Tissue-Based MicroRNAs as Predictors of Biochemical Recurrence after Radical Prostatectomy: What Can We Learn from Past Studies?
Abstract
:1. Introduction
2. Literature Search Strategy
2.1. Medical Subject Heading (MeSH) Terms and Keywords
2.2. Defining BCR as the Clinical Endpoint
3. Overview of the Evaluated Studies
3.1. Number of Annual Publications and Type of Tissue Samples Used in the Studies
3.2. Characteristics of the Studies Evaluated in This Review
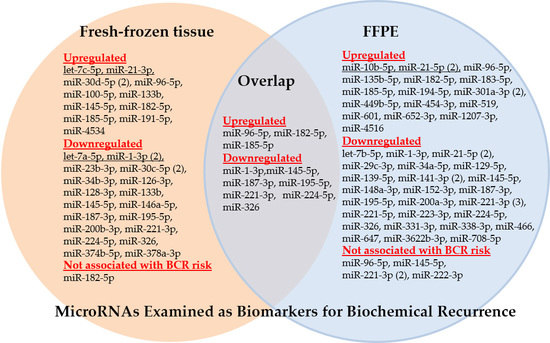
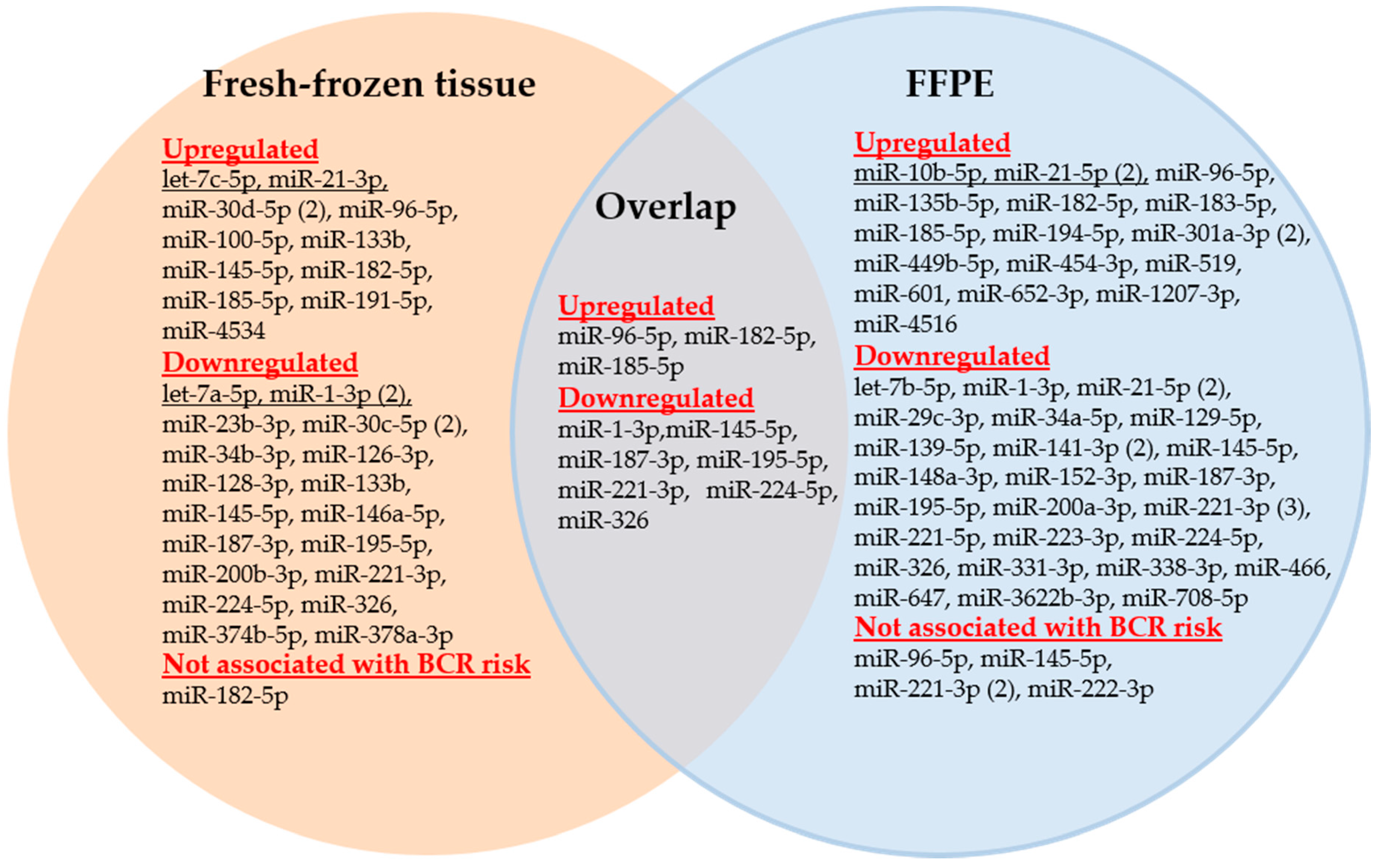
3.2.1. Dysregulated miRNAs with Association to Biochemical Recurrence
3.2.2. miR-221-3p, miR-21-5p, miR-145-5p, miR-1-3p, and miR-96-5p, the Most Frequently Analyzed miRNA-Based BCR Markers
- miR-221-3p. Three of the four studies confirmed the downregulated expression of miR-221 as a useful BCR predictor and independent factor in multivariate analyses with the standard clinicopathological variables (Study nos. 3, 31, and 46; [24,50,83]). Kristensen et al. [50] (Study 46) validated miR-221 in two independent BCR cohorts and an additional external validation using a publicly available data set as part of their 3-miRNA signature while Spahn et al. [24] (Study 3) proved the usefulness of this miRNA especially in high-risk PCa patients. Thus, these studies can be assessed as successful approaches from the discovery phase to validation by clinical assessment with the aim to develop a potential clinical tool as suggested in Table 1. The miRNA tool miQ that was primarily developed for diagnostic purposes included the also downregulated 5p strand of miR-221 in predicting BCR (Study 17, [67]). Strong correlations were observed in these studies between the increased expression of miR-221 and the tumor stage, Gleason score, and the pre-operative PSA level. In contrast, these correlations were not found in Study 9 with the missing predictor evidence of miR-221 [58]. However, this failure could also be caused by the short follow-up period of less than two years in this study.
- miR-21-5p. Increased and decreased expression of this miRNA was suggested as a potential BCR predictor in two studies (Table 4). Correlations were described between the increased expression of miR-21-5p as a BCR predictor and the standard clinicopathological variables (Study nos. 11 and 29; [60,81] while these data were not reported in the controversial studies with the decreased miRNA expression (Study nos. 14 and 31; [64,83]). After adjustment with clinicopathological factors, decreased miRNA expression failed to be an independent BCR risk factor (Study 31, [83]) or was only appropriate in obese patients (Study 14, [64]). Only one of the three studies with upregulated expression in tumor tissue clearly proved miR-21 as an independent factor for shorter BCR-free survival in multivariate analysis (Study 11, [60]).
