Systematic Understanding of Mechanisms of a Chinese Herbal Formula in Treatment of Metabolic Syndrome by an Integrated Pharmacology Approach
Abstract
:1. Introduction
2. Results and Discussion
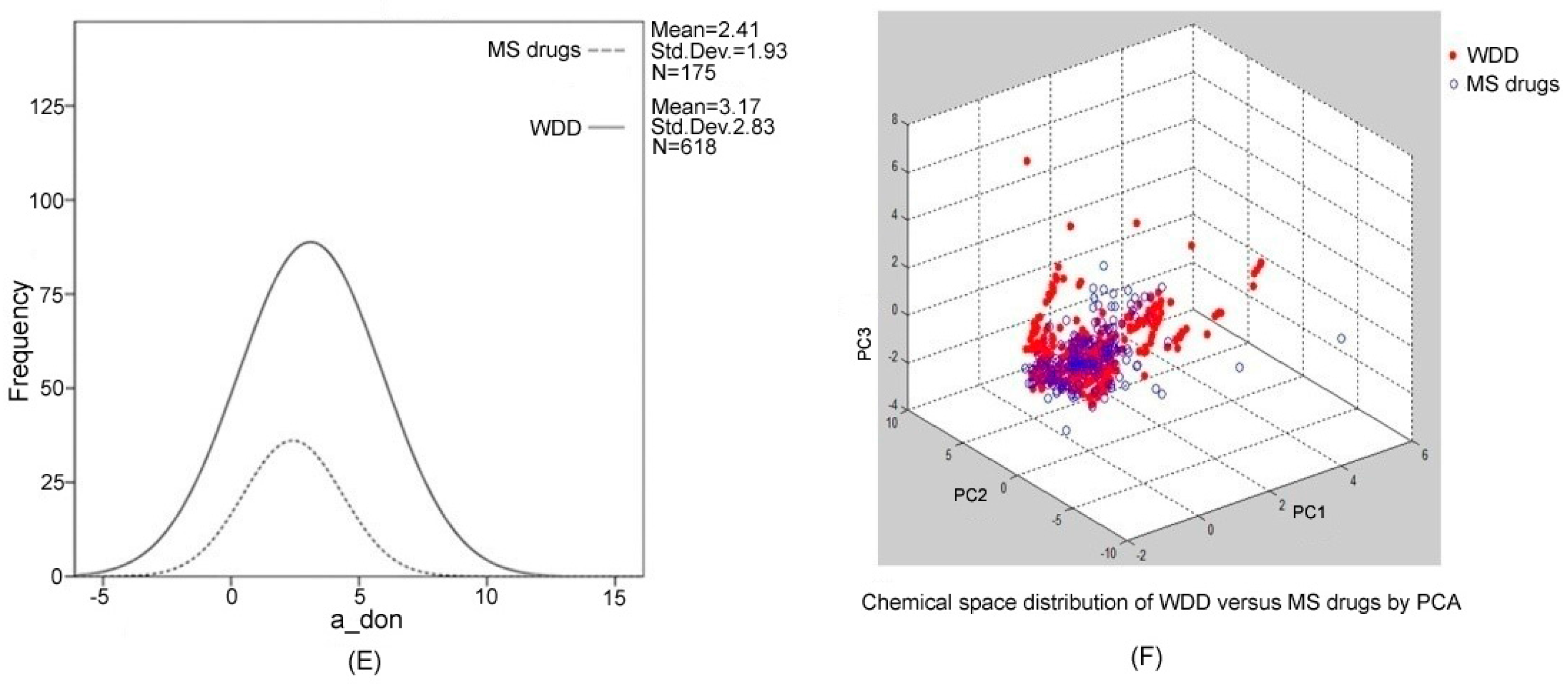
2.1. Chemical Diversity and Drug-Likeness Analysis of Wendan Decoction (WDD)
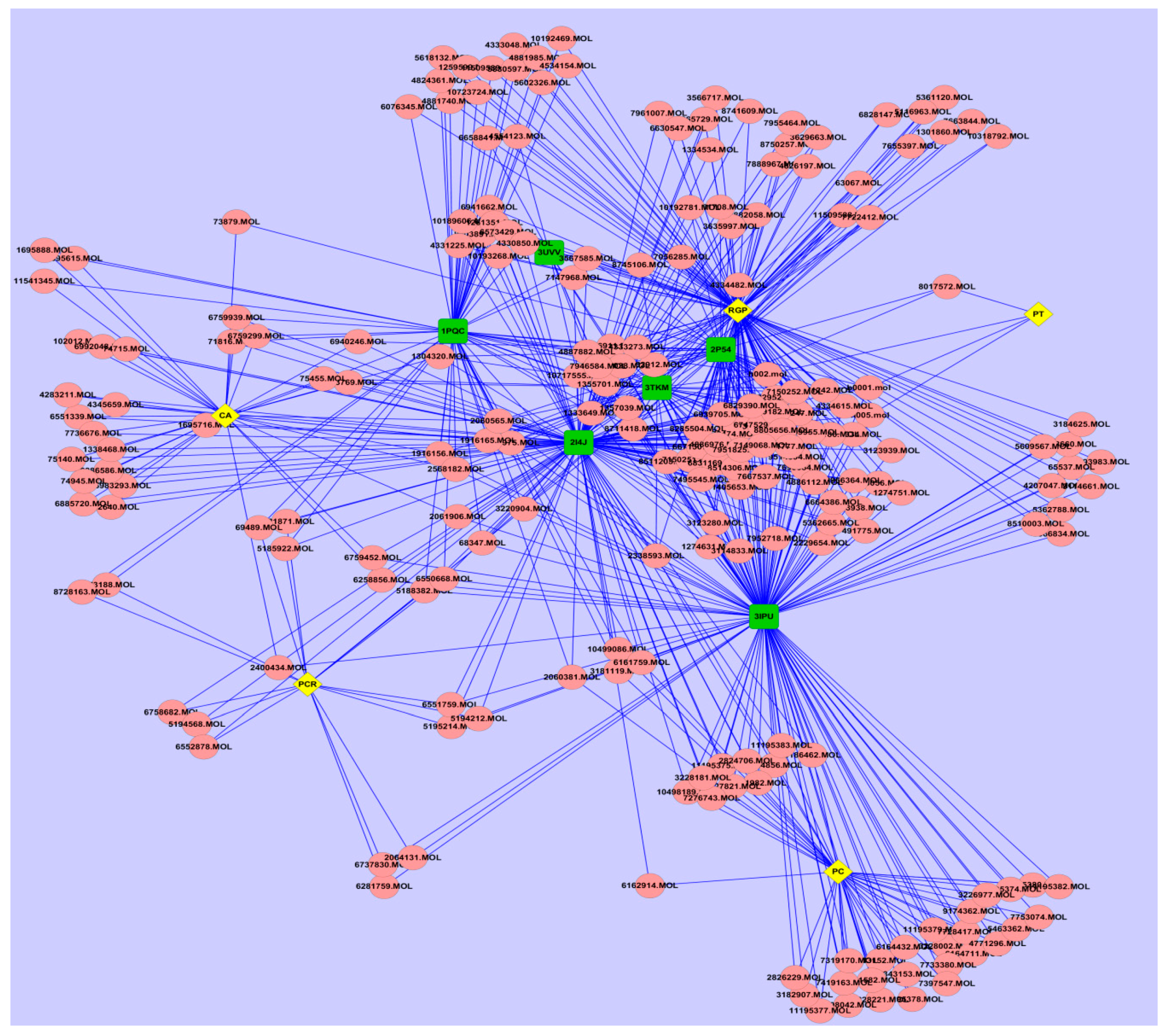
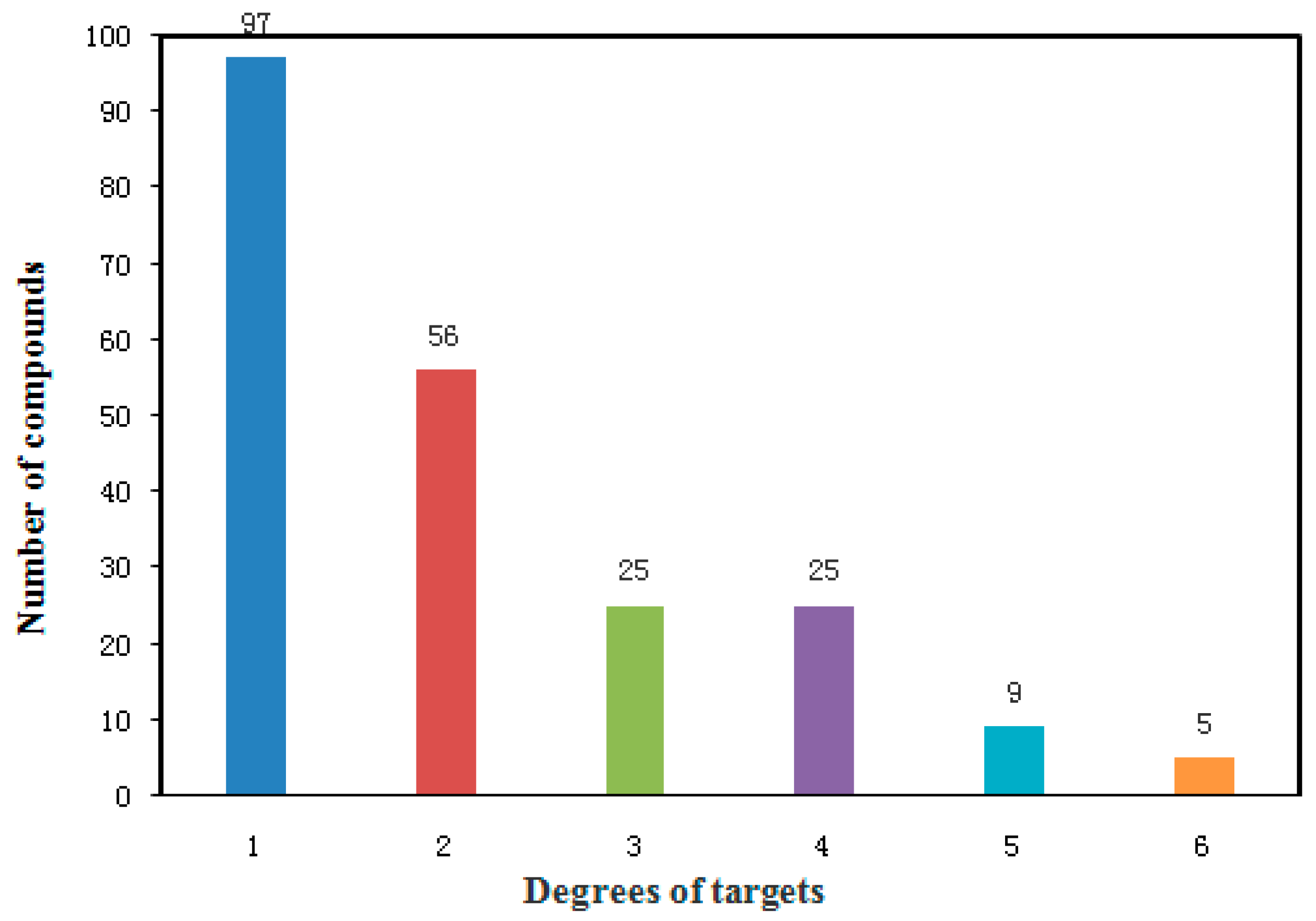
2.2. Potential Targetsand Bioactive Compounds of WDD
2.3. The Potential Pathways Affected by WDD
3. Materials and Methods
3.1. Chemical Structures Collection and Structural Classification
3.2. Chemical Space and Drug-Likeness Calculations
3.3. Target Prediction and Bioactive Compound Screening for WDD
3.4. Network Construction and Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moreira, G.C.; Cipullo, J.P.; Ciorlia, L.A.; Cesarino, C.B.; Vilela-Martin, J.F. Prevalence of metabolic syndrome: Association with risk factors and cardiovascular complications in an urban population. PLoS ONE 2013, 9, e105056. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.E.; Zhang, C.L.; Zhen, Q. Metabolic syndrome in children (review). Exp. Ther. Med. 2016, 12, 2390–2394. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 943162. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Feldman, E.L. Insulin resistance as a key link for the increased risk of cognitive impairment in the metabolic syndrome. Exp. Mol. Med. 2015, 47, e149. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Abundis, E.; Villar, M.D.; Perez-Rubio, K.G.; Zuniga, L.Y.; Cortez-Navarrete, M.; Ramirez-Rodriguez, A.; Gonzalez-Ortiz, M. Novel nutraceutic therapies for the treatment of metabolic syndrome. World J. Diabetes 2016, 7, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhang, A.; Zhang, H.; Sun, H.; Wang, X. The application of metabolomics in traditional Chinese medicine opens up a dialogue between Chinese and Western medicine. Phytother. Res. 2015, 29, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lv, Q.; Xie, D.; Shi, T.; Wen, C. Deciphering the potential pharmaceutical mechanism of Chinese traditional medicine (Gui-zhi-shao-yao-zhi-mu) on rheumatoid arthritis. Sci. Rep. 2016, 6, 22602. