MRI Dynamically Evaluates the Therapeutic Effect of Recombinant Human MANF on Ischemia/Reperfusion Injury in Rats
Abstract
:1. Introduction
2. Results
2.1. Effects of MANF Protein on the Mortality, Body Weight, and Neurological Score of Rats with MCAO
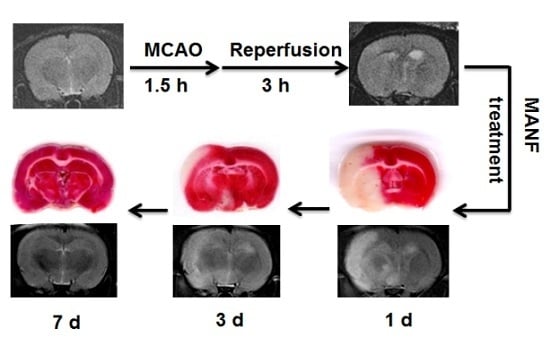
2.2. The Early MRI Manifestation of Ischemia/Reperfusion Injury and the Selection of Proper Time for MANF Administration
2.3. MRI Manifestation of MANF Restoration on Focal Cerebral Ischemia
2.4. MRI Manifestation Was Confirmed by TTC Staining in Evaluating the Therapeutic Effect of MANF on Focal Cerebral Ischemia
2.5. MRI Manifestation Was Confirmed by Histologic Analyses in Evaluating the Therapeutic Effect of MANF on Focal Cerebral Ischemia
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Induction of Focal Cerebral Ischemia/Reperfusion Injury
4.4. Drug Administration
4.5. Neurological Function Test
4.6. Magnetic Resonance Imaging
4.7. 2,3,5-Triphenyltetrazolium Chloride (TTC) Staining
4.8. Immunohistochemistry
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mukherjee, D.; Patil, C.G. Epidemiology and the global burden of stroke. World Neurosurg. 2011, 76, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J.; et al. Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation 2016, 133, 38–360. [Google Scholar] [CrossRef] [PubMed]
- Messe, S.R.; Fonarow, G.C.; Smith, E.E.; Kaltenbach, L.; Olson, D.M.; Kasner, S.E.; Schwamm, L.H. Use of tissue-type plasminogen activator before and after publication of the european cooperative acute stroke study III in get with the guidelines-stroke. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Kleindorfer, D.; Kissela, B.; Schneider, A.; Woo, D.; Khoury, J.; Miller, R.; Alwell, K.; Gebel, J.; Szaflarski, J.; Pancioli, A.; et al. Eligibility for recombinant tissue plasminogen activator in acute ischemic stroke: A population-based study. Stroke 2004, 35, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Z.; Reeves, M.J.; Jacobs, B.S.; Birbeck, G.L.; Kothari, R.U.; Hickenbottom, S.L.; Mullard, A.J.; Wehner, S.; Maddox, K.; Majid, A.; et al. IV tissue plasminogen activator use in acute stroke: Experience from a statewide registry. Neurology 2006, 66, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Apostolou, A.; Shen, Y.; Liang, Y.; Luo, J.; Fang, S. Armet, a UPR-upregulated protein, inhibits cell proliferation and ER stress-induced cell death. Exp. Cell Res. 2008, 314, 2454–2467. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Sundvik, M.; Rozov, S.; Priyadarshini, M.; Panula, P. MANF regulates dopaminergic neuron development in larval zebrafish. Dev. Biol. 2012, 370, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Hellman, M.; Arumae, U.; Yu, L.Y.; Lindholm, P.; Peranen, J.; Saarma, M.; Permi, P. Mesencephalic astrocyte-derived neurotrophic factor (MANF) has a unique mechanism to rescue apoptotic neurons. J. Biol. Chem. 2011, 286, 2675–2680. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, Y.; Cheng, L.; Liu, B.; Zhang, W.; Guo, Y.J.; Nie, L. Mesencephalic astrocyte-derived neurotrophic factor inhibits oxygen-glucose deprivation-induced cell damage and inflammation by suppressing endoplasmic reticulum stress in rat primary astrocytes. J. Mol. Neurosci. 