- miR-145-5p. Both a study with increased (Study 5, [52]) and two studies with decreased expression of miR-145 estimated this miRNA as a potential BCR predictor or part of a significant prediction signature (Study nos. 15 and 17; [65,67]). It cannot be excluded that these discrepant findings were caused by analytical reasons, as two studies calculated the expression of miR-145 with normalizers (RNU43 and SNORD48) that were criticized regarding their suitability as reference genes [116]. Another study with decreased miR-145-5p expression (Study 9, [58]) was not able to confirm miR-145-5p as a BCR predictor in Kaplan-Meier analysis. However, it should be noted that the above-mentioned very short follow-up period in that study makes a true assessment difficult.
- miR-1-3p. Three studies examined the potential BCR capability of downregulated miR-1. Two studies (Study nos. 8 and 53; [55,107]) identified miR-1 as an independent BCR predictor after adjustment with the conventional clinicopathological factors. However, the additional benefit was not demonstrated when miR-1-3p was included in the model based only on clinicopathological factors. miR-1 was also demonstrated to be a successful BCR predictor in the third study (Study 24, [74]), but its clinical accuracy was exceeded by the pre-operative PSA value. The inconsistent documentation of clinicopathological variables in these studies makes it impossible to attribute this uniform BCR predictor result to congruent clinical characteristics between the studies.
- miR-96-5p. In two studies (Study nos. 2 and 17; [23,67]), increased levels of this miRNA in PCa tissue were successfully identified as a single BCR predictor or part of a BCR predictor combination. A third study (Study 9, [58]) did not confirm an association of the recurrence-free survival and the miR-9-5p expression level.
3.2.3. Multiple miRNAs as Signatures or in Combination with Other Analytes
4. Critical Assessment of the Recent Situation of miRNA-Based BCR Prediction
4.1. Analytical Considerations
4.2. Study Design Considerations
4.3. Divergences between BCR Outcome and the Functional Role of miRNAs
5. Future Directions
- No study has thus been able to comply with the suggested requirements specified in the final development phase “Validation of clinical usability” (Table 2) to establish a robust BCR tool for clinical practice using miRNAs. In addition, few studies can be valued as successfully finished in the second development phase due to the lack of internal validation in most of the studies (Table 2 and Table 5).