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.M.; Xu, J.H.; Ling, W.; Li, Y.; Zhang, X.X.; Dai, Z.K.; Sui, Y.; Zhao, H.L. Efficacy of the Wendan decoction, a Chinese herbal formula, for metabolic syndrome. Altern. Ther. Health Med. 2015, 21, 54–67. [Google Scholar] [PubMed]
- Xu, J.H.; Huang, Y.M.; Ling, W.; Li, Y.; Wang, M.; Chen, X.Y.; Sui, Y.; Zhao, H.L. Wendan decoction for hemorrhagic stroke and ischemic stroke. Complement. Ther. Med. 2015, 23, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Huang, Y.; Xu, J.H.; Li, Y.; Huang, Y.M.; Ling, H.B.; Sui, Y.; Zhao, H.L. Consistent efficacy of Wendan decoction for the treatment of digestive reflux disorders. Am. J. Chin. Med. 2015, 43, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Liu, L. The clinical effect of the treatment of metabolic syndrome by HuanglianWendan decoction. Chin. J. Integr. Med. Cardio Cerebrovasc. 2015, 5, 581–582. [Google Scholar]
- Guan, H.; Yuan, X.; Yu, S.; Xie, N. Clinical observation of HuanglianWendan decoction in treating metabolic syndrome. J. Liaoning Univ. Tradit. Chin. Med. 2012, 14, 88–89. (In Chinese) [Google Scholar]
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China. Part 1; Chemical Industry Press: Beijing, China, 2010. [Google Scholar]
- Yang, R.; Wang, L.Q.; Yuan, B.C.; Liu, Y. The pharmacological activities of licorice. Planta Medica 2015, 81, 1654–1669. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L. Network pharmacology. Nat. Biotechnol. 2007, 25, 1110–1111. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Sheng, Z.; Yan, X.; Cao, Z.; Tang, K. In Silico Insight into Potential Anti-Alzheimer’s Disease Mechanisms of Icariin. Int. J. Mol. Sci. 2016, 17, 113. