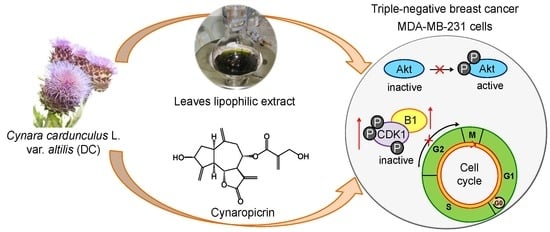
Antiproliferative Effects of Cynara cardunculus L. var. altilis (DC) Lipophilic Extracts
Abstract
:1. Introduction
2. Results
2.1. Inhibitory Effects of Cultivated Cardoon Lipophilic Extracts and Its Major Compounds on MDA-MB-231 Cellular Viability
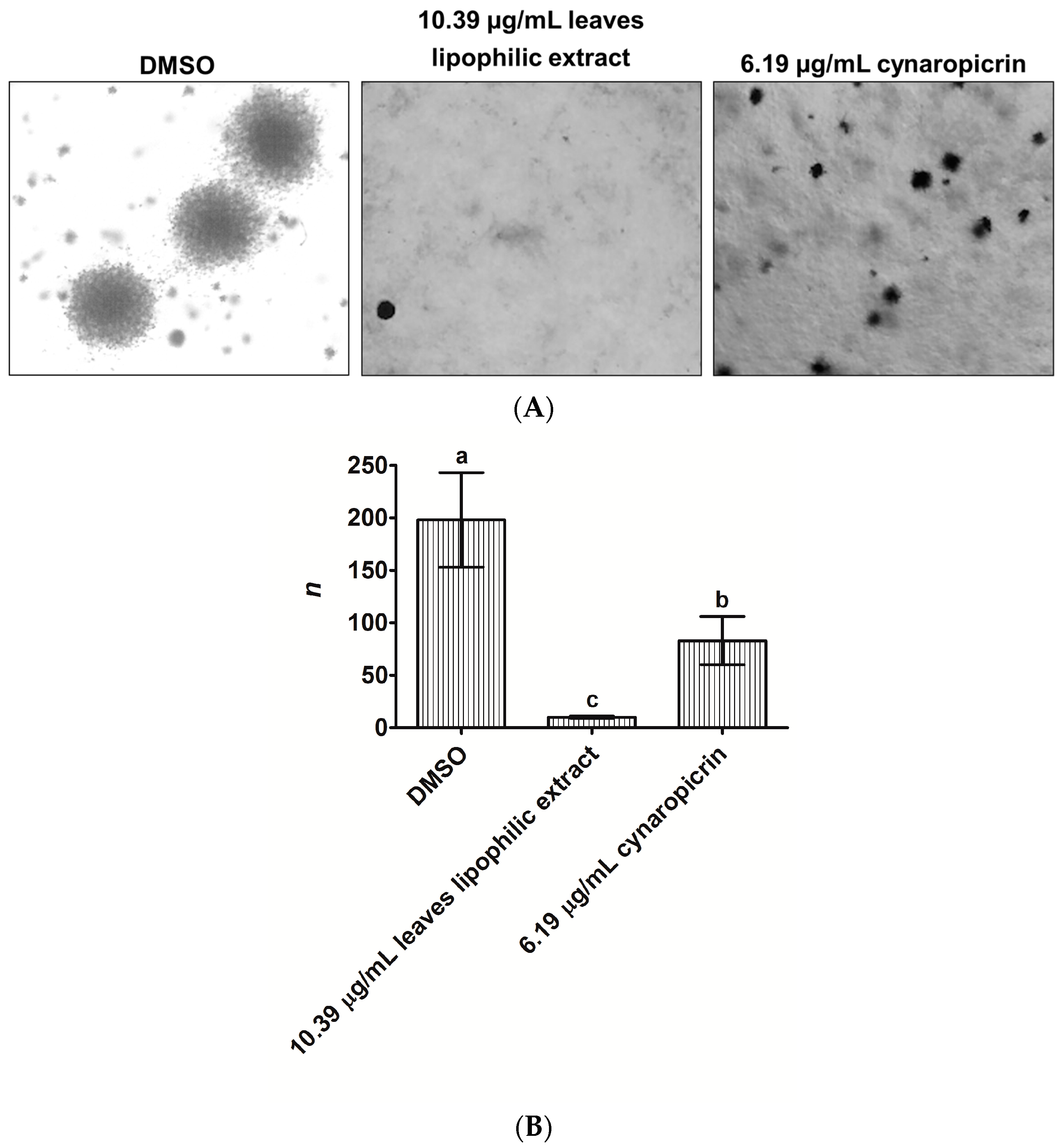
2.2. Effects of Cultivated Cardoon Leaves Lipophilic Extract and Cynaropicrin on MDA-MB-231 Cells Colonies Formation Using Anchorage-Independent Growth Assay
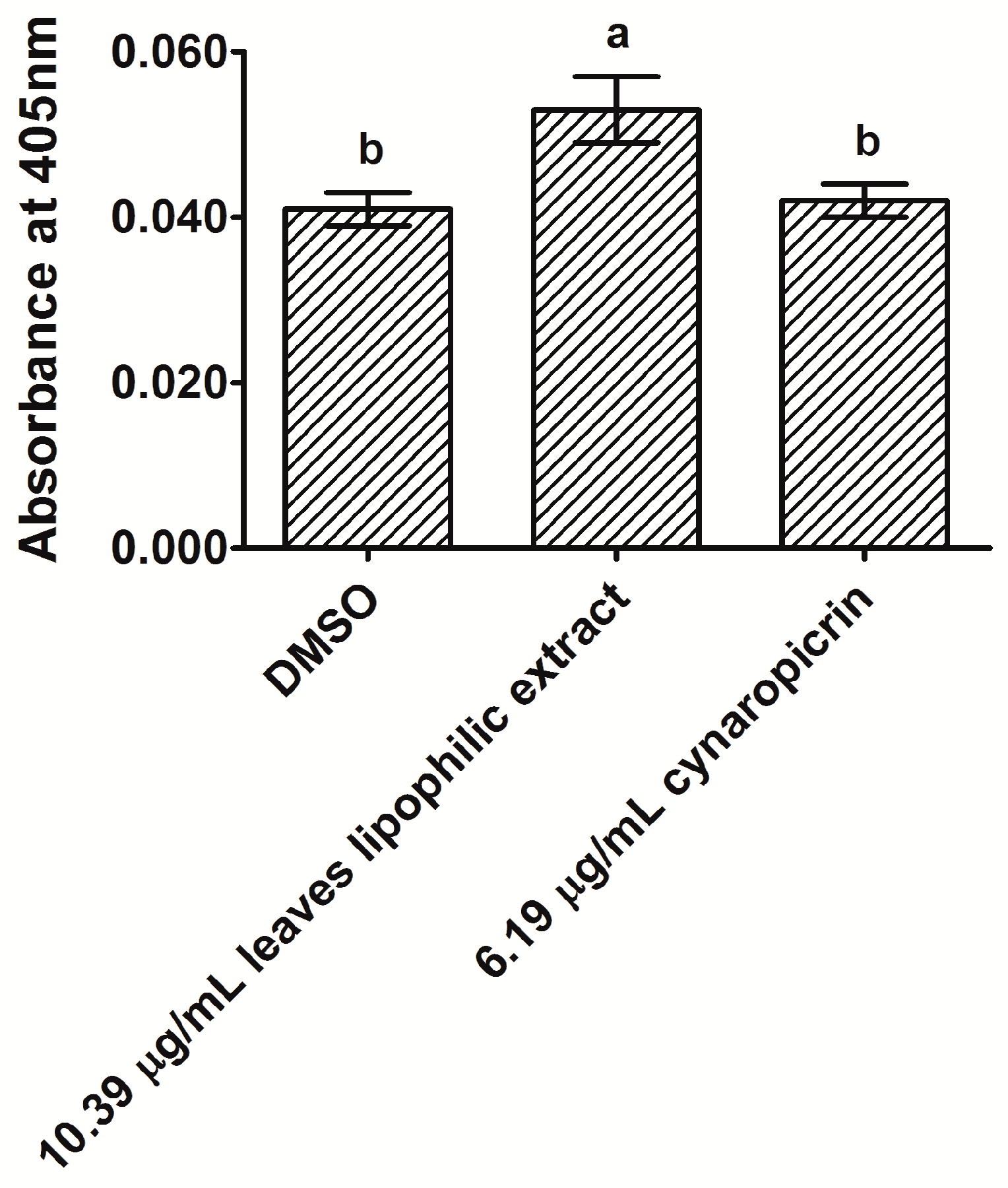
2.3. Analysis of Caspase-3 Activity in MDA-MB-231 Cells after Exposure to Cultivated Cardoon Leaves Lipophilic Extract and Cynaropicrin
2.4. Effects of Cultivated Cardoon Leaves Lipophilic Extract and Cynaropicrin in MDA-MB-231 Cells Distribution through Cell Cycle Phases
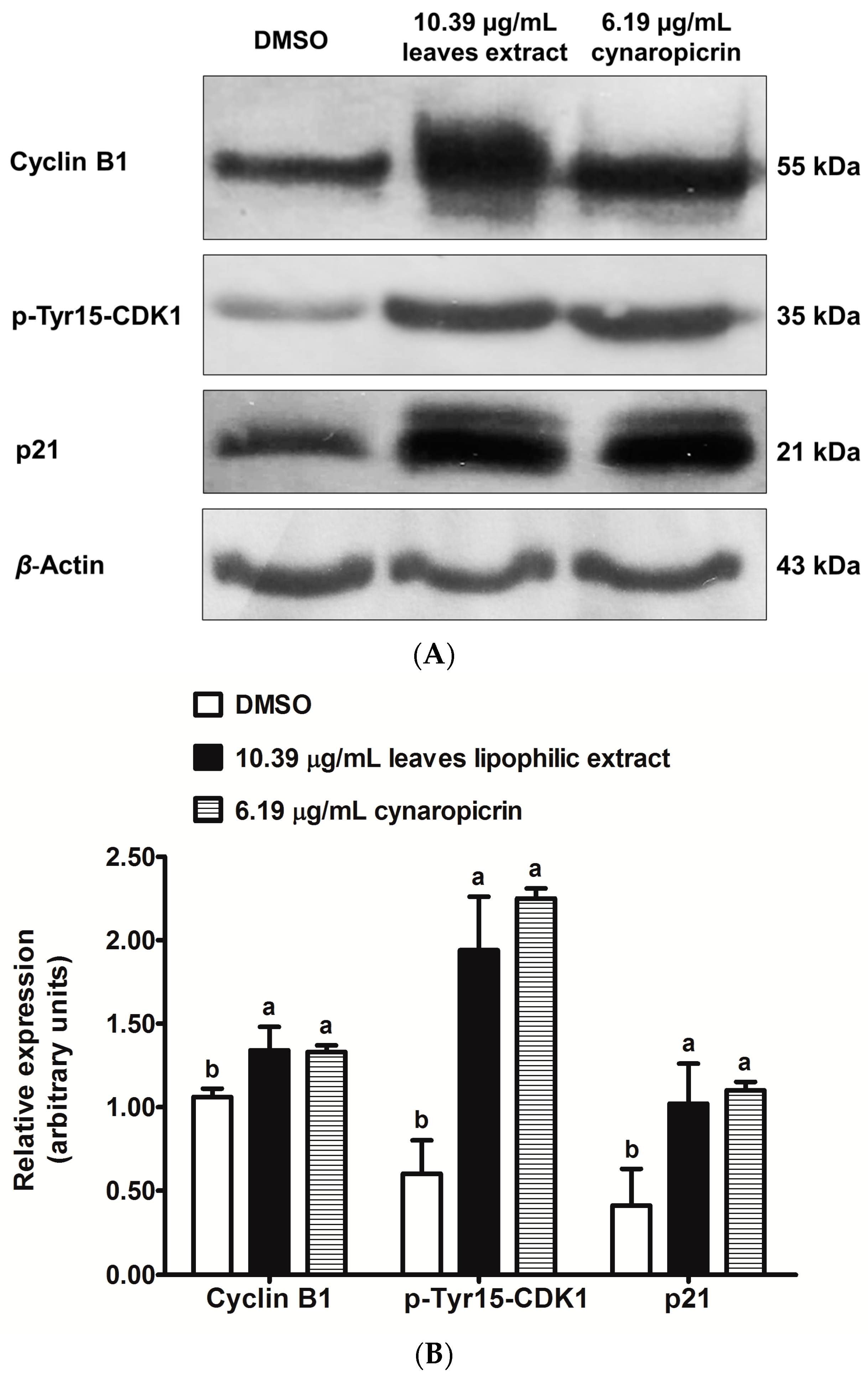
2.5. Effects of Cultivated Cardoon Leaves Lipophilic Extract and Cynaropicrin on p21Waf1/Cip1, Phospho(Tyr15)-CDK1 and Cyclin B1 Protein Expressions in MDA-MB-231 Cells
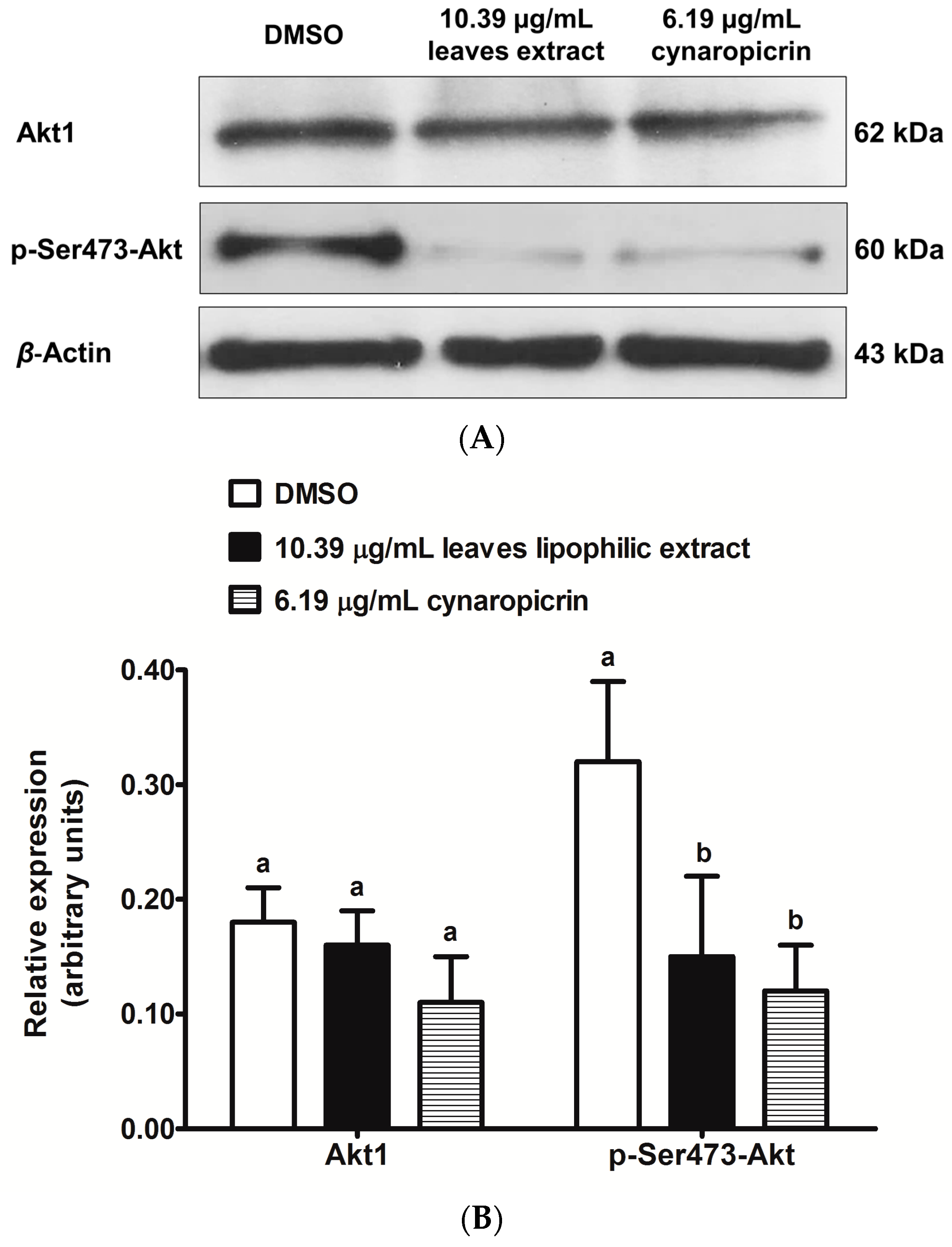
2.6. Effects of Cultivated Cardoon Leaves Lipophilic Extract and Cynaropicrin on Akt Signaling Pathway in MDA-MB-231 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extracts Preparation and Chemical Characterization
4.3. Cell Culture
4.4. Cellular Viability by MTT Assay
4.5. Soft Agar Colony Formation Assay
4.6. Caspase-3 Activity Analysis
4.7. Cell Distribution at G1, S, and G2 Cell Cycle Phases by Flow Cytometry
4.8. Western Blotting
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- International Agency for Research on Cancer. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed on 22 June 2016).