- The evaluation and comparison of analytical and clinical conditions in the various studies provided a wealth of experience in the assessment of study design features. Based on these experiences, critical study deficiencies could be identified (see Section 4, comments to Table 5), and future directions could be elaborated to overcome these shortcomings. In the following, we focus on some essential issues.
6. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
Abbreviations
| APLN | Apelin |
| AUA | American Urological Association |
| AUC | Area under the ROC curve |
| BCL9 | B-cell CLL/lymphoma 9 |
| BCR | Biochemical recurrence |
| BPH | Benign prostatic hyperplasia |
| CoxM | Multivariate Cox regression analysis |
| CoxU | Univariate Cox regression analysis |
| EAU | European Association of Urology |
| FFPE | Formalin-fixed, paraffin-embedded |
| HR | Hazard ratio |
| ISUP | International Society of Urological Pathology |
| KMA | Kaplan-Meier analysis |
| MeSH | Medical Subject Heading of the U.S. National Library of Medicine |
| miQ | miRNA index quote |
| MIQE | Minimum information for publication of quantitative real-time PCR experiments |
| miRNA, miR | microRNA |
| MW | Mann-Whitney U-test |
| MYPT1 | Protein phosphatase 1 regulatory subunit 12A (official symbol: PPP1R12A) |
| PCa | Prostate carcinoma |
| PSA | Prostate-specific antigen |
| RB1CC1 | RB1 inducible coiled-coil 1 |
| REMARK | Reporting Recommendations for Tumor Marker Prognostic Studies |
| RFS | Biochemical recurrence-free survival |
| RM | Reference method, in general the reference gene |
| ROC | Receiver-operating characteristic curve |
| ROCK1 | Rho associated coiled-coil containing protein kinase 1 |
| RP | Radical prostatectomy |
| RT-qPCR | Reverse transcription-quantitative polymerase chain reaction |
| SOCS | Cytokine inducible SH2 containing protein (official symbol: CISH) |
| STARD | Standards for Reporting of Diagnostic Accuracy |
| TCGA | The Cancer Genome Atlas |
| TNM | Classification of malignant tumors describing the involment of the primary tumor, regional lymph nodes and the distant metastatic spread |
| TRIB1 | Tribbles pseudokinase 1 |
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Andriole, G.L.; Crawford, E.D.; Grubb III, R.L.; Buys, S.S.; Chia, D.; Church, T.R.; Fouad, M.N.; Gelmann, E.P.; Kvale, P.A.; Reding, D.J.; et al. Mortality results from a randomized prostate-cancer screening trial. N. Engl. J. Med. 2009, 360, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Schroder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.; Ciatto, S.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Lilja, H.; Zappa, M.; et al. Screening and prostate-cancer mortality in a randomized European study. N. Engl. J. Med. 2009, 360, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Shariat, S.F.; Karakiewicz, P.I.; Roehrborn, C.G.; Kattan, M.W. An updated catalog of prostate cancer predictive tools. Cancer 2008, 113, 3075–3099. [Google Scholar] [CrossRef] [PubMed]
- Lughezzani, G.; Budaus, L.; Isbarn, H.; Sun, M.; Perrotte, P.; Haese, A.; Chun, F.K.; Schlomm, T.; Steuber, T.; Heinzer, H.; et al. Head-to-head comparison of the three most commonly used preoperative models for prediction of biochemical recurrence after radical prostatectomy. Eur. Urol. 2010, 57, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Punnen, S.; Freedland, S.J.; Presti, J.C., Jr.; Aronson, W.J.; Terris, M.K.; Kane, C.J.; Amling, C.L.; Carroll, P.R.; Cooperberg, M.R. Multi-institutional validation of the CAPRA-S score to predict disease recurrence and mortality after radical prostatectomy. Eur. Urol. 2014, 65, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Burke, H.B. Predicting clinical outcomes using molecular biomarkers. Biomark. Cancer 2016, 8, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.E. Risk stratification of prostate cancer 2016. Scand. J. Clin. Lab. Investig. Suppl. 2016, 245, S54–S59. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Reszka, R.; Kamlage, B.; Bethan, B.; Lein, M.; Stephan, C.; Kristiansen, G. Tissue metabolite profiling identifies differentiating and prognostic biomarkers for prostate carcinoma. Int. J. Cancer 2013, 133, 2914–2924. [Google Scholar] [CrossRef] [PubMed]
