The Establishment of an Assay to Measure DNA Polymerase-Catalyzed Repair of UVB-Induced DNA Damage in Skin Cells and Screening of DNA Polymerase Enhancers from Medicinal Plants
Abstract
:1. Introduction
2. Results
2.1. Cellular Polymerase (Pol) Activity in Ultraviolet B (UVB)-Irradiated Normal Human Epidermal Keratinocytes (NHEKs)
2.2. Medicinal Plant Pol Activity Enhancer Screen in UVB-Irradiated NHEKs
2.3. Effect of Rose Myrtle Extract on Cellular Pol Activity in UVB-Irradiated NHEKs
2.4. Isolation of a Cellular Pol Component from Rose Myrtle Extract Active against UVB-Irradiated NHEKs
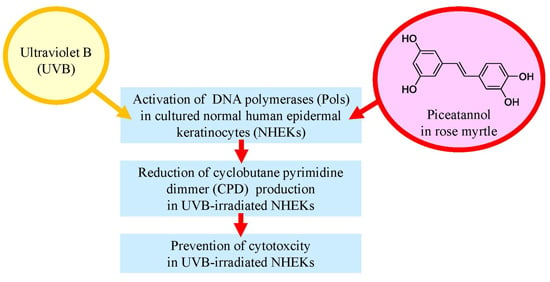
2.5. Effect of Rose Myrtle Extract and Piceatannol on Pol Activity in UVB-Irradiated NHEKs
2.6. Effect of Rose Myrtle Extract and Piceatannol on Cyclobutane Pyrimidine Dimmer (CPD) Production in UVB-Irradiated NHEKs
2.7. Effect of Rose Myrtle Extract and Piceatannol on Cell Viability in UVB-Irradiated NHEKs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Measurement of Cellular Pol Activity in NHEKs
4.4. Purification and Identification of a Cellular Pol Enhancer
4.5. Measurement of CPD Production
4.6. Measurement of Cell Viability
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| Pol | DNA polymerase |
| NHEKs | normal human epidermal keratinocytes |
| UV | ultraviolet |
| CPDs | cyclobutane pyrimidine dimmers |
| TT-CPDs | TT-cis-syn CPDs |
| TdT | terminal-deoxynucleotidyl transferase |
| TLS | translesion DNA synthesis |
| KGM | keratinocyte growth medium |
| dTTP | 2′-deoxythymidine-5′-triphosphate |
| DMSO | dimethyl sulfoxide |
| dNTP | 2′-deoxynucleotide-5′-triphosphate |
| ELISA | enzyme-linked immunosorbent assay |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| SEM | standard error of the mean |
| NER | nucleotide excision repair |
| XP | xeroderma pigmentosum |
| BER | base excision repair |
References
- Berneburg, M.; Plettenberg, H.; Krutmann, J. Photoaging of human skin. Photodermatol. Photoimmunol. Photomed. 2000, 16, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Verschooten, L.; Claerhout, S.; van Laethem, A.; Agostinis, P.; Garmyn, M. New strategies of photoprotection. Photochem. Photobiol. 2006, 82, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, S.; Takahashi, Y. Photoaging and DNA repair. J. Dermatol. Sci. 2008, 50, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Hübscher, U.; Maga, G.; Spadari, S. Eukaryotic DNA polymerases. Annu. Rev. Biochem. 2002, 71, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.S.; Takata, K.; Wood, R.D. DNA polymerases and cancer. Nat. Rev. Cancer 2011, 11, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Autier, P. Sunscreen abuse for intentional sun exposure. Br. J. Dermatol. 2009, 161, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, P.; Krutmann, J. What is needed for a sunscreen to provide complete protection. Skin Ther. Lett. 2010, 15, 4–5. [Google Scholar]
- Matsui, M.S.; Hsia, A.; Miller, J.D.; Hanneman, K.; Scull, H.; Cooper, K.D.; Baron, E. Non-sunscreen photoprotection: Antioxidants add value to a sunscreen. J. Investig. Dermatol. Symp. Proc. 2009, 14, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Mizushina, Y. Specific inhibitors of mammalian DNA polymerase species. Biosci. Biotechnol. Biochem. 2009, 73, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Mizushina, Y. Screening of novel bioactive compounds from food components and nutrients. J. Jpn. Soc. Nutr. Food Sci. 2011, 64, 377–384. [Google Scholar] [CrossRef]
- Mizushina, Y.; Tanaka, N.; Yagi, H.; Kurosawa, T.; Onoue, M.; Seto, H.; Horie, T.; Aoyagi, N.; Yamaoka, M.; Matsukage, A.; et al. Fatty acids selectively inhibit eukaryotic DNA polymerase activities in vitro. Biochim. Biophys. Acta 1996, 1308, 256–262. [Google Scholar] [CrossRef]