- Bosch, A.; Eroles, P.; Zaragoza, R.; Viña, J.R.; Lluch, A. Triple-negative breast cancer: Molecular features, pathogenesis, treatment and current lines of research. Cancer Treat. Rev. 2010, 36, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Anders, C.K.; Carey, L.A. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin. Breast Cancer 2009, 9, S73–S81. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, E.K.J. The protective effect of the Mediterranean diet: Focus on cancer and cardiovascular risk. Med. Princ. Pract. 2011, 20, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Ronco, A.; de Stefani, E.; Boffetta, P.; Deneo-Pellegrini, H.; Mendilaharsu, M.; Leborgne, F. Vegetables, fruits, and related nutrients and risk of breast cancer: A case-control study in Uruguay. Nutr. Cancer 1999, 35, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Gammon, M.D.; Santella, R.M.; Gaudet, M.M.; Britton, J.A.; Teitelbaum, S.L.; Terry, M.B.; Nowell, S.; Davis, W.; Garza, C.; et al. Associations between breast cancer risk and the catalase genotype, fruit and vegetable consumption, and supplement use. Am. J. Epidemiol. 2005, 162, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.; Odhav, B.; Bhoola, K.D. Natural products for cancer prevention: A global perspective. Pharmacol. Ther. 2003, 99, 1–13. [Google Scholar] [CrossRef]
- Ullah, M.; Bhat, S.; Husain, E.; Abu-Duhier, F.; Hadi, S.M.; Sarkar, F.; Ahmad, A. Cancer chemopreventive pharmacology of phytochemicals derived from plants of dietary and non-dietary origin: Implication for alternative and complementary approaches. Phytochem. Rev. 2014, 13, 811–833. [Google Scholar] [CrossRef]
