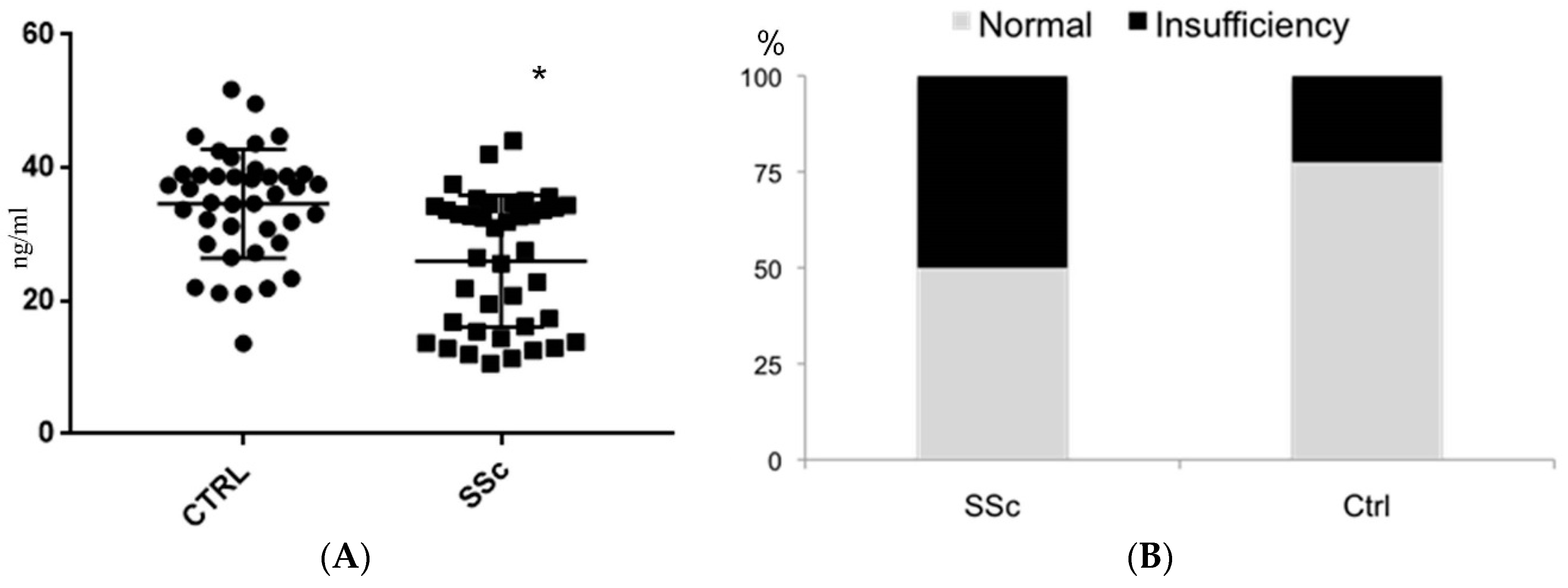
2. Results
3. Discussion
4. Material and Methods
4.1. Study Population and Recruitment
4.2. Clinical Parameters and Biochemical Data
4.3. Statistical Analyses
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Gabrielli, A.; Avvedimento, E.V.; Krieg, T. Scleroderma. N. Engl. J. Med. 2009, 360, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Abraham, D. Systemic sclerosis: A prototypic multisystem fibrotic disorder. J. Clin. Investig. 2007, 117, 557–567. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, K.G.; Dawson, N.A.; Huang, Q.; Dunne, J.V.; Levings, M.K.; Broady, R. Regulatory T cells produce profibrotic cytokines in the skin of patients with systemic sclerosis. J. Allergy Clin. Immunol. 2015, 135, 946. [Google Scholar] [CrossRef] [PubMed]
- Veldman, C.M.; Cantorna, M.T.; Luca, H.F.D.E. Expression of 1,25-dihydroxyvitamin D3 receptor in the immune system. Arch. Biochem. Biophys. 2000, 374, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.W.; Kouttab, N.; Ford, D.; Maizel, A.L. Vitamin D-mediated gene regulation in phenotypically defined B cells subpopulations. Endocrinology 2000, 141, 3225–3234. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M. Further emergent evidence for the vitamin D endocrine system involvement in autoimmune rheumatic disease risk and prognosis. Ann. Rheum. Dis. 2013, 72, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Zerr, P.; Vollath, S.; Palumbo-Zerr, K.; Tomcik, M.; Huang, J.; Distler, A.; Beyer, C.; Dees, C.; Gela, K.; Distler, O.; et al. Vitamin D receptor regulates TGF-β signalling in systemic sclerosis. Ann. Rheum. Dis. 2015, 74, e20. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, K.; Wessner, B.; Laggner, U.; Ploder, M.; Tamandl, D.; Friedl, J.; Zügel, U.; Steinmeyer, A.; Pollak, A.; Roth, E.; et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur. J. Immunol. 2006, 36, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Delvin, E.; Souberbielle, J.C.; Viard, J.P.; Salle, B. Role of Vitamin D in acquired immune and autoimmune diseases. Crit. Rev. Clin. Lab. Sci. 2014, 51, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.; Dos Santos, A.S.; Santos, M.; da Silva Chakr, R.M.; Monticielo, O.A. Vitamin D and systemic lupus erythematosus: State of the art. Clin. Rheumatol. 2014, 33, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Costenbader, K.H.; Feskanich, D.; Holmes, M.; Karlson, E.W.; Benito-Garcia, E. Vitamin D intake and risks of systemic lupus erythematosus and rheumatoid arthritis in women. Ann. Rheum. Dis. 2008, 67, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Rossini, M.; Gatti, D.; Viapiana, O.; Caimmi, C.; Idolazzi, L.; Fracassi, E.; Adami, S. Vitamin D and rheumatic diseases. Reumatismo 2014, 66, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Merlino, L.A.; Curtis, J.; Mikuls, T.R.; Cerhan, J.R.; Criswell, L.A.; Saag, K.G.; Iowa Women’s Health Study. Vitamin D intake is inversely associated with rheumatoid arthritis: Results from the Iowa Women’s Health Study. Arthritis Rheum. 