- Ross-Adams, H.; Lamb, A.D.; Dunning, M.J.; Halim, S.; Lindberg, J.; Massie, C.M.; Egevad, L.A.; Russell, R.; Ramos-Montoya, A.; Vowler, S.L.; et al. Integration of copy number and transcriptomics provides risk stratification in prostate cancer: A discovery and validation cohort study. EBioMedicine 2015, 2, 1133–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalonde, E.; Alkallas, R.; Chua, M.L.; Fraser, M.; Haider, S.; Meng, A.; Zheng, J.; Yao, C.Q.; Picard, V.; Orain, M.; et al. Translating a prognostic DNA genomic classifier into the clinic: Retrospective validation in 563 localized prostate tumors. Eur. Urol. 2017, 72, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Latour, M.; Lattouf, J.B.; Widmer, H.; Zorn, K.C.; Mes-Masson, A.M.; Ouellet, V.; Saad, G.; Prakash, A.; Choudhury, S.; et al. Biopsy based proteomic assay predicts risk of biochemical recurrence after radical prostatectomy. J. Urol. 2017, 197, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Filella, X.; Foj, L. Prostate cancer detection and prognosis: From prostate specific antigen (PSA) to exosomal biomarkers. Int. J. Mol. Sci. 2016, 17, 1784. [Google Scholar] [CrossRef] [PubMed]
- Luu, H.N.; Lin, H.Y.; Sorensen, K.D.; Ogunwobi, O.O.; Kumar, N.; Chornokur, G.; Phelan, C.; Jones, D.; Kidd, L.; Batra, J.; et al. miRNAs associated with prostate cancer risk and progression. BMC Urol. 2017, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- Shukla, K.K.; Misra, S.; Pareek, P.; Mishra, V.; Singhal, B.; Sharma, P. Recent scenario of microRNA as diagnostic and prognostic biomarkers of prostate cancer. Urol. Oncol. 2017, 35, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Fendler, A.; Stephan, C.; Yousef, G.M.; Jung, K. miRNAs as regulators of signal transduction in urological tumors. Clin. Chem. 2011, 57, 954–968. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, D.; Meller, S.; Uhl, B.; Ralla, B.; Stephan, C.; Jung, K.; Ellinger, J.; Kristiansen, G. Nucleic acid-based tissue biomarkers of urologic malignancies. Crit. Rev. Clin. Lab. Sci. 2014, 51, 173–199. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, G.; Cava, C.; Castiglioni, I. MicroRNAs as biomarkers for diagnosis, prognosis and theranostics in prostate cancer. Int. J. Mol. Sci. 2016, 17, 421. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Junker, K.; Heinzelmann, J. Prognostic and predictive miRNA biomarkers in bladder, kidney and prostate cancer: Where do we stand in biomarker development? J. Cancer Res. Clin. Oncol. 2016, 142, 1673–1695. [Google Scholar] [CrossRef] [PubMed]
- Gill, B.S.; Alex, J.M.; Navgeet; Kumar, S. Missing link between microRNA and prostate cancer. Tumour. Biol. 2016, 37, 5683–5704. [Google Scholar] [CrossRef] [PubMed]
- Kurozumi, A.; Goto, Y.; Okato, A.; Ichikawa, T.; Seki, N. Aberrantly expressed microRNAs in bladder cancer and renal cell carcinoma. J. Hum. Genet. 2017, 62, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.W.; Fulgham, P.; Jay, C.; Chen, P.; Khalil, I.; Liu, S.; Senzer, N.; Eklund, A.C.; Han, J.; Nemunaitis, J. MicroRNA profile analysis of human prostate cancers. Cancer Gene Ther. 2009, 16, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.; Jung, M.; Mollenkopf, H.J.; Wagner, I.; Stephan, C.; Jentzmik, F.; Miller, K.; Lein, M.; Kristiansen, G.; Jung, K. Diagnostic and prognostic implications of microRNA profiling in prostate carcinoma. Int. J. Cancer 2010, 126, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Spahn, M.; Kneitz, S.; Scholz, C.J.; Stenger, N.; Rudiger, T.; Strobel, P.; Riedmiller, H.; Kneitz, B. Expression of microRNA-221 is progressively reduced in aggressive prostate cancer and metastasis and predicts clinical recurrence. Int. J. Cancer 2010, 127, 394–403. [Google Scholar] [PubMed]