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, X.D.; Niu, Y.Y.; Duan, D.D.; Yang, X.; Hao, J.; Zhu, C.H.; Chen, D.; Wang, K.X.; Qin, X.M.; et al. Molecular targets of chinese herbs: A clinical study of hepatoma based on network pharmacology. Sci. Rep. 2016, 6, 24944. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, X.; Lai, X.; Kang, J.; Gan, H.; Gao, Y. Structural Investigation for optimization of anthranilic acid derivatives as partial FXR agonists by in silico approaches. Int. J. Mol. Sci. 2016, 17, 536–551. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef] [PubMed]
- Nogara, P.A.; Saraiva, R.D.A.; Bueno, D.C.; Lissner, L.J.; Corte, C.L.D.; Braga, M.M.; Rosemberg, D.B.; Rocha, J.B. Virtual screening of acetylcholinesterase inhibitors using the Lipinski’s rule of five and zinc databank. BioMed Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.P.; Hersey, A.; Montanari, D.; Overington, J. Probing the links between in vitro potency, ADMET and physicochemical parameters. Nat. Rev. Drug Discov. 2011, 10, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Li, Y.; Song, Y.; Li, D.; Sun, H.; Hou, T. ADMET evaluation in drug discovery: 15 accurate prediction of rat oral acute toxicity using relevance vector machine and consensus modeling. J. Cheminform. 2016, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Stratton, C.F.; Newman, D.J.; Tan, D.S. Cheminformatic comparison of approved drugs from natural product versus synthetic origins. Bioorg. Med. Chem. Lett. 2015, 25, 4802–4807. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.T.; Marshall, G.R. Virtual screening for lead discovery. Methods Mol. Biol. 2011, 716, 1–22. [Google Scholar] [PubMed]
- Monsalve, F.A.; Pyarasani, R.D.; Delgado-Lopez, F.; Moore-Carrasco, R. Peroxisome proliferator-activated receptor targets for the treatment of metabolic diseases. Mediat. Inflamm. 2013, 2013, 549–627. [Google Scholar] [CrossRef] [PubMed]