2004, 50, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Otsa, K.; Uprus, M.; Paolino, S.; Seriolo, B. Vitamin D in rheumatoid arthritis. Autoimmun. Rev. 2007, 7, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Hollan, I.; Dessein, P.H.; Ronda, N.; Wasko, M.C.; Svenungsson, E.; Agewall, S.; Cohen-Tervaert, J.W.; Maki-Petaja, K.; Grundtvig, M.; Karpouzas, G.A.; et al. Prevention of cardiovascular disease in rheumatoid arthritis. Autoimmun. Rev. 2015, 14, 952–969. [Google Scholar] [CrossRef] [PubMed]
- Atteritano, M.; Sorbara, S.; Bagnato, G.; Miceli, G.; Sangari, D.; Morgante, S.; Visalli, E.; Bagnato, G. Bone mineral density, bone turnover markers and fractures in patients with systemic sclerosis: A case control study. PLoS ONE 2013, 8, e66991. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Watanabe, M.; Ishido, N.; Katsumata, Y.; Kagawa, T.; Hidaka, Y.; Iwatani, Y. The functional polymorphisms of VDR, GC and CYP2R1 are involved in the pathogenesis of autoimmune thyroid diseases. Clin. Exp. Immunol. 2014, 178, 262–269. [Google Scholar] [CrossRef] [PubMed]
- D’Aurizio, F.; Villalta, D.; Metus, P.; Doretto, P.; Tozzoli, R. Is Vitamin D a player or not in the pathohystology of autoimmune thyroid diseases? Autoimmun. Rev. 2015, 14, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Weinstock-Guttman, B.; Mehta, B.K.; Ramanathan, M.; Karmon, Y.; Henson, L.J.; Halper, J.; Riskind, P. Vitamin D and multiple sclerosis. Neurologist 2012, 18, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, A.; Mutlu, G.Y.; Özsu, E.; Çizmecioğlu, F.M.; Hatun, Ş. Vitamin D deficiency in children and adolescents with type 1 diabetes. J. Clin. Res. Pediatr. Endocrinol. 2011, 3, 179–183. [Google Scholar] [PubMed]
- Lucisano, S.; Buemi, M.; Passantino, A.; Aloisi, C.; Cernaro, V.; Santoro, D. New insights on the role of vitamin D in the progression of renal damage. Kidney Blood Press Res. 2013, 37, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Arnson, Y.; Amital, H.; Shoenfeld, Y. Vitamin D and autoimmunity: New aetiological and therapeutic considerations. Ann. Rheum. Dis. 2007, 66, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Motrich, R.D.; van Etten, E.; Depovere, J.; Riera, C.M.; Rivero, V.E.; Mathieu, C. Impact of vitamin D receptor activity on experimental autoimmune prostatitis. J. Autoimmun. 2009, 32, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Kanazawa, Y.; Motohashi, Y.; Yamada, S.; Maruyama, T.; Ikegami, H. Evidence for association between vitamin D receptor BsmI polymorphism and type 1 diabetes in Japanese. J. Autoimmun. 2008, 30, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Ibn Yacoub, Y.; Amine, B.; Laatiris, A.; Wafki, F.; Znat, F.; Hajjaj-Hassouni, N. Bone density in Moroccan women with systemic scleroderma and its relationships with disease-related parameters and vitamin D status. Rheumatol. Int. 2012, 32, 3143–3148. [Google Scholar] [CrossRef] [PubMed]