- Fendler, A.; Jung, M.; Stephan, C.; Honey, R.J.; Stewart, R.J.; Pace, K.T.; Erbersdobler, A.; Samaan, S.; Jung, K.; Yousef, G.M. miRNAs can predict prostate cancer biochemical relapse and are involved in tumor progression. Int. J. Oncol. 2011, 39, 1183–1192. [Google Scholar] [PubMed]
- Amling, C.L.; Blute, M.L.; Bergstralh, E.J.; Seay, T.M.; Slezak, J.; Zincke, H. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: Continued risk of biochemical failure after 5 years. J. Urol. 2000, 164, 101–105. [Google Scholar] [CrossRef]
- Han, M.; Partin, A.W.; Zahurak, M.; Piantadosi, S.; Epstein, J.I.; Walsh, P.C. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J. Urol. 2003, 169, 517–523. [Google Scholar] [CrossRef]
- Lein, M.; Brux, B.; Jung, K.; Henke, W.; Koenig, F.; Stephan, C.; Schnorr, D.; Loening, S.A. Elimination of serum free and total prostate-specific antigen after radical retropubic prostatectomy. Eur. J. Clin. Chem. Clin. Biochem. 1997, 35, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; de, S.M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Cookson, M.S.; Aus, G.; Burnett, A.L.; Canby-Hagino, E.D.; D’Amico, A.V.; Dmochowski, R.R.; Eton, D.T.; Forman, J.D.; Goldenberg, S.L.; Hernandez, J.; et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: The American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J. Urol. 2007, 177, 540–545. [Google Scholar] [PubMed]
- Amling, C.L.; Bergstralh, E.J.; Blute, M.L.; Slezak, J.M.; Zincke, H. Defining prostate specific antigen progression after radical prostatectomy: What is the most appropriate cut point? J. Urol. 2001, 165, 1146–1151. [Google Scholar] [CrossRef]
- Suardi, N.; Porter, C.R.; Reuther, A.M.; Walz, J.; Kodama, K.; Gibbons, R.P.; Correa, R.; Montorsi, F.; Graefen, M.; Huland, H.; et al. A nomogram predicting long-term biochemical recurrence after radical prostatectomy. Cancer 2008, 112, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Walz, J.; Chun, F.K.; Klein, E.A.; Reuther, A.; Saad, F.; Graefen, M.; Huland, H.; Karakiewicz, P.I. Nomogram predicting the probability of early recurrence after radical prostatectomy for prostate cancer. J. Urol. 2009, 181, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Aktas, B.K.; Ozden, C.; Bulut, S.; Tagci, S.; Erbay, G.; Gokkaya, C.S.; Baykam, M.M.; Memis, A. Evaluation of biochemical recurrence-free survival after radical prostatectomy by cancer of the prostate risk assessment post-surgical (CAPRA-S) score. Asian Pac. J. Cancer Prev. 2015, 16, 2527–2530. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Jeong, C.W.; Choi, W.S.; Park, Y.H.; Cho, S.Y.; Lee, S.; Lee, S.B.; Ku, J.H.; Hong, S.K.; Byun, S.S.; et al. Pre- and post-operative nomograms to predict recurrence-free probability in korean men with clinically localized prostate cancer. PLoS ONE 2014, 9, e100053. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Cammann, H.; Meyer, H.-A.; Jung, K.; Lu, H.; Leva, N.; Magheli, A.; Stephan, C.; Busch, J. Risk prediction models for biochemical recurrence after radical prostatectomy using prostate-specific anitigen and Gleason score. Asian J. Androl. 2014, 16, 897–901. [Google Scholar] [PubMed]
- Stephenson, A.J.; Kattan, M.W.; Eastham, J.A.; Dotan, Z.A.; Bianco, F.J., Jr.; Lilja, H.; Scardino, P.T. Defining biochemical recurrence of prostate cancer after radical prostatectomy: A proposal for a standardized definition. J. Clin. Oncol. 2006, 24, 3973–3978. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.C.; Li, J.; Klink, J.C.; Kattan, M.W.; Klein, E.A.; Stephenson, A.J. Optimal definition of biochemical recurrence after radical prostatectomy depends on pathologic risk factors: Identifying candidates for early salvage therapy. Eur. Urol. 2014, 66, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Tosoian, J.J.; Pierorazio, P.M. Optimizing use of serum prostate specific antigen to define biochemical recurrence—Is there a method to the madness? J. Urol. 2016, 195, 1648–1649. [Google Scholar] [CrossRef] [PubMed]
