Effect of AQP9 Expression in Androgen-Independent Prostate Cancer Cell PC3
Abstract
:1. Introduction
2. Result
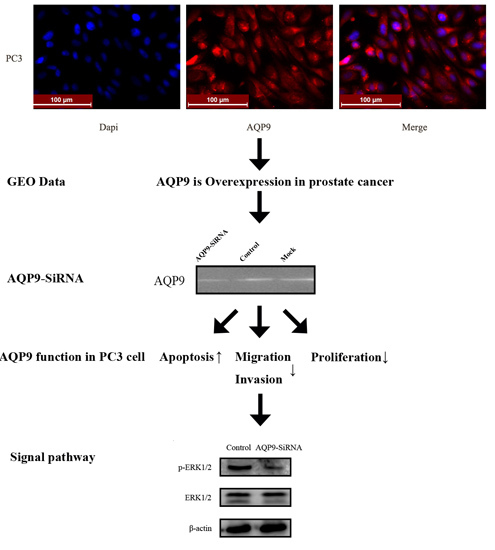
2.1. Aquaporin 9 (AQP9) Expression in Prostate Cancer Cells
2.2. Knockdown of AQP9 Suppressed the Proliferation of Prostate Cancer Cells
2.3. Knockdown of AQP9 Induced Apoptosis in Prostate Cancer Cells
2.4. Silencing of AQP9 Inhibited the Migration and Invasion of Prostate Cancer Cells
2.5. ERK Pathway Is Required for AQP9-Mediated Motility and Invasion
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Quantitative Real-Time PCR and Western Blot
4.3. Small Interfering (Si) RNA Treatment
4.4. Cell Proliferation Assay, Wounding Healing Assay and Invasion Assay
4.5. Immunofluorescence Microscopy
4.6. Statistical Analysis and Gene Set Enrichment Analysis (GSEA)
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PCa | Prostate cancer |
| AQP | Aquaporin |
| siRNA | Small interfering RNA |
| ERK | Extracellular signal-regulated kinase |
| GSEA | Gene set enrichment analysis |
References
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef] [PubMed]
- Sluka, P.; Davis, I.D. Cell mates: Paracrine and stromal targets for prostate cancer therapy. Nat. Rev. Urol. 2013, 10, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. More than just water channels: Unexpected cellular roles of aquaporins. J. Cell Sci. 2005, 118 Pt 15, 3225–3232. [Google Scholar] [CrossRef] [PubMed]
- Bloch, O.; Papadopoulos, M.C.; Manley, G.T.; Verkman, A.S. Aquaporin-4 gene deletion in mice increases focal edema associated with staphylococcal brain abscess. J. Neurochem. 2005, 95, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin-4 and brain edema. Pediatr. Nephrol. 2007, 22, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, B.; Zhang, L.; Chen, L.; Sun, G.; Zhang, Q.; Wang, J.; Zhi, X.; Wang, L.; Xu, Z.; et al. The proliferation impairment induced by AQP3 deficiency is the result of glycerol uptake and metabolism inhibition in gastric cancer cells. Tumour Biol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Xia, R. The emerging role of aquaporin 5 (AQP5) expression in systemic malignancies. Tumour Biol. 2014, 35, 6191–6192. [Google Scholar] [CrossRef] [PubMed]
- El Hindy, N.; Bankfalvi, A.; Herring, A.; Adamzik, M.; Lambertz, N.; Zhu, Y.; Siffert, W.; Sure, U.; Sandalcioglu, I.E. Correlation of aquaporin-1 water channel protein expression with tumor angiogenesis in human astrocytoma. Anticancer Res. 2013, 33, 609–613. [Google Scholar] [PubMed]
- Wei, M.; Shi, R.; Zeng, J.; Wang, N.; Zhou, J.; Ma, W. The over-expression of aquaporin-1 alters erythroid gene expression in human erythroleukemia K562 cells. Tumour Biol. 2015, 36, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tanji, N.; Sasaki, T.; Kikugawa, T.; Song, X.; Yokoyama, M. Androgens upregulate aquaporin 9 expression in the prostate. Int. J. Urol. 2008, 15, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Nicchia, G.P.; Frigeri, A.; Nico, B.; Ribatti, D.; Svelto, M. Tissue distribution and membrane localization of aquaporin-9 water channel: Evidence for sex-linked differences in liver. J. Histochem. Cytochem. 2001, 49, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Taira, K.; Morris, K.V. siRNA induced transcriptional gene silencing in mammalian cells. Cell Cycle 2005, 4, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Shankar, E.; Song, K.; Corum, S.L.; Bane, K.L.; Wang, H.; Kao, H.Y.; Danielpour, D. Signaling Network Controlling Androgenic Repression of c-Fos in Prostate Adenocarcinoma Cells. J. Biol Chem. 2016, 291, 5512–5526. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Hao, J.W.; Zhou, R.J.; Zhang, X.S.; Yan, T.Z.; Ding, D.G.; Shan, L. Meta-analysis of gene expression data identifies causal genes for prostate cancer. Asian Pac. J. Cancer Prev. 2013, 14, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Saadoun, S. Key roles of aquaporins in tumor biology. Biochim. Biophys. Acta 2015, 1848 10 Pt B, 2576–2583. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Yan, C.X.; Chen, X.J.; Zhu, Y.S. Expression of aquaglyceroporins in epithelial ovarian tumours and their clinical significance. J. Int. Med. Res. 2011, 39, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Fossdal, G.; Vik-Mo, E.O.; Sandberg, C.; Varghese, M.; Kaarbø, M.; Telmo, E.; Langmoen, I.A.; Murrell, W. AQP 9 and brain tumour stem cells. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Soler, N.; Bagnis, C.; Sabolic, I.; Tyszkowski, R.; McKee, M.; van Hoek, A.; Breton, S.; Brown, D. Aquaporin 9 expression along the male reproductive tract. Biol. Reprod. 2001, 65, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Soler, N.; Isnard-Bagnis, C.; Herak-Kramberger, C.; Sabolic, I.; van Hoek, A.; Brown, D.; Breton, S. Expression of aquaporin 9 in the adult rat epididymal epithelium is modulated by androgens. Biol. Reprod. 2002, 66, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Zhao, G.; Li, Y.; Li, H.; Zhao, X.; Pannone, G.; Bufo, P.; Santoro, A.; Sanguedolce, F.; Tortorella, S.; et al. Role of Runx2 phosphorylation in prostate cancer and association with metastatic disease. Oncogene 2016, 35, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.; Wang, W.; Sun, D.; Jiang, Y.; Tian, S.; Li, J.; Yang, X.; Shi, R. Bone morphogenetic protein 2 mediates epithelial-mesenchymal transition via AKT and ERK signaling pathways in gastric cancer. Tumour Biol. 2015, 36, 2773–2778. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Ma, W.; Hu, Z.; Zang, L.; Tian, Z.; Zhang, K. Filamin A (FLNA) modulates chemosensitivity to docetaxel in triple-negative breast cancer through the MAPK/ERK pathway. Tumour Biol. 2016, 37, 5107–5115. [Google Scholar] [CrossRef] [PubMed]
- Nico, B.; Ribatti, D. Aquaporins in tumor growth and angiogenesis. Cancer Lett. 2010, 294, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Huo, C.; Kao, Y.-H.; Chuu, C.-P. Androgen receptor inhibits epithelial-mesenchymal transition, migration, and invasion of PC-3 prostate cancer cells. Cancer Lett. 2015, 369, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qi, K.; Wang, Z.; Gu, M.; Chen, G.; Guo, F.; Wang, Z. Golgi phosphoprotein 3 regulates metastasis of prostate cancer via matrix metalloproteinase 9. Int. J. Clin. Exp. Pathol. 2015, 8, 3691–3700. [Google Scholar] [PubMed]







© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Zhu, L.; Zheng, B.; Wang, J.; Song, X.; Zheng, W.; Wang, L.; Yang, D.; Wang, J. Effect of AQP9 Expression in Androgen-Independent Prostate Cancer Cell PC3. Int. J. Mol. Sci. 2016, 17, 738. https://doi.org/10.3390/ijms17050738
Chen Q, Zhu L, Zheng B, Wang J, Song X, Zheng W, Wang L, Yang D, Wang J. Effect of AQP9 Expression in Androgen-Independent Prostate Cancer Cell PC3. International Journal of Molecular Sciences. 2016; 17(5):738. https://doi.org/10.3390/ijms17050738
Chicago/Turabian StyleChen, Qiwei, Liang Zhu, Bo Zheng, Jinliang Wang, Xishuang Song, Wei Zheng, Lina Wang, Deyong Yang, and Jianbo Wang. 2016. "Effect of AQP9 Expression in Androgen-Independent Prostate Cancer Cell PC3" International Journal of Molecular Sciences 17, no. 5: 738. https://doi.org/10.3390/ijms17050738
APA StyleChen, Q., Zhu, L., Zheng, B., Wang, J., Song, X., Zheng, W., Wang, L., Yang, D., & Wang, J. (2016). Effect of AQP9 Expression in Androgen-Independent Prostate Cancer Cell PC3. International Journal of Molecular Sciences, 17(5), 738. https://doi.org/10.3390/ijms17050738