- Veríssimo, P.; Esteves, C.; Faro, C.; Pires, E. The vegetable rennet of Cynara cardunculus L. contains two proteinases with chymosin and pepsin-like specificities. Biotechnol. Lett. 1995, 17, 621–626. [Google Scholar] [CrossRef]
- Portis, E.; Barchi, L.; Acquadro, A.; Macua, J.I.; Lanteri, S. Genetic diversity assessment in cultivated cardoon by AFLP (amplified fragment length polymorphism) and microsatellite markers. Plant Breed. 2005, 124, 299–304. [Google Scholar] [CrossRef]
- Adzet, T.; Camarasa, J.; Laguna, J.C. Hepatoprotective activity of polyphenolic compounds from Cynara scolymus against CCl4 toxicity in isolated rat hepatocytes. J. Nat. Prod. 1987, 50, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Saénz Rodriguez, T.; Garcia Gimenez, D.; de la Puerta Vazquez, R. Choleretic activity and biliary elimination of lipids and bile acids induced by an artichoke leaf extract in rats. Phytomedicine 2002, 9, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, R. Choleretic and anticholestatic activities of flavonoids of artichoke (Cynara cardunculus L. subsp. scolymus (L.) Hayek). Acta Hortic. 2005, 681, 429–436. [Google Scholar] [CrossRef]
- Brown, J.E.; Rice-Evans, C.A. Luteolin-rich artichoke extract protects low density lipoprotein from oxidation in vitro. Free Radic. Res. 1998, 29, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Velez, Z.; Campinho, M.; Guerra, Â.; García, L.; Ramos, P.; Guerreiro, O.; Felício, L.; Schmitt, F.; Duarte, M. Biological characterization of Cynara cardunculus L. methanolic extracts: Antioxidant, anti-proliferative, anti-migratory and anti-angiogenic activities. Agriculture 2012, 2, 472–492. [Google Scholar] [CrossRef]