- Toussi, A.; Stewart-Merrill, S.B.; Boorjian, S.A.; Psutka, S.P.; Thompson, R.H.; Frank, I.; Tollefson, M.K.; Gettman, M.T.; Carlson, R.E.; Rangel, L.J.; et al. Standardizing the definition of biochemical recurrence after radical prostatectomy-what prostate specific antigen cut point best predicts a durable increase and subsequent systemic progression? J. Urol. 2016, 195, 1754–1759. [Google Scholar] [CrossRef] [PubMed]
- Pound, C.R.; Partin, A.W.; Eisenberger, M.A.; Chan, D.W.; Pearson, J.D.; Walsh, P.C. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999, 281, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Weinstein, M.; Tomaszewski, J.E.; Schultz, D.; Rhude, M.; Rocha, S.; Wein, A.; Richie, J.P. Predicting prostate specific antigen outcome preoperatively in the prostate specific antigen era. J. Urol. 2001, 166, 2185–2188. [Google Scholar] [CrossRef]
- Freedland, S.J.; Sutter, M.E.; Dorey, F.; Aronson, W.J. Defining the ideal cutpoint for determining PSA recurrence after radical prostatectomy. Urology 2003, 61, 365–369. [Google Scholar] [CrossRef]
- Ward, J.F.; Moul, J.W. Biochemical recurrence after definitive prostate cancer therapy. Part I: Defining and localizing biochemical recurrence of prostate cancer. Curr. Opin. Urol. 2005, 15, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Stephan, C.; Klaas, M.; Müller, C.; Schnorr, D.; Loening, S.A.; Jung, K. Interchangeability of measurements of total and free prostate-specific antigen in serum with 5 frequently used assay combinations: An update. Clin. Chem. 2006, 52, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Stephan, C.; Kahrs, A.-M.; Klotzek, S.; Reiche, J.; Müller, C.; Lein, M.; Deger, S.; Miller, K.; Jung, K. Toward metrological traceability in the determination of prostate-specific antigen (PSA): Calibrating Beckman Coulter Hybritech Access PSA assays to WHO standards compared with the traditional Hybritech standards. Clin. Chem. Lab. Med. 2008, 46, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Boorjian, S.A.; Thompson, R.H.; Tollefson, M.K.; Rangel, L.J.; Bergstralh, E.J.; Blute, M.L.; Karnes, R.J. Long-term risk of clinical progression after biochemical recurrence following radical prostatectomy: The impact of time from surgery to recurrence. Eur. Urol. 2011, 59, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Herranz-Amo, F.; Molina-Escudero, R.; Ogaya-Pinies, G.; Ramirez-Martin, D.; Verdu-Tartajo, F.; Hernandez-Fernandez, C. Prediction of biochemical recurrence after radical prostatectomy. New tool for selecting candidates for adjuvant radiation therapy. Actas Urol. Esp. 2016, 40, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Salas, I.; Rubio-Briones, J.; Calatrava, A.; Mancarella, C.; Masia, E.; Casanova, J.; Fernandez-Serra, A.; Rubio, L.; Ramirez-Backhaus, M.; Arminan, A.; et al. Identification of miR-187 and miR-182 as biomarkers of early diagnosis and prognosis in patients with prostate cancer treated with radical prostatectomy. J. Urol. 2014, 192, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, H.; Thomsen, A.R.; Haldrup, C.; Dyrskjot, L.; Hoyer, S.; Borre, M.; Mouritzen, P.; Orntoft, T.F.; Sorensen, K.D. Novel diagnostic and prognostic classifiers for prostate cancer identified by genome-wide microRNA profiling. Oncotarget 2016, 7, 30760–30771. [Google Scholar] [CrossRef] [PubMed]
- Fendler, A.; Stephan, C.; Yousef, G.M.; Kristiansen, G.; Jung, K. The translational potential of microRNAs as biofluid markers of urologic tumors. Nat. Rev. Urol. 2016, 13, 734–752. [Google Scholar] [CrossRef] [PubMed]