- Maqdasy, S.; Trousson, A.; Tauveron, I.; Volle, D.H.; Baron, S.; Lobaccaro, J.M.A. Once and for all, LXRα and LXRβ are gatekeepers of the endocrine system. Mol. Asp. Med. 2016, 49, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Sadasivuni, M.K.; Reddy, B.M.; Singh, J.; Anup, M.O.; Sunil, V.; Lakshmi, M.N.; Yogeshwari, S.; Chacko, S.K.; Pooja, T.L.; Dandu, A.; et al. CNX-013-B2, a unique pan tissue acting rexinoid, modulates several nuclear receptors and controls multiple risk factors of the metabolic syndrome without risk of hypertriglyceridemia, hepatomegaly and body weight gain in animal models. Diabetol. Metab. Syndr. 2014, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.B. The Intervention Effect of huanglianwendan Decoction on NF-κB, PPAR and Glycolipids Metabolism in Metabolic Syndrome Rats. Ph.D. Thesis, Heilongjiang University of Traditional Chinese Medicine, Haerbin, China, 2011. [Google Scholar]
- Wang, Y.; Liu, Z.; Li, C.; Li, D.; Ouyang, Y.; Yu, J.; Guo, S.; He, F.; Wang, W. Drug target prediction based on the herbs components : The study on the multitargets pharmacological mechanism of Qishenkeli acting on the coronary heart disease. Evid-Based Complement. Altern. 2012, 2012, 69853. [Google Scholar] [CrossRef] [PubMed]
- Janani, C.; Kumari, B.D.R. PPARγ gene-A review. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Mandard, S.; Patsouris, D. Nuclear control of the inflammatory response in mammals by peroxisome proliferator-activated receptors. PPAR Res. 2013, 2013, 613864. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Nassiri-Asl, M. Pharmacological Effects of Glycyrrhiza spp. and Its Bioactive Constituents: Update and Review. Phytother. Res. 2015, 29, 1868–1886. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Mimaki, Y.; Honda, S.; Tanaka, H.; Yokota, S.; Mae, T. Phenolics from Glycyrrhizaglabra roots and their PPARγ ligand-binding activity. Bioorg. Med. Chem. 2010, 18, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Pubchem Database. Available online: http://www.ncbi.nlm.nih.gov/pcassay/ (accessed on 25 February 2016).