- Yasukawa, K.; Matsubara, H.; Sano, Y. Inhibitory effect of the flowers of artichoke (Cynara cardunculus) on TPA-induced inflammation and tumor promotion in two-stage carcinogenesis in mouse skin. J. Nat. Med. 2010, 64, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Zhang, H.X.; Lo, R. Phenolic compounds from the leaf extract of artichoke (Cynara scolymus L.) and their antimicrobial activities. J. Agric. Food Chem. 2004, 52, 7272–7278. [Google Scholar] [CrossRef] [PubMed]
- Kukic, J.; Popovic, V.; Petrovic, S.; Mucaji, P.; Ciric, A.; Stoikovic, D.; Sokovic, M. Antioxidant and antimicrobial activity of Cynara cardunculus extracts. Food Chem. 2008, 107, 861–868. [Google Scholar] [CrossRef]
- Mileo, A.M.; di Venere, D.; Linsalata, V.; Fraioli, R.; Miccadei, S. Artichoke polyphenols induce apoptosis and decrease the invasive potential of the human breast cancer cell line MDA-MB-231. J. Cell. Physiol. 2012, 227, 3301–3309. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.A.B.; Santos, S.A.O.; Guerra, Â.R.; Guerreiro, O.; Freire, C.S.R.; Rocha, S.M.; Duarte, M.F.; Silvestre, A.J.D. Phenolic composition and antioxidant activity of different morphological parts of Cynara cardunculus L. var. altilis (DC). Ind. Crops Prod. 2014, 61, 460–471. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Guerra, Â.R.; Guerreiro, O.; Freire, C.S.R.; Silva, A.M.S.; Duarte, M.F.; Silvestre, A.J.D. Lipophilic extracts of Cynara cardunculus L. var. altilis (DC): A source of valuable bioactive terpenic compounds. J. Agric. Food Chem. 2013, 61, 8420–8429. [Google Scholar] [PubMed]
