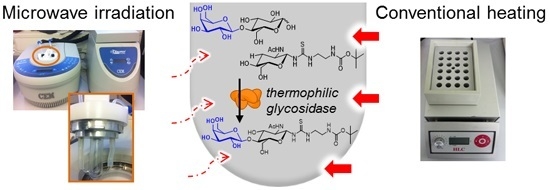
Microwave-Assisted Synthesis of Glycoconjugates by Transgalactosylation with Recombinant Thermostable β-Glycosidase from Pyrococcus †
Abstract
:1. Introduction

2. Results and Discussion
2.1. Production and Characterization of Recombinant β-Glycosidase
| Aryl Glycosides | Relative Activity (%) [c] |
|---|---|
| p-Nitrophenyl-β-d-glucopyranoside (pNP-β-Glc) [a] | 100 |
| p-Nitrophenyl-β-d-galactopyranoside (pNP-β-Gal) [a] | 89 |
| o-Nitrophenyl-β-d-galactopyranoside (oNP-β-Gal) [a] | 69 |
| p-Nitrophenyl-β-d-mannopyranoside (pNP-β-Man) [b] | 39 |
| p-Nitrophenyl-β-d-xylopyranoside (pNP-β-Xyl) [a] | 15 |
2.2. Hydrolytic Activity of Recombinant β-Glycosidase from Pyrococcus under MWI
| Reaction Condition | Relative Activity (%) | Temperature (°C) |
|---|---|---|
| TH 85 °C | 100.0 | 85 °C |
| TH 30 °C | 6.2 ± 3.0 | 30 °C |
| MWI 100 W | 4.3 ± 1.4 | 8–12 °C |
| MWI 200 W | 10.3 ± 2.9 | 10–14 °C |
| MWI 300 W | 21.3 ± 2.9 | 20–32 °C |

2.3. Transglycosylation Reactions with Recombinant β-Glycosidase from Pyrococcus under Thermal Heating

| Peak | Retention Time (min) | Relative Peak Area (%) | m/z Found [M − H]− | m/z Calculated [M] | Product |
|---|---|---|---|---|---|
| 1 | 9.6 | 3.8 | 745.2 | 746.7 | Gal-Gal-GlcNAc-linker-tBoc |
| 2 | 11.3 | 3.3 | 583.1 | 584.6 | Gal-GlcNAc-linker-tBoc |
| 3 | 12.3 | 0.3 | 745.2 | 746.7 | Gal-Gal-GlcNAc-linker-tBoc |
| 4 | 13.4 | 5.8 | 745.2 | 746.7 | Gal-Gal-GlcNAc-linker-tBoc |
| 5 | 14.9 | 41.5 | 583.2 | 584.6 | Gal(β1,4)GlcNAc-linker-tBoc |
2.4. Transglycosylation Reactions with Recombinant β-Glycosidase from Pyrococcus under MWI



3. Materials and Methods
3.1. Gene Cloning
3.2. Production of Recombinant Glycosidase
3.3. Determination of Enzymatic Activities
3.4. Microwave System Equipment
3.5. Synthesis of Glycoconjugates
3.6. Product Analysis and Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bojarová, P.; Kren, V. Glycosidases: A key to tailored carbohydrates. Trends Biotechnol. 2009, 27, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Haensler, M.; Hahn, U.; Jakubke, H.D. Reverse action of ribonuclease T1 in frozen aqueous systems. Biol. Chem. 1997, 378, 115–118. [Google Scholar] [PubMed]
- Zeuner, B.; Jers, C.; Mikkelsen, J.D.; Meyer, A.S. Methods for Improving enzymatic Trans-glycosylation for Synthesis of Human Milk Oligosaccharide Biomimetics. J. Agric. Food Chem. 2014, 62, 9615–9631. [Google Scholar] [CrossRef] [PubMed]
- Cobucci-Ponzano, B.; Perugino, G.; Trincone, A.; Mazzone, M.; di Lauro, B.; Giordano, A.; Rossi, M.; Moracci, M. Applications in Biocatalysis of Glycosyl Hydrolases from the Hyperthermophilic Archaeon Sulfolobus solfataricus. Biocatal. Biotransform. 2003, 21, 215–221. [Google Scholar] [CrossRef]
- Bruckmann, A.; Krebs, A.; Bolm, C. Organocatalytic reactions: Effects of ball milling, microwave and ultrasound irradiation. Green Chem. 2008, 10, 1131–1141. [Google Scholar] [CrossRef]