- Leite, K.R.; Tomiyama, A.; Reis, S.T.; Sousa-Canavez, J.M.; Sanudo, A.; Dall'Oglio, M.F.; Camara-Lopes, L.H.; Srougi, M. MicroRNA-100 expression is independently related to biochemical recurrence of prostate cancer. J. Urol. 2011, 185, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Johnson, B.A.; Osunkoya, A.O.; Lai, Y.H.; Zhou, W.; Abramovitz, M.; Xia, M.; Bouzyk, M.B.; Nam, R.K.; Sugar, L.; et al. Protein-coding and microRNA biomarkers of recurrence of prostate cancer following radical prostatectomy. Am. J. Pathol. 2011, 179, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Barron, N.; Keenan, J.; Gammell, P.; Martinez, V.G.; Freeman, A.; Masters, J.R.; Clynes, M. Biochemical relapse following radical prostatectomy and miR-200a levels in prostate cancer. Prostate 2012, 72, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Hudson, R.S.; Yi, M.; Esposito, D.; Watkins, S.K.; Hurwitz, A.A.; Yfantis, H.G.; Lee, D.H.; Borin, J.F.; Naslund, M.J.; Alexander, R.B.; et al. MicroRNA-1 is a candidate tumor suppressor and prognostic marker in human prostate cancer. Nucleic Acids Res. 2012, 40, 3689–3703. [Google Scholar] [CrossRef] [PubMed]
- Ambs, S.; Prueitt, R.L.; Yi, M.; Hudson, R.S.; Howe, T.M.; Petrocca, F.; Wallace, T.A.; Liu, C.G.; Volinia, S.; Calin, G.A.; et al. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 2008, 68, 6162–6170. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.G.; Ha, Y.R.; Kim, S.J.; Kang, S.H.; Park, H.S.; Lee, J.G.; Cheon, J.; Kim, C.H. Do microRNA 96, 145 and 221 expressions really aid in the prognosis of prostate carcinoma? Asian J. Androl. 2012, 14, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Uemura, H.; Nagahama, K.; Okudela, K.; Furuya, M.; Ino, Y.; Ito, Y.; Hirano, H.; Inayama, Y.; Aoki, I.; et al. Identification of miR-30d as a novel prognostic maker of prostate cancer. Oncotarget 2012, 3, 1455–1471. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, R.S.; Li, Y.H.; Zhong, S.; Chen, Y.Y.; Zhang, C.M.; Hu, M.M.; Shen, Z.J. miR-21 as an independent biochemical recurrence predictor and potential therapeutic target for prostate cancer. J. Urol. 2012, 187, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, D.; Sha, J.; Sun, P.; Huang, Y. MicroRNA-21 directly targets MARCKS and promotes apoptosis resistance and invasion in prostate cancer cells. Biochem. Biophys. Res. Commun. 2009, 383, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Majid, S.; Dar, A.A.; Saini, S.; Arora, S.; Shahryari, V.; Zaman, M.S.; Chang, I.; Yamamura, S.; Tanaka, Y.; Deng, G.; et al. miR-23b represses proto-oncogene Src kinase and functions as methylation-silenced tumor suppressor with diagnostic and prognostic significance in prostate cancer. Cancer Res. 2012, 72, 6435–6446. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Majid, S.; Shahryari, V.; Arora, S.; Yamamura, S.; Chang, I.; Zaman, M.S.; Deng, G.; Tanaka, Y.; Dahiya, R. miRNA-708 control of CD44+ prostate cancer-initiating cells. Cancer Res. 2012, 72, 3618–3630. [Google Scholar] [CrossRef] [PubMed]
- Amankwah, E.K.; Anegbe, E.; Park, H.; Pow-Sang, J.; Hakam, A.; Park, J.Y. miR-21, miR-221 and miR-222 expression and prostate cancer recurrence among obese and non-obese cases. Asian J. Androl. 2013, 15, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Avgeris, M.; Stravodimos, K.; Fragoulis, E.G.; Scorilas, A. The loss of the tumour-suppressor miR-145 results in the shorter disease-free survival of prostate cancer patients. Br. J. Cancer 2013, 108, 2573–2581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.C.; Han, Z.D.; Dai, Q.S.; Ling, X.H.; Fu, X.; Lin, Z.Y.; Deng, Y.H.; Qin, G.Q.; Cai, C.; Chen, J.H.; et al. Global analysis of the differentially expressed miRNAs of prostate cancer in Chinese patients. BMC Genomics 2013, 14, 757. [Google Scholar] [CrossRef] [PubMed]
- Larne, O.; Martens-Uzunova, E.; Hagman, Z.; Edsjo, A.; Lippolis, G.; den Berg, M.S.; Bjartell, A.; Jenster, G.; Ceder, Y. miQ—A novel microRNA based diagnostic and prognostic tool for prostate cancer. Int. J. Cancer 2013, 132, 2867–2875. [Google Scholar] [CrossRef] [PubMed]
- Martens-Uzunova, E.S.; Jalava, S.E.; Dits, N.F.; van Leenders, G.J.; Moller, S.; Trapman, J.; Bangma, C.H.; Litman, T.; Visakorpi, T.; Jenster, G. Diagnostic and prognostic signatures from the small non-coding RNA transcriptome in prostate cancer. Oncogene 2012, 31, 978–991. [Google Scholar] [CrossRef] [PubMed]