- Ramos, P.; Guerra, A.; Guerreiro, O.; Santos, S.; Oliveira, H.; Freire, C.; Rocha, S.; Silvestre, A.; Duarte, M.F. Antitumoral and antioxidant activities of lipophilic and phenolic extracts from Cynara cardunculus L. var. altilis (DC). Planta Med. 2014, 80, P1L16. [Google Scholar] [CrossRef]
- Takasaki, M.; Konoshima, T.; Tokuda, H.; Masuda, K.; Arai, Y.; Shiojima, K.; Ageta, H. Anti-carcinogenic activity of Taraxacum plant. II. Biol. Pharm. Bull. 1999, 22, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Soares, B.; Guerreiro, O.; Ramos, P.; Oliveira, H.; Silvestre, A.; Freire, C.; Duarte, M.F. Anti-tumoral activity of lipophilic Eucalyptus bark extracts, enriched on triterpenic acids, against breast cancer cells. Planta Med. 2014, 80, SL41. [Google Scholar] [CrossRef]
- Ha, T.J.; Jang, D.S.; Lee, J.R.; Lee, K.D.; Lee, J.; Hwang, S.W.; Jung, H.J.; Nam, S.H.; Park, K.H.; Yang, M.S. Cytotoxic effects of sesquiterpene lactones from the flowers of Hemisteptia lyrata B. Arch. Pharm. Res. 2003, 26, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Ghantous, A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N.A.; Darwiche, N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today 2010, 15, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Baik, K.U.; Jung, J.H.; Park, M.H. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, from Saussurea lappa. Eur. J. Pharmacol. 2000, 398, 399–407. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, A.R.; Jung, J.H.; Chun, T.; Rhee, M.H.; Yoo, E.S. Cytotoxic and pro-apoptotic activities of cynaropicrin, a sesquiterpene lactone, on the viability of leukocyte cancer cell lines. Eur. J. Pharmacol. 