- Herrero, M.A.; Kremsner, J.M.; Kappe, C.O. Nonthermal microwave effects revisited: On the importance of internal temperature monitoring and agitation in microwave chemistry. J. Org. Chem. 2008, 73, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Nadagouda, M.N.; Varma, R.S. Microwave-Assisted Chemistry: A Rapid and Sustainable Route to Synthesis of Organics and Nanomaterials. Aust. J. Chem. 2009, 62, 16–26. [Google Scholar] [CrossRef]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-Assisted Chemistry: Synthetic Applications for Rapid Assembly of Nanomaterials and Organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O.; Pieber, B.; Dallinger, D. Microwave Effects in Organic Synthesis: Myth or Reality? Angew. Chem. Int. Ed. 2013, 52, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Bachu, P.; Gibson, J.S.; Sperry, J.; Brimble, M.A. The influence of microwave irradiation on lipase-catalyzed kinetic resolution of racemic secondary alcohols. Tetrahedron Asymmetry 2007, 18, 1618–1624. [Google Scholar] [CrossRef]
- Rejasse, B.; Lamare, S.; Legoy, M.D.; Besson, T. Influence of microwave irradiation on enzymatic properties: Applications in enzyme chemistry. J. Enzym. Inhib. Med. Chem. 2007, 22, 518–526. [Google Scholar] [CrossRef]
- Young, D.D.; Nichols, J.; Kelly, R.M.; Deiters, A. Microwave activation of enzymatic catalysis. J. Am. Chem. Soc. 2008, 130, 10048–10049. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.O.M.A.; Antunes, O.A.C.; Kroutil, W.; Kappe, C.O. Kinetic Resolution of rac-1-Phenylethanol with Immobilized Lipases: A Critical Comparison of Microwave and Conventional Heating Protocols. J. Org. Chem. 2009, 74, 6157–6162. [Google Scholar] [CrossRef] [PubMed]
- Kidwai, M.; Mothsra, P.; Gupta, N.; Kumar, S.S.; Gupta, R. Green Enzymatic Synthesis of l-Ascorbyl Fatty Acid Ester: An antioxidant. Synth. Commun. 2009, 39, 1143–1151. [Google Scholar] [CrossRef]
- Risso, M.; Mazzini, M.; Kroeger, S.; Saenz-Mendez, P.; Seoane, G.; Gamenara, D. Microwave-assisted solvent-free lipase catalyzed transesterification of β-ketoesters. Green Chem. Lett. Rev. 2012, 5, 539–543. [Google Scholar] [CrossRef]
- Yu, D.H.; Wang, Y.Y.; Wang, C.M.; Ma, D.X.; Fang, X.X. Combination use of microwave irradiation and ionic liquid in enzymatic isomerization of xylose to xylulose. J. Mol. Catal. B 2012, 79, 8–14. [Google Scholar] [CrossRef]
- Maugard, T.; Gaunt, D.; Legoy, M.D.; Besson, T. Microwave-assisted synthesis of galacto-oligosaccharides from lactose with immobilized β-galactosidase from Kluyveromyces lactis. Biotechnol. Lett. 2003, 25, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Lundell, K.; Kurki, T.; Lindroos, M.; Kanerva, L.T. Room temperature ionic liquids in the kinetic resolution of adrenaline-type aminoethanols by Burkholderia cepacia lipase under normal and microwave conditions. Adv. Synth. Catal. 2005, 347, 1110–1118. [Google Scholar] [CrossRef]
- Ren, H.J.; Zhan, Y.; Fang, X.X.; Yu, D.H. Enhanced catalytic activity and thermal stability of 2,4-dichlorophenol hydroxylase by using microwave irradiation and imidazolium ionic liquid for 2,4-dichlorophenol removal. RSC Adv. 2014, 4, 62631–62638. [Google Scholar] [CrossRef]
