Differential Apoptosis Radiosensitivity of Neural Progenitors in Adult Mouse Hippocampus
Abstract
:1. Introduction
2. Results
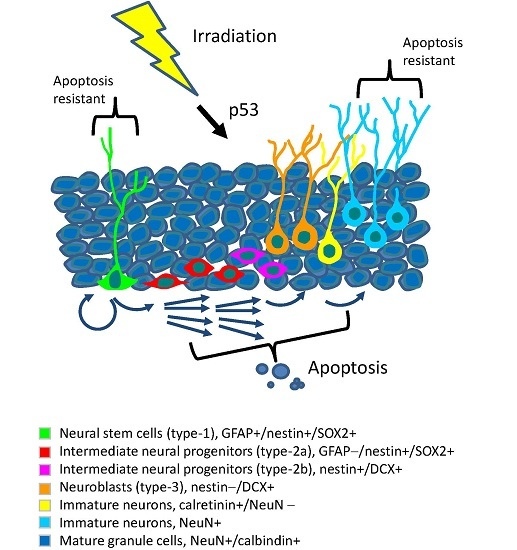
2.1. Neuroblasts and Immature Neurons Undergo Radiation-Induced Apoptosis
2.2. Proliferating Early NPCs but Not Newborn NPCs Undergo Radiation-Induced Apoptosis
2.3. There Is Differential Activation of p53 in NPCs after Irradiation
2.4. Radiation-Induced Apoptosis Is Not Influenced by p21 Status
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Irradiation
4.3. Bromodeoxyuridine (BrdU) Incorporation
4.4. Histopathology and Immunohistochemistry
4.5. Stereological Analysis
4.6. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Bonaguidi, M.A.; Song, J.; Ming, G.L.; Song, H. A unifying hypothesis on mammalian neural stem cell properties in the adult hippocampus. Curr. Opin. Neurobiol. 2012, 22, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Shors, T.J.; Miesegaes, G.; Beylin, A.; Zhao, M.; Rydel, T.; Gould, E. Neurogenesis in the adult is involved in the formation of trace memories. Nature 2001, 410, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Rampon, C.; Tang, Y.P.; Shrom, D.; Jin, J.; Kyin, M.; Sopher, B.; Miller, M.W.; Ware, C.B.; Martin, G.M.; et al. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron 2001, 32, 911–926. [Google Scholar] [CrossRef]
- Sahay, A.; Scobie, K.N.; Hill, A.S.; O’Carroll, C.M.; Kheirbek, M.A.; Burghardt, N.S.; Fenton, A.A.; Dranovsky, A.; Hen, R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 2011, 472, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Akers, K.G.; Martinez-Canabal, A.; Restivo, L.; Yiu, A.P.; de Cristofaro, A.; Hsiang, H.L.; Wheeler, A.L.; Guskjolen, A.; Niibori, Y.; Shoji, H.; et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 2014, 344, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, H.; Kempermann, G.; Gage, F.H. Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 2000, 1, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Lugert, S.; Basak, O.; Knuckles, P.; Haussler, U.; Fabel, K.; Gotz, M.; Haas, C.A.; Kempermann, G.; Taylor, V.; Giachino, C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 2010, 6, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.L.; Palmer, T. Radiation injury and neurogenesis. Curr. Opin. Neurol. 2003, 16, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Fike, J.R.; Rosi, S.; Limoli, C.L. Neural precursor cells and central nervous system radiation sensitivity. Semin. Radiat. Oncol. 2009, 19, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Blanpain, C.; Mohrin, M.; Sotiropoulou, P.A.; Passegue, E. DNA-damage response in tissue-specific and cancer stem cells. Cell Stem Cell 2011, 8, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Chow, B.M.; Li, Y.Q.; Wong, C.S. Radiation-induced apoptosis in the adult central nervous system is p53-dependent. Cell Death Differ. 2000, 7, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Li, Y.Q.; Aubert, I.; Wong, C.S. Endothelial cells regulate p53-dependent apoptosis of neural progenitors after irradiation. Cell Death Dis. 2012, 3, e324. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.; Encinas, J.M.; Deudero, J.J.; Chancey, J.H.; Enikolopov, G.; Overstreet-Wadiche, L.S.; Tsirka, S.E.; Maletic-Savatic, M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 2010, 7, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Mizumatsu, S.; Monje, M.L.; Morhardt, D.R.; Rola, R.; Palmer, T.D.; Fike, J.R. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003, 63, 4021–4027. [Google Scholar] [PubMed]
- Bonaguidi, M.A.; Wheeler, M.A.; Shapiro, J.S.; Stadel, R.P.; Sun, G.J.; Ming, G.L.; Song, H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 2011, 145, 1142–1155. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Aubert, I.; Wong, C.S. Abrogation of early apoptosis does not alter late inhibition of hippocampal neurogenesis after irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; Russo, A.; Cook, J.A.; Straus, K.L.; Glatstein, E. Radiobiology and clinical application of halogenated pyrimidine radiosensitizers. Int. J. Radiat. Biol. 1989, 56, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.D.; Doan, N.B.; Imura, T.; Bush, T.G.; Sofroniew, M.V. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat. Neurosci. 2004, 7, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Dranovsky, A.; Picchini, A.M.; Moadel, T.; Sisti, A.C.; Yamada, A.; Kimura, S.; Leonardo, E.D.; Hen, R. Experience dictates stem cell fate in the adult hippocampus. Neuron 2011, 70, 908–923. [Google Scholar] [CrossRef] [PubMed]