2004, 492, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Lhinhatrakool, T.; Sutthivaiyakit, S. 19-Nor and 18, 20-Epoxy-cardenolides from the leaves of Calotropis gigantea. J. Nat. Prod. 2006, 69, 1249–1251. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.K.; Wu, A.T.H.; Geethangili, M.; Huang, M.-T.; Chao, W.-J.; Wu, C.-H.; Deng, W.-P.; Yeh, C.-T.; Tzeng, Y.-M. Identification of antrocin from Antrodia camphorata as a selective and novel class of small molecule inhibitor of Akt/mTOR signaling in metastatic breast cancer MDA-MB-231 cells. Chem. Res. Toxicol. 2011, 24, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Lee, H.J.; Kim, C.Y.; Lee, S.B.; Tunsag, J.; Batsuren, D.; Nho, C.W. The chemopreventive effects of Saussurea salicifolia through induction of apoptosis and phase II detoxification enzyme. Biol. Pharm. Bull. 2007, 30, 2352–2359. [Google Scholar] [CrossRef] [PubMed]
- Abukhdeir, A.M.; Park, B.H. p21 and p27: Roles in carcinogenesis and drug resistance. Expert Rev. Mol. Med. 2008, 10, e19. [Google Scholar] [CrossRef] [PubMed]
- Cazzalini, O.; Scovassi, A.I.; Savio, M.; Stivala, L.A.; Prosperi, E. Multiple roles of the cell cycle inhibitor p21CDKN1A in the DNA damage response. Mutat. Res. Mutat. Res. 2010, 704, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Charrier-Savournin, F.B.; Château, M.-T.; Gire, V.; Sedivy, J.; Piette, J.; Dulić, V. p21-mediated nuclear retention of cyclin B1-Cdk1 in response to genotoxic stress. Mol. Biol. Cell 2004, 15, 3965–3976. [Google Scholar] [CrossRef] [PubMed]