- Kamerke, C.; Pattky, M.; Huhn, C.; Elling, L. Synthesis of UDP-activated oligosaccharides with commercial β-galactosidase from Bacillus circulans under microwave irradiation. J. Mol. Catal. B 2012, 79, 27–34. [Google Scholar] [CrossRef]
- Kamerke, C.; Pattky, M.; Huhn, C.; Elling, L. Synthesis of nucleotide-activated disaccharides with recombinant β3-galactosidase C from Bacillus circulans. J. Mol. Catal. B 2013, 89, 73–81. [Google Scholar] [CrossRef]
- Porcelli, M.; Cacciapuoti, G.; Fusco, S.; Massa, R.; dAmbrosio, G.; Bertoldo, C.; DeRosa, M.; Zappia, V. Non-thermal effects of microwaves on proteins: Thermophilic enzymes as model system. FEBS Lett. 1997, 402, 102–106. [Google Scholar] [CrossRef]
- La Cara, F.; Scarfi, M.R.; D’Auria, S.; Massa, R.; d’Ambrosio, G.; Franceschetti, G.; Rossi, M.; de Rosa, M. Different effects of microwave energy and conventional heat on the activity of a thermophilic β-galactosidase from Bacillus acidocaldarius. Bioelectromagnetics 1999, 20, 172–176. [Google Scholar] [CrossRef]
- Nagashima, I.; Sugiyama, J.I.; Sakuta, T.; Sasaki, M.; Shimizu, H. Efficiency of 2.45 and 5.80 GHz microwave irradiation for a hydrolysis reaction by thermostable β-Glucosidase HT1. Biosci. Biotechnol. Biochem. 2014, 78, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, S.; Maciunska, J.; Synowiecki, J. Cloning and nucleotide sequence of the thermostable β-galactosidase gene from Pyrococcus woesei in Escherichia coli and some properties of the isolated enzyme. Mol. Biotechnol. 1998, 10, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, S.; Sobiewska, G.; Maciunska, J.; Synowiecki, J.; Kur, J. Cloning, expression, and purification of the His(6)-tagged thermostable β-galactosidase from Pyrococcus woesei in Escherichia coli and some properties of the isolated enzyme. Protein Expr. Purif. 2000, 19, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Synowiecki, J.; Maciunska, J. Isolation and some properties of the thermostable β-galactosidase of Pyrococcus woesei expressed in Escherichia coli. J. Food Biochem. 2002, 26, 49–62. [Google Scholar] [CrossRef]
- Wanarska, M.; Kur, J.; Pladzyk, R.; Turkiewicz, M. Thermostable Pyrococcus woesei β-d-galactosidase—High level expression, purification and biochemical properties. Acta Biochim. Pol. 2005, 52, 781–787. [Google Scholar] [PubMed]
- Xiong, A.S.; Peng, R.H.; Zhuang, J.; Li, X.; Xue, Y.; Liu, J.G.; Gao, F.; Cai, B.; Chen, J.M.; Yao, Q.H. Directed evolution of a β-galactosidase from Pyrococcus woesei resulting in increased thermostable β-glucuronidase activity. Appl. Microbiol. Biotechnol. 2007, 77, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.W.; Bylina, E.J.; Swanson, R.V.; Kelly, R.M. Comparison of a β-glucosidase and a β-mannosidase from the hyperthermophilic archaeon Pyrococcus furiosus—Purification, characterization, gene cloning, and sequence analysis. J. Biol. Chem. 1996, 271, 23749–23755. [Google Scholar] [PubMed]
- Hamilton-Brehm, S.D.; Schut, G.J.; Adams, M.W.W. Metabolic and evolutionary relationships among Pyrococcus species: Genetic exchange within a hydrothermal vent environment. J. Bacteriol. 2005, 187, 7492–7499. [Google Scholar] [CrossRef] [PubMed]