- Encinas, J.M.; Michurina, T.V.; Peunova, N.; Park, J.H.; Tordo, J.; Peterson, D.A.; Fishell, G.; Koulakov, A.; Enikolopov, G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 2011, 8, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Insinga, A.; Cicalese, A.; Faretta, M.; Gallo, B.; Albano, L.; Ronzoni, S.; Furia, L.; Viale, A.; Pelicci, P.G. DNA damage in stem cells activates p21, inhibits p53, and induces symmetric self-renewing divisions. Proc. Natl. Acad. Sci. USA 2013, 110, 3931–3936. [Google Scholar] [CrossRef] [PubMed]
- Kippin, T.E.; Martens, D.J.; van der Kooy, D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005, 19, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Meletis, K.; Wirta, V.; Hede, S.M.; Nister, M.; Lundeberg, J.; Frisen, J. p53 suppresses the self-renewal of adult neural stem cells. Development 2006, 133, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Armesilla-Diaz, A.; Bragado, P.; del Valle, I.; Cuevas, E.; Lazaro, I.; Martin, C.; Cigudosa, J.C.; Silva, A. p53 regulates the self-renewal and differentiation of neural precursors. Neuroscience 2009, 158, 1378–1389. [Google Scholar] [CrossRef] [PubMed]
- Gil-Perotin, S.; Haines, J.D.; Kaur, J.; Marin-Husstege, M.; Spinetta, M.J.; Kim, K.H.; Duran-Moreno, M.; Schallert, T.; Zindy, F.; Roussel, M.F.; et al. Roles of p53 and p27Kip1 in the regulation of neurogenesis in the murine adult subventricular zone. Eur. J. Neurosci. 2011, 34, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Marques-Torrejon, M.A.; Porlan, E.; Banito, A.; Gomez-Ibarlucea, E.; Lopez-Contreras, A.J.; Fernandez-Capetillo, O.; Vidal, A.; Gil, J.; Torres, J.; Farinas, I. Cyclin-dependent kinase inhibitor p21 controls adult neural stem cell expansion by regulating Sox2 gene expression. Cell Stem Cell 2013, 12, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Roque, T.; Haton, C.; Etienne, O.; Chicheportiche, A.; Rousseau, L.; Martin, L.; Mouthon, M.A.; Boussin, F.D. Lack of a p21waf1/cip-dependent G1/S checkpoint in neural stem and progenitor cells after DNA damage in vivo. Stem Cells 2012, 30, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Tronche, F.; Kellendonk, C.; Kretz, O.; Gass, P.; Anlag, K.; Orban, P.C.; Bock, R.; Klein, R.; Schutz, G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 1999, 23, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Marino, S.; Vooijs, M.; van Der Gulden, H.; Jonkers, J.; Berns, A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000, 14, 994–1004. [Google Scholar] [PubMed]
- Roughton, K.; Kalm, M.; Blomgren, K. Sex-dependent differences in behavior and hippocampal neurogenesis after irradiation to the young mouse brain. Eur. J. Neurosci. 2012, 36, 2763–2772. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: London, UK, 2001. [Google Scholar]








| Cell Type (Phenotypic Markers) | Number of Cells | |
|---|---|---|
| 0 Gy | 17 Gy | |
| Type 1, neural stem cells (nestin+/GFAP+) | 1826 ± 178 | 1887 ± 248 |
| Type 1, neural stem cells (SOX2+/GFAP+) | 980 ± 84 | 831 ± 252 |
| Type 2a, early INPs (nestin+/DCX−) | 1782 ± 392 | 1222 ± 340 |
| Type 2a, early INPs (SOX2+/Mash1+) | 2228 ± 354 | 1753 ± 564 |
| Type 2b, late INPs (nestin+/DCX+) | 650 ± 121 | 265 ± 53 * |
| Type 3, neuroblasts (nestin+/DCX+) | 15,517 ± 240 | 3646 ± 56 ** |
| Immature neurons (DCX+/calretinin+) | 1476 ± 1075 | 3 ± 3 ** |
| Mature neurons (NeuN+/calbindin+) | 37,112 ± 2980 | 48,347 ± 11,897 |
| Mature neurons (NeuN+) | 428,274 ± 38,591 | 393,498 ± 25,634 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-Q.; Cheng, Z.; Wong, S. Differential Apoptosis Radiosensitivity of Neural Progenitors in Adult Mouse Hippocampus. Int. J. Mol. Sci. 2016, 17, 970. https://doi.org/10.3390/ijms17060970
Li Y-Q, Cheng Z, Wong S. Differential Apoptosis Radiosensitivity of Neural Progenitors in Adult Mouse Hippocampus. International Journal of Molecular Sciences. 2016; 17(6):970. https://doi.org/10.3390/ijms17060970
Chicago/Turabian StyleLi, Yu-Qing, Zoey Cheng, and Shun Wong. 2016. "Differential Apoptosis Radiosensitivity of Neural Progenitors in Adult Mouse Hippocampus" International Journal of Molecular Sciences 17, no. 6: 970. https://doi.org/10.3390/ijms17060970