- Rozenblat, S.; Grossman, S.; Bergman, M.; Gottlieb, H.; Cohen, Y.; Dovrat, S. Induction of G2/M arrest and apoptosis by sesquiterpene lactones in human melanoma cell lines. Biochem. Pharmacol. 2008, 75, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, P.T.; Hay, N. The two TORCs and Akt. Dev. Cell 2007, 12, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Hanada, M.; Feng, J.; Hemmings, B.A. Structure, regulation and function of PKB/AKT—A major therapeutic target. Biochim. Biophys. Acta-Proteins Proteom. 2004, 1697, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Oka, D.; Nishimura, K.; Shiba, M.; Nakai, Y.; Arai, Y.; Nakayama, M.; Takayama, H.; Inoue, H.; Okuyama, A.; Nonomura, N. Sesquiterpene lactone parthenolide suppresses tumor growth in a xenograft model of renal cell carcinoma by inhibiting the activation of NF-κB. Int. J. Cancer 2007, 120, 2576–2581. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-J.; Jung, J.-Y.; Jeong, J.H.; Cho, S.-D. Induction of apoptosis by parthenolide in human oral cancer cell lines and tumor xenografts. Oral Oncol. 2015, 51, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Tsai, A.-C.; Peng, C.-Y.; Chang, Y.-L.; Lee, K.-H.; Teng, C.-M.; Pan, S.-L. Dehydrocostuslactone suppresses angiogenesis in vitro and in vivo through inhibition of Akt/GSK-3β and mTOR signaling pathways. PLoS ONE 2012, 7, e31195. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]

| Cultivated Cardoon Extract/Pure Compound | IC50 for Antiproliferative Activity (µg/mL) 1 |
|---|---|
| Leaves lipophilic extract | 10.39 ± 0.41 b |
| Florets lipophilic extract | 315.22 ± 67.88 a |