2013, 51, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Q.; Liu, L.C.; Wang, F.C.; Liang, Y.; Cha, D.Q.; Zhang, J.J.; Shen, Y.J.; Wang, H.P.; Fang, S.; Shen, Y.X. Induction profile of MANF/ARMET by cerebral ischemia and its implication for neuron protection. J. Cereb. Blood Flow Metab. 2010, 30, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Glembotski, C.C.; Thuerauf, D.J.; Huang, C.; Vekich, J.A.; Gottlieb, R.A.; Doroudgar, S. Mesencephalic astrocyte-derived neurotrophic factor protects the heart from ischemic damage and is selectively secreted upon sarco/endoplasmic reticulum calcium depletion. J. Biol. Chem. 2012, 287, 25893–25904. [Google Scholar] [CrossRef] [PubMed]
- Matlik, K.; Yu, L.Y.; Eesmaa, A.; Hellman, M.; Lindholm, P.; Peranen, J.; Galli, E.; Anttila, J.; Saarma, M.; Permi, P.; et al. Role of two sequence motifs of mesencephalic astrocyte-derived neurotrophic factor in its survival-promoting activity. Cell Death Dis. 2015, 6, e2032. [Google Scholar] [CrossRef] [PubMed]
- Airavaara, M.; Shen, H.; Kuo, C.C.; Peranen, J.; Saarma, M.; Hoffer, B.; Wang, Y. Mesencephalic astrocyte-derived neurotrophic factor reduces ischemic brain injury and promotes behavioral recovery in rats. J. Comp. Neurol. 2009, 515, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Shen, Y.; Chen, Y.; Chen, L.; Wang, L.; Wang, H.; Xu, S.; Fang, S.; Fu, Y.; Yu, Y.; et al. Mesencephalic astrocyte-derived neurotrophic factor prevents neuron loss via inhibiting ischemia-induced apoptosis. J. Neurol. Sci. 2014, 344, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Aronowski, J.; Strong, R.; Grotta, J.C. Reperfusion injury: Demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J. Cereb. Blood Flow Metab. 1997, 17, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Benedek, A.; Moricz, K.; Juranyi, Z.; Gigler, G.; Levay, G.; Harsing, L.G., Jr.; Matyus, P.; Szenasi, G.; Albert, M. Use of TTC staining for the evaluation of tissue injury in the early phases of reperfusion after focal cerebral ischemia in rats. Brain Res. 2006, 1116, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Schabitz, W.R.; Schade, H.; Heiland, S.; Kollmar, R.; Bardutzky, J.; Henninger, N.; Muller, H.; Carl, U.; Toyokuni, S.; Sommer, C.; et al. Neuroprotection by hyperbaric oxygenation after experimental focal cerebral ischemia monitored by MRI. Stroke 2004, 35, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Kollmar, R.; Schabitz, W.R.; Heiland, S.; Georgiadis, D.; Schellinger, P.D.; Bardutzky, J.; Schwab, S. Neuroprotective effect of delayed moderate hypothermia after focal cerebral ischemia: An MRI study. Stroke 2002, 33, 1899–1904. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Yan, X.; Li, S.; Sun, Y.; Xu, L.; Shi, Z.; Wu, M.; Lu, Y.; Dong, L.; Liu, R.; et al. Methylene blue ameliorates ischemia/reperfusion-induced cerebral edema: An MRI and transmission electron microscope study. Acta Neurochir. 2016, 121, 227–236. [Google Scholar]
- Pillai, D.R.; Dittmar, M.S.; Baldaranov, D.; Heidemann, R.M.; Henning, E.C.; Schuierer, G.; Bogdahn, U.; Schlachetzki, F. Cerebral ischemia-reperfusion injury in rats—A 3T MRI study on biphasic blood–brain barrier opening and the dynamics of edema formation. J. Cereb. Blood Flow Metab. 2009, 29, 1846–1855. [Google Scholar] [CrossRef] [PubMed]