- Rios Fernández, R.; Fernández Roldán, C.; Callejas Rubio, J.L.; Ortego Centeno, N. Vitamin D deficiency in a cohort of patients with systemic scleroderma from the south of Spain. J. Rheumatol. 2010, 37, 1355. [Google Scholar] [CrossRef] [PubMed]
- Caramaschi, P.; Gassa, A.D.; Ruzzenente, O.; Volpe, A.; Ravagnani, V.; Tinazzi, I.; Barausse, G.; Bambara, L.M.; Biasi, D. Very Low levels of vitamin D in systemic sclerosis patients. Clin. Rheumatol. 2010, 29, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, V.; Modoni, S.; Pileri, M.; di Giorgio, A.; Chiodini, I.; Minisola, S.; Vieth, R.; Scillitani, A. Longitudinal evaluation of vitamin D status in healthy subjects from southern Italy: Seasonal and gender differences. Osteoporos. Int. 2001, 12, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Szodoray, P.; Nakken, B.; Gaal, J.; Jonsson, R.; Szegedi, A.; Zold, E.; Szegedi, G.; Brun, J.G.; Gesztelyi, R.; Zeher, M.; et al. The complex role of vitamin D in autoimmune diseases. Scand. J. Immunol. 2008, 68, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Arnson, Y.; Amital, H.; Agmon-Levin, N.; Alon, D.; Sánchez-Castañón, M.; López-Hoyos, M. Serum 25-OH vitamin D concentrations are linked with various clinical aspects in patients with systemic sclerosis: A retrospective cohort study and review of the literature. Autoimmun. Rev. 2011, 10, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, S.; Hersberger, M.; Fischler, M.; Huber, L.C.; Senn, O.; Treder, U.; Speich, R.; Schmid, C. Bone mineral density and secondary hyperparathyroidism in pulmonary hypertension. Open Respir. Med. J. 2009, 3, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.; Uyan, U.; Keçeoçlu, S.; Demir, C. The Relationship between Vitamin D Deficiency and Pulmonary Hypertension. Prague Med. Rep. 2013, 114, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shoshan, M.; Amir, S.; Dang, D.T.; Dang, L.H.; Weisman, Y.; Mabjeesh, N.J. 1α,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol. Cancer Ther. 2007, 6, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M.; Chacon, M.; Alger, L.; Wang, J.; Taraseviciene-Stewart, L.; Kasahara, Y.; Cool, C.D.; Bishop, A.E.; Geraci, M.; Semenza, G.L.; et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: Evidence for a process of disordered angiogenesis. J. Pathol. 2001, 195, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Terrier, B.; Derian, N.; Schoindre, Y.; Chaara, W.; Geri, G.; Zahr, N.; Mariampillai, K.; Rosenzwajg, M.; Carpentier, W.; Musset, L.; et al. Restoration of regulatory and effector T cell balance and B cell homeostasis in systemic lupus erythematosus patients through vitamin D supplementation. Arthritis Res. Ther. 2012, 14, R221. [Google Scholar] [CrossRef] [PubMed]