| Cynaropicrin | 6.19 ± 0.57 b |
| Group | MDA-MB-231 Cells (%) 1 | ||
|---|---|---|---|
| G0/G1 | S | G2 | |
| DMSO | 42.5 ± 2.8 a | 45.0 ± 2.2 a | 12.6 ± 3.0 c |
| 10.39 µg/mL leaves lipophilic extract | 15.8 ± 3.1 b | 24.8 ± 2.1 b | 59.5 ± 1.1 a |
| 6.19 µg/mL cynaropicrin | 41.2 ± 3.8 a | 21.0 ± 3.7 b | 37.8 ± 3.1 b |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, P.A.B.; Guerra, Â.R.; Guerreiro, O.; Santos, S.A.O.; Oliveira, H.; Freire, C.S.R.; Silvestre, A.J.D.; Duarte, M.F. Antiproliferative Effects of Cynara cardunculus L. var. altilis (DC) Lipophilic Extracts. Int. J. Mol. Sci. 2017, 18, 63. https://doi.org/10.3390/ijms18010063
Ramos PAB, Guerra ÂR, Guerreiro O, Santos SAO, Oliveira H, Freire CSR, Silvestre AJD, Duarte MF. Antiproliferative Effects of Cynara cardunculus L. var. altilis (DC) Lipophilic Extracts. International Journal of Molecular Sciences. 2017; 18(1):63. https://doi.org/10.3390/ijms18010063
Chicago/Turabian StyleRamos, Patrícia A. B., Ângela R. Guerra, Olinda Guerreiro, Sónia A. O. Santos, Helena Oliveira, Carmen S. R. Freire, Armando J. D. Silvestre, and Maria F. Duarte. 2017. "Antiproliferative Effects of Cynara cardunculus L. var. altilis (DC) Lipophilic Extracts" International Journal of Molecular Sciences 18, no. 1: 63. https://doi.org/10.3390/ijms18010063
APA StyleRamos, P. A. B., Guerra, Â. R., Guerreiro, O., Santos, S. A. O., Oliveira, H., Freire, C. S. R., Silvestre, A. J. D., & Duarte, M. F. (2017). Antiproliferative Effects of Cynara cardunculus L. var. altilis (DC) Lipophilic Extracts. International Journal of Molecular Sciences, 18(1), 63. https://doi.org/10.3390/ijms18010063