- Dhungana, H.; Malm, T.; Denes, A.; Valonen, P.; Wojciechowski, S.; Magga, J.; Savchenko, E.; Humphreys, N.; Grencis, R.; Rothwell, N.; et al. Aging aggravates ischemic stroke-induced brain damage in mice with chronic peripheral infection. Aging Cell 2013, 12, 842–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Y.; Zhu, W.; Zhang, Q.; Wang, W. Observation of post-MCAO cortical inflammatory edema in rats by 7.0 Tesla MRI. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014, 34, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Bederson, J.B.; Pitts, L.H.; Tsuji, M.; Nishimura, M.C.; Davis, R.L.; Bartkowski, H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke 1986, 17, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Schafer, D.P.; McCullough, L.D. TTC, Fluoro-Jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J. Neurosci. Methods 2009, 179, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Komine-Kobayashi, M.; Tanaka, R.; Liu, M.; Mizuno, Y.; Urabe, T. Edaravone reduces early accumulation of oxidative products and sequential inflammatory responses after transient focal ischemia in mice brain. Stroke 2005, 36, 2220–2225. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Sun, A.; Wang, Y.; Cha, D.; Wang, H.; Wang, F.; Feng, L.; Fang, S.; Shen, Y. Upregulation of mesencephalic astrocyte-derived neurotrophic factor in glial cells is associated with ischemia-induced glial activation. J. Neuroinflamm. 2012, 9, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.; Zhu, J.; Sousa-Victor, P.; Konjikusic, M.; Riley, R.; Chew, S.; Qi, Y.; Jasper, H.; Lamba, D.A. Immune modulation by MANF promotes tissue repair and regenerative success in the retina. Science 2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Feng, L.; Wang, X.; Du, J.; Chen, Y.; Yang, W.; Zhou, C.; Cheng, L.; Shen, Y.; Fang, S.; et al. Mesencephalic astrocyte-derived neurotrophic factor is involved in inflammation by negatively regulating the NF-κB pathway. Sci. Rep. 2015, 5, 8133–8146. [Google Scholar] [CrossRef] [PubMed]
- Neumann-Haefelin, T.; Kastrup, A.; de Crespigny, A.; Yenari, M.A.; Ringer, T.; Sun, G.H.; Moseley, M.E. Serial MRI after transient focal cerebral ischemia in rats: Dynamics of tissue injury, blood–brain barrier damage, and edema formation. Stroke 2000, 31, 1965–1972. [Google Scholar] [CrossRef] [PubMed]
- Swanson, R.A.; Morton, M.T.; Tsao-Wu, G.; Savalos, R.A.; Davidson, C.; Sharp, F.R. A semiautomated method for measuring brain infarct volume. J. Cereb. Blood Flow Metab. 1990, 10, 290–293. [Google Scholar] [CrossRef] [PubMed]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-Y.; Song, M.-M.; Bi, S.-X.; Shen, Y.-J.; Shen, Y.-X.; Yu, Y.-Q. MRI Dynamically Evaluates the Therapeutic Effect of Recombinant Human MANF on Ischemia/Reperfusion Injury in Rats. Int. J. Mol. Sci. 2016, 17, 1476. https://doi.org/10.3390/ijms17091476
Wang X-Y, Song M-M, Bi S-X, Shen Y-J, Shen Y-X, Yu Y-Q. MRI Dynamically Evaluates the Therapeutic Effect of Recombinant Human MANF on Ischemia/Reperfusion Injury in Rats. International Journal of Molecular Sciences. 2016; 17(9):1476. https://doi.org/10.3390/ijms17091476
Chicago/Turabian StyleWang, Xian-Yun, Meng-Meng Song, Si-Xing Bi, Yu-Jun Shen, Yu-Xian Shen, and Yong-Qiang Yu. 2016. "MRI Dynamically Evaluates the Therapeutic Effect of Recombinant Human MANF on Ischemia/Reperfusion Injury in Rats" International Journal of Molecular Sciences 17, no. 9: 1476. https://doi.org/10.3390/ijms17091476