- Ritterhouse, L.L.; Crowe, S.R.; Niewold, T.B.; Kamen, D.L.; Macwana, S.R.; Roberts, V.C.; Dedeke, A.B.; Harley, J.B.; Scofield, R.H.; Guthridge, J.M.; et al. Vitamin D deficiency is associated with an increased autoimmune response in healthy individuals and in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2011, 70, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- LeRoy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A. Jr.; Rowell, N.; Wollheim, F. Scleroderma (systemic sclerosis): Classification, subset and pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar] [PubMed]
- Valentini, G.; Silman, A.J.; Veale, D. Assessment of disease activity. Clin. Exp. Rheumatol. 2003, 21 (Suppl. 29), S39–S41. [Google Scholar] [PubMed]
- Valentini, G.; Della Rossa, A.; Bombardieri, S.; Bencivelli, W.; Silman, A.J.; D’Angelo, S.; Cerinic, M.M.; Belch, J.F.; Black, C.M.; Bruhlmann, P.; et al. European multicentre study to define disease activity criteria for systemic sclerosis. II. Identification of disease activity variables and development of preliminary activity indexes. Ann. Rheum. Dis. 2001, 60, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Valentini, G.; Bencivelli, W.; Bombardieri, S.; D’Angelo, S.; Della Rossa, A.; Silman, A.J.; Black, C.M.; Czirjak, L.; Nielsen, H.; Vlachoyiannopoulos, P.G. European multicentre study to define disease activity criteria for systemic sclerosis. III. Assessment of the construct validity of the preliminary activity criteria. Ann. Rheum. Dis. 2003, 62, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Valentini, G. The assessment of the patient with systemic sclerosis. Autoimmun. Rev. 2003, 2, 370–376. [Google Scholar] [CrossRef]
- Medsger, T.A., Jr.; Bombardieri, S.; Czirjak, L.; Scorza, R.; Della Rossa, A.; Bencivelli, W. Assessment of disease severity and prognosis. Clin. Exp. Rheumatol. 2003, 3, 42–46. [Google Scholar]
- Hoeper, M.M.; Bogaard, H.J.; Condiffe, R.; Frantz, R.; Khanna, D.; Kurzyna, M.; Langleben, D.; Manes, A.; Satoh, T.; Torres, F.; et al. Definitions and diagnosis of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62, D4250. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | SSc (n = 40) | Controls (n = 40) | p |
|---|---|---|---|
| Sex (Male/Female) | 2/38 | 2/38 | 1.00 |
| Age (Years) | 58.47 ± 14.04 | 57.96 ± 12.87 | 0.61 |
| Body Mass Index (kg/m2) | 25.33 ± 4.43 | 24.87 ± 4.54 | 0.26 |
| Smoking status (%) | |||
| Current | 8 | 10 | 0.88 |
| Former | 18 | 22 | 0.67 |
| Never | 74 | 70 | 0.82 |
| Sunlight exposure (%) | |||
| <5 h/week | 70 | 65 | 0.67 |
| >5 h/week | 25 | 27.5 | 0.52 |
| >10 h/week | 5 | 7.5 | 0.45 |