- Shirataki, Y.; Motohashi, N.; Tani, S.; Sakagami, H.; Satoh, K.; et al. In vitro biological activity of prenylflavanones. Anticancer Res. 2001, 21, 275–280. [Google Scholar] [PubMed]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Merz, M.K., Jr. Prediction of aqueous solubility of a diverse set of compounds using quantitative structure-property relationships. J. Med. Chem. 2003, 46, 3572–3580. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.W. The Study of Regulating PI3K Pathway to Improve Insulin Resistance of Metabolic Syndrome Rats by Huanglianwendan Decoction. Ph.D. Thesis, Heilongjiang University of Traditional Chinese Medicine, Haerbin, China, 2012. [Google Scholar]
- Wang, C.R. Impact and mechanism on LKB1/AMPK signal pathway in the rats of metabolic syndrome treated via warming the kidney and strengthening the spleen. World J. Integr. Tradit. Western Med. 2016, 11, 338–340. [Google Scholar]
- Finelli, C.; Tarantino, G. Have guidelines addressing physical activity been established in nonalcoholic fatty liver disease? World J. Gastroenterol. 2012, 18, 6790–6800. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Jiang, K.; Li, J.; Tao, Y. Clinical observation on treating 50 cases of NAFLD with the ChaihuWendan decoction. Clin. J. Chin. Med. 2012, 4, 3–5. [Google Scholar]
- Sun, K.L.; Park, K.K.; Park, J.H.Y.; Lim, S.S.; Chung, W.Y. The Inhibitory Effect of Roasted Licorice Extract on Human Metastatic Breast Cancer Cell-Induced Bone Destruction. Phytother. Res. 2013, 27, 1776–1783. [Google Scholar]
- Editorial committee of State Administration of Traditional Chinese Medicine in China. Chinese Materia Medica, 1st ed.; Shanghai Scientific &Techincal Publishers: Shanghai, China, 1999; pp. 30–9000. [Google Scholar]
- Zhou, J.; Xie, G.; Yan, X. Traditional Chinese Medicines Molecular Structures, 1st ed.; Chemical Industry Press: Beijing, China, 2004; pp. 10–1000. [Google Scholar]
- DrugBank Database. Available online: http://www.drugbank.ca/ (accessed on 12 December 2015).