- Grubiak, K.; Synowiecki, J. Application of the cells of Escherichia coli transformant containing gene encoding thermostable β-galactosidase from Pyrococcus woesei for the production of galactosylfructose. BioTechnologia 2009, 1, 152–162. [Google Scholar]
- Rech, C.; Rosencrantz, R.R.; Krenek, K.; Pelantova, H.; Bojarova, P.; Roemer, C.E.; Hanisch, F.G.; Kren, V.; Elling, L. Combinatorial One-Pot Synthesis of Poly-N-acetyllactosamine Oligosaccharides with Leloir-Glycosyltransferases. Adv. Synth. Catal. 2011, 353, 2492–2500. [Google Scholar] [CrossRef]
- Henze, M.; Schmidtke, S.; Hoffmann, N.; Steffens, H.; Pietruszka, J.; Elling, L. Combination of Glycosyltransferases and a Glycosynthase in Sequential and One-Pot Reactions for the Synthesis of Type1 and Type2 N-Acetyllactosamine Oligomers. ChemCatChem 2015, 7, 3131–3139. [Google Scholar] [CrossRef]
- Henze, M.; You, D.J.; Kamerke, C.; Hoffmann, N.; Angkawidjaja, C.; Ernst, S.; Pietruszka, J.; Kanaya, S.; Elling, L. Rational design of a glycosynthase by the crystal structure of β-galactosidase from Bacillus circulans (BgaC) and its use for the synthesis of N-acetyllactosamine type 1 glycan structures. J. Biotechnol. 2014, 191, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Engels, L.; Elling, L. Wbgl: A novel bacterial α1,2-fucosyltransferase for the synthesis of 2’-fucosyllactose. Glycobiology 2014, 24, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Sauerzapfe, B.; Krenek, K.; Schmiedel, J.; Wakarchuk, W.W.; Pelantova, H.; Kren, V.; Elling, L. Chemo-enzymatic synthesis of poly-N-acetyllactosamine (poly-LacNAc) structures and their characterization for CGL2-galectin-mediated binding of ECM glycoproteins to biomaterial surfaces. Glycoconj. J. 2009, 26, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Slamova, K.; Marhol, P.; Bezouska, K.; Lindkvist, L.; Hansen, S.G.; Kren, V.; Jensen, H.H. Synthesis and biological activity of glycosyl-1H-1,2,3-triazoles. Bioorg. Med. Chem. Lett. 2013, 23, 6197–6197. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henze, M.; Merker, D.; Elling, L. Microwave-Assisted Synthesis of Glycoconjugates by Transgalactosylation with Recombinant Thermostable β-Glycosidase from Pyrococcus. Int. J. Mol. Sci. 2016, 17, 210. https://doi.org/10.3390/ijms17020210
Henze M, Merker D, Elling L. Microwave-Assisted Synthesis of Glycoconjugates by Transgalactosylation with Recombinant Thermostable β-Glycosidase from Pyrococcus. International Journal of Molecular Sciences. 2016; 17(2):210. https://doi.org/10.3390/ijms17020210
Chicago/Turabian StyleHenze, Manja, Dorothee Merker, and Lothar Elling. 2016. "Microwave-Assisted Synthesis of Glycoconjugates by Transgalactosylation with Recombinant Thermostable β-Glycosidase from Pyrococcus" International Journal of Molecular Sciences 17, no. 2: 210. https://doi.org/10.3390/ijms17020210
APA StyleHenze, M., Merker, D., & Elling, L. (2016). Microwave-Assisted Synthesis of Glycoconjugates by Transgalactosylation with Recombinant Thermostable β-Glycosidase from Pyrococcus. International Journal of Molecular Sciences, 17(2), 210. https://doi.org/10.3390/ijms17020210