| Supplementation with calcium, n | 0 | 0 | 1.00 |
| Supplementation with vitamin D, n | 0 | 0 | 1.00 |
| Characteristics | SSc (n = 40) |
|---|---|
| Pattern of Scleroderma, limited/diffuse (%) | 12/28 (30/70) |
| Duration of disease (Years) | 9.11 ± 6.74 |
| Anticentromere antibodies, n (%) | 6 (15%) |
| Modified Rodnan Skin Score | 10.45 ± 6.74 |
| Interstitial Lung Disease, n (%) | 17 (44%) |
| Renal Crisis, n (%) | 0 (0) |
| Malabsorption, n (%) | 7 (17.5) |
| Musculo-skeletal involvement, n (%) | 24 (60) |
| Pulmonary Hypertension, n (%) | 9 (24%) |
| Creatinine (mg/dL) | 0.98 ± 0.12 |
| Total Proteins (g/dL) | 7.24 ± 0.52 |
| Albumin (g/dL) | 3.88 ± 0.31 |
| Fibrinogen (mg/dL) | 287.11 ± 33.74 |
| Variables | 25(OH)D3 < 30 ng/mL | 25(OH)D3 > 30 ng/mL | Test | p |
|---|---|---|---|---|
| Subset Diffuse | 17 (85%) | 11 (55%) | x2 Yates = 2.98 | 0.08 |
| Rodnan Skin Score > 10 | 13 (65%) | 5 (25%) | x2 Yates = 4.95 | 0.02 * |
| Digital ulcers | 8 (40%) | 6 (33%) | x2 Yates = 0.11 | 0.74 |
| pH < 7.38 | 3 (15%) | 0 (0%) | x2 Yates = 1.44 | 0.22 |
| pO2 < 80 mmHg | 4 (20%) | 1 (5%) | x2 Yates = 0.91 | 0.33 |
| pCO2 < 35 mmHg | 4 (20%) | 1 (5%) | x2 Yates = 0.91 | 0.33 |
| SO2 < 93% | 1 (5%) | 1 (5%) | x2 Yates = 0.53 | 0.46 |
| Systolic Pulmonary Artery Pressure > 35 mmHg | 8 (40%) | 1 (5%) | x2 Yates = 5.16 | 0.02 * |
| Hearth Rate < 60 | 1 (5%) | 1 (5%) | x2 Yates = 0.53 | 0.46 |
| PQ interval > 0.20 seconds | 1 (5%) | 0 (0%) | x2 Yates = 0.53 | 0.22 |
| Long QT interval | 2 (10%) | 2 (10%) | x2 Yates = 0.28 | 0.59 |
| Diffusing Capacity oft he Lung for Carbone Monoxide < 75% | 2 (10%) | 2 (10%) | x2 Yates = 0.28 | 0.59 |
| Anticentromere antibodies | 4 (20%) | 2 (10%) | x2 Yates = 0.20 | 0.65 |
| Anti SCL70 antibodies | 10 (50%) | 4 (20%) | x2 Yates = 2.75 | 0.09 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atteritano, M.; Santoro, D.; Corallo, G.; Visalli, E.; Buemi, M.; Catalano, A.; Lasco, A.; Bitto, A.; Squadrito, F. Skin Involvement and Pulmonary Hypertension Are Associated with Vitamin D Insufficiency in Scleroderma. Int. J. Mol. Sci. 2016, 17, 2103. https://doi.org/10.3390/ijms17122103
Atteritano M, Santoro D, Corallo G, Visalli E, Buemi M, Catalano A, Lasco A, Bitto A, Squadrito F. Skin Involvement and Pulmonary Hypertension Are Associated with Vitamin D Insufficiency in Scleroderma. International Journal of Molecular Sciences. 2016; 17(12):2103. https://doi.org/10.3390/ijms17122103
Chicago/Turabian StyleAtteritano, Marco, Domenico Santoro, Giorgio Corallo, Elisa Visalli, Michele Buemi, Antonino Catalano, Antonino Lasco, Alessandra Bitto, and Francesco Squadrito. 2016. "Skin Involvement and Pulmonary Hypertension Are Associated with Vitamin D Insufficiency in Scleroderma" International Journal of Molecular Sciences 17, no. 12: 2103. https://doi.org/10.3390/ijms17122103