- Chen, M.; Yang, X.; Lai, X.; Gao, Y. 2D and 3D QSAR Models for Identifying Diphenylpyridylethanamine based Inhibitors against Cholesteryl Ester Transfer Protein. Bioorg. Med. Chem. Lett. 2015, 25, 4487–4495. [Google Scholar] [CrossRef] [PubMed]
- Bickerton, G.R.; Paolini, G.V.; Besnard, J.; Muresan, S.; Hopkins, A.L. Quantifying the chemical beauty of drugs. Nat. Chem. 2012, 4, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility B and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Gallastegui, N.; Mackinnon, J.A.; Fletterick, R.J.; Estebanez-Perpina, E. Advances in our structural understanding of orphan nuclear receptors. Trends Biochem. Sci. 2015, 40, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwicowski, B.; Ideker, T. Cytoscape: A Software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]


| Subclasses | Number of Ingredients | Categories |
|---|---|---|
| Lignin | 1 | Phenylpropanoids |
| Coumarin | 47 | Phenylpropanoids |
| Flavan-3-ol | 1 | Flavonoids |
| Isoflavanone | 10 | Flavonoids |
| Anthocyanidin | 8 | Flavonoids |
| Chalcone | 16 | Flavonoids |
| Flavonol | 19 | Flavonoids |
| Isoflavone | 44 | Flavonoids |
| Flavone | 63 | Flavonoids |
| Flavanone | 53 | Flavonoids |
| Dihydrochalcone | 69 | Flavonoids |
| Imidazole | 1 | Alkaloids |
| Piperidines | 4 | Alkaloids |
| Quinolines | 4 | Alkaloids |
| Indoles | 5 | Alkaloids |
| Pyrrolidines | 12 | Alkaloids |
| Six carbon aldose | 99 | Sugars |
| Five carbon aldose | 103 | Sugars |
| Annular monoterpene | 90 | Terpenoids |
| Open chain monoterpene | 10 | Terpenoids |
| Diterpene | 96 | Terpenoids |
| Descriptors | Meaning | Median | Mean | Std. Deviation |
|---|---|---|---|---|
| Weight | Molecular weight | 388.47 | 413.40 | 179.80 |
| a_acc | Number of hydrogen bond acceptor atoms | 5 | 5.59 | 4.08 |
| a_don | Number of hydrogen bond donor atoms | 2 | 3.17 | 2.83 |
| b_rotN | Number of rotatable bonds | 5 | 5.26 | 3.80 |
| logP(o/w) | Log of the octanol/water partition coefficient | 3.28 | 3.24 | 2.83 |
| Herbs | Hit Targets | Number of Bioactive Compounds |
|---|---|---|
| Radix Glycyrrhizae Preparata | PPARα, PPARβ, PPARγ, LXRα, LXRβ, RXRα | 118 |
| Poria Cocos | PPARα, PPARβ, PPARγ, LXRα, LXRβ | 42 |
| Citrus Aurantium | PPARα, PPARβ, PPARγ, LXRα, LXRβ | 35 |
| Pericarpium CitriReticulatae | PPARα, PPARβ, PPARγ, LXRα, LXRβ | 18 |
| Pinellia Ternata | PPARα, PPARβ, PPARγ, LXRα, LXRβ | 3 |
| Code | Node | Degree | Betweenness |
|---|---|---|---|
| 2P54 | PPARα | 67 | 0.0537 |
| 2I4J | PPARγ | 145 | 0.3741 |
| 3UVV | RXRα | 9 | 0.0007 |
| 3IKM | PPARΔ | 48 | 0.0235 |
| 3IPU | LXRα | 128 | 0.3283 |
| 1PQC | LXRβ | 62 | 0.0921 |
| RGP | Radix Glycyrrhizae Preparata | 118 | 0.2311 |
| PC | Poria Cocos | 42 | 0.0264 |
| CA | Citrus Aurantium | 35 | 0.0232 |
| PCR | Pericarpium Citri Reticulatae | 18 | 0.0074 |