- Mizushina, Y.; Yoshida, S.; Matsukage, A.; Sakaguchi, K. The inhibitory action of fatty acids on DNA polymerase β. Biochim. Biophys. Acta 1997, 1336, 509–521. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Nonaka, G.; Nishioka, I.; Nishizawa, M.; Yamagishi, T. Studies on rhubarb (Rhei Rhizoma). XIV. Isolation and characterization of stilbene glucosides from chinese rhubarb. Chem. Pharm. Bull. 1988, 36, 1545–1549. [Google Scholar] [CrossRef]
- Marrot, L.; Meunier, J.R. Skin DNA photodamage and its biological consequences. J. Am. Acad. Dermatol. 2008, 58, S139–S148. [Google Scholar] [CrossRef] [PubMed]
- Shiratake, S.; Nakahara, T.; Iwahashi, H.; Onodera, T.; Mizushina, Y. Rose myrtle (Rhodomyrtus tomentosa) extract and its component, piceatannol, enhance the activity of DNA polymerase and suppress the inflammatory response elicited by UVB-induced DNA damage in skin cells. Mol. Med. Rep. 2015, 12, 5857–5864. [Google Scholar] [CrossRef] [PubMed]
- O’Day, C.L.; Burgers, P.M.; Taylor, J.S. PCNA-induced DNA synthesis past cis-syn and trans-syn-I thymine dimers by calf thymus DNA polymerase delta in vitro. Nucleic Acids Res. 1992, 20, 5403–5406. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.D.; Araujo, S.J.; Ariza, R.R.; Batty, D.P.; Biggerstaff, M.; Evans, E.; Gaillard, P.H.; Gunz, D.; Koberle, B.; Kuraoka, I.; et al. DNA damage recognition and nucleotide excision repair in mammalian cells. Cold Spring Harb. Symp. Quant. Biol. 2000, 65, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Bootsma, D.; Weeda, G.; Vermeulen, W.; van Vuuren, H.; Troelstra, C.; van der Spek, P.; Hoeijmakers, J. Nucleotide excision repair syndromes: Molecular basis and clinical symptoms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1995, 347, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Masutani, C.; Kusumoto, R.; Yamada, A.; Dohmae, N.; Yokoi, M.; Yuasa, M.; Araki, M.; Iwai, S.; Takio, K.; Hanaoka, F. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase eta. Nature 1999, 399, 700–704. [Google Scholar] [PubMed]
- Johnson, R.E.; Washington, M.T.; Prakash, S.; Prakash, L. Fidelity of human DNA polymerase η. J. Biol. Chem. 2000, 275, 7447–7450. [Google Scholar] [CrossRef] [PubMed]
- Servant, L.; Cazaux, C.; Bieth, A.; Iwai, S.; Hanaoka, F.; Hoffmann, J.S. A role for DNA polymerase β in mutagenic UV lesion bypass. J. Biol. Chem. 2002, 277, 50046–50053. [Google Scholar] [CrossRef] [PubMed]
- Gailani, M.R.; Leffell, D.J.; Ziegler, A.; Gross, E.G.; Brash, D.E.; Bale, A.E. Relationship between sunlight exposure and a key genetic alteration in basal cell carcinoma. J. Natl. Cancer Inst. 1996, 88, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Pagès, V.; Fuchs, R.P. How DNA lesions are turned into mutations within cells? Oncogene 2002, 21, 8957–8966. [Google Scholar] [CrossRef] [PubMed]
- Lindell, T.J.; Weinberg, F.; Morris, P.W.; Roeder, R.G.; Rutter, W.J. Specific inhibition of nuclear RNA polymerase II by α-amanitin. Science 1970, 170, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]








© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeoka, S.; Nakahara, T.; Iwahashi, H.; Mizushina, Y. The Establishment of an Assay to Measure DNA Polymerase-Catalyzed Repair of UVB-Induced DNA Damage in Skin Cells and Screening of DNA Polymerase Enhancers from Medicinal Plants. Int. J. Mol. Sci. 2016, 17, 667. https://doi.org/10.3390/ijms17050667
Ikeoka S, Nakahara T, Iwahashi H, Mizushina Y. The Establishment of an Assay to Measure DNA Polymerase-Catalyzed Repair of UVB-Induced DNA Damage in Skin Cells and Screening of DNA Polymerase Enhancers from Medicinal Plants. International Journal of Molecular Sciences. 2016; 17(5):667. https://doi.org/10.3390/ijms17050667
Chicago/Turabian StyleIkeoka, Sawako, Tatsuo Nakahara, Hiroyasu Iwahashi, and Yoshiyuki Mizushina. 2016. "The Establishment of an Assay to Measure DNA Polymerase-Catalyzed Repair of UVB-Induced DNA Damage in Skin Cells and Screening of DNA Polymerase Enhancers from Medicinal Plants" International Journal of Molecular Sciences 17, no. 5: 667. https://doi.org/10.3390/ijms17050667