| PT | Pinellia Ternata | 3 | 0.0001 |
| Chemical Name | Herb Source | Degree | Bioactivity |
|---|---|---|---|
| Glabrol | Radix Glycyrrhizae Preparata | 6 | Activation of PPARγ and Drd3, and inhibition of TPNT1 and PTP1B. |
| Euchrestaflavanone A | Radix Glycyrrhizae Preparata | 6 | Inhibition of TPNT1 and PTP1B, and cytotoxic activity, antimicrobial activity |
| Euchrenone a5 | Radix Glycyrrhizae Preparata | 6 | Activation of PPARγ |
| Glyinflanin D | Radix Glycyrrhizae Preparata | 6 | Unreported |
| 1-(7-Hydroxy-2,2-dimethyl-2H-chromen-6-yl)-3-(4-hydroxy-3-(3-methylbut-2-en-1-yl)phenyl)propane-1,3-dione | Radix Glycyrrhizae Preparata | 6 | Cytotoxic activity |
| Pathway ID | Term | Pathway Class | Degree |
|---|---|---|---|
| hsa03320 | PPAR signaling pathway | Endocrine system | 6 |
| hsa04919 | Thyroid hormone signaling pathway | Endocrine system | 1 |
| hsa04920 | Adipocytokine signaling pathway | Endocrine system | 2 |
| hsa0492 | Glucagon signaling pathway | Endocrine system | 1 |
| hsa04932 | Non-alcoholic fatty liver disease (NAFLD) | Endocrine and metabolic diseases | 4 |
| hsa04024 | cAMP signaling pathway | Signal transduction | 1 |
| hsa04151 | PI3K-Akt signaling pathway | Signal transduction | 1 |
| hsa04152 | AMPK signaling pathway | Signal transduction | 1 |
| hsa04310 | Wnt signaling pathway | Signal transduction | 1 |
| hsa04380 | Osteoclast differentiation | Development | 1 |
| hsa04976 | Bile secretion | Digestive system | 1 |
| hsa05016 | Huntington’s disease | Neurodegenerative diseases | 1 |
| hsa05160 | Hepatitis C | Infectious diseases | 4 |
| hsa05200 | Pathways in cancer | Cancers | 3 |
| hsa05202 | Transcriptional misregulation in cancer | Cancers | 2 |
| hsa05216 | Thyroid cancer | Cancers | 2 |
| hsa05221 | Acute myeloid leukemia | Cancers | 1 |
| hsa05222 | Small cell lung cancer | Cancers | 1 |
| hsa05223 | Non-small cell lung cancer | Cancers | 1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Yang, F.; Yang, X.; Lai, X.; Gao, Y. Systematic Understanding of Mechanisms of a Chinese Herbal Formula in Treatment of Metabolic Syndrome by an Integrated Pharmacology Approach. Int. J. Mol. Sci. 2016, 17, 2114. https://doi.org/10.3390/ijms17122114
Chen M, Yang F, Yang X, Lai X, Gao Y. Systematic Understanding of Mechanisms of a Chinese Herbal Formula in Treatment of Metabolic Syndrome by an Integrated Pharmacology Approach. International Journal of Molecular Sciences. 2016; 17(12):2114. https://doi.org/10.3390/ijms17122114
Chicago/Turabian StyleChen, Meimei, Fafu Yang, Xuemei Yang, Xinmei Lai, and Yuxing Gao. 2016. "Systematic Understanding of Mechanisms of a Chinese Herbal Formula in Treatment of Metabolic Syndrome by an Integrated Pharmacology Approach" International Journal of Molecular Sciences 17, no. 12: 2114. https://doi.org/10.3390/ijms17122114