- Lichner, Z.; Fendler, A.; Saleh, C.; Nasser, A.N.; Boles, D.; Al-Haddad, S.; Kupchak, P.; Dharsee, M.; Nuin, P.S.; Evans, K.R.; et al. MicroRNA signature helps distinguish early from late biochemical failure in prostate cancer. Clin. Chem. 2013, 59, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Majid, S.; Dar, A.A.; Saini, S.; Shahryari, V.; Arora, S.; Zaman, M.S.; Chang, I.; Yamamura, S.; Tanaka, Y.; Chiyomaru, T.; et al. miRNA-34b inhibits prostate cancer through demethylation, active chromatin modifications, and AKT pathways. Clin. Cancer Res. 2013, 19, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Spahn, M.; Kneitz, S.; Scholz, C.J.; Joniau, S.; Stroebel, P.; Riedmiller, H.; Kneitz, B. Distinct microRNA expression profile in prostate cancer patients with early clinical failure and the impact of let-7 as prognostic marker in high-risk prostate cancer. PLoS ONE 2013, 8, e65064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Liu, Z.; Yang, Z.; Xiao, L.; Wang, F.; He, Y.; Su, P.; Wang, J.; Jing, B. Association of microRNA-126 expression with clinicopathological features and the risk of biochemical recurrence in prostate cancer patients undergoing radical prostatectomy. Diagn. Pathol. 2013, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Avgeris, M.; Stravodimos, K.; Scorilas, A. Loss of miR-378 in prostate cancer, a common regulator of KLK2 and KLK4, correlates with aggressive disease phenotype and predicts the short-term relapse of the patients. Biol. Chem. 2014, 395, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Karatas, O.F.; Guzel, E.; Suer, I.; Ekici, I.D.; Caskurlu, T.; Creighton, C.J.; Ittmann, M.; Ozen, M. miR-1 and miR-133b are differentially expressed in patients with recurrent prostate cancer. PLoS ONE 2014, 9, e98675. [Google Scholar] [CrossRef] [PubMed]
- Katz, B.; Reis, S.T.; Viana, N.I.; Morais, D.R.; Moura, C.M.; Dip, N.; Silva, I.A.; Iscaife, A.; Srougi, M.; Leite, K.R. Comprehensive study of gene and microRNA expression related to epithelial-mesenchymal transition in prostate cancer. PLoS ONE 2014, 9, e113700. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wan, X.; Chen, H.; Yang, S.; Liu, Y.; Mo, W.; Meng, D.; Du, W.; Huang, Y.; Wu, H.; et al. Identification of miR-133b and RB1CC1 as independent predictors for biochemical recurrence and potential therapeutic targets for prostate cancer. Clin. Cancer Res. 2014, 20, 2312–2325. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.Y.; Huang, Y.Q.; Zhang, Y.Q.; Han, Z.D.; He, H.C.; Ling, X.H.; Fu, X.; Dai, Q.S.; Cai, C.; Chen, J.H.; et al. MicroRNA-224 inhibits progression of human prostate cancer by downregulating TRIB1. Int. J. Cancer 2014, 135, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.H.; Han, Z.D.; Xia, D.; He, H.C.; Jiang, F.N.; Lin, Z.Y.; Fu, X.; Deng, Y.H.; Dai, Q.S.; Cai, C.; et al. MicroRNA-30c serves as an independent biochemical recurrence predictor and potential tumor suppressor for prostate cancer. Mol. Biol. Rep. 2014, 41, 2779–2788. [Google Scholar] [CrossRef] [PubMed]
- He, H.C.; Zhu, J.G.; Chen, X.B.; Chen, S.M.; Han, Z.D.; Dai, Q.S.; Ling, X.H.; Fu, X.; Lin, Z.Y.; Deng, Y.H.; et al. MicroRNA-23b downregulates peroxiredoxin III in human prostate cancer. FEBS Lett 2012, 586, 2451–2458. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.W.; Lin, T.X.; Xu, K.W.; Dong, W.; Ling, X.H.; Jiang, F.N.; Chen, G.; Zhong, W.D.; Huang, J. MicroRNA-335 acts as a candidate tumor suppressor in prostate cancer. Pathol. Oncol. Res. 2013, 19, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Melbo-Jorgensen, C.; Ness, N.; Andersen, S.; Valkov, A.; Donnem, T.; Al-Saad, S.; Kiselev, Y.; Berg, T.; Nordby, Y.; Bremnes, R.M.; et al. Stromal expression of miR-21 predicts biochemical failure in prostate cancer patients with Gleason score 6. PLoS ONE 2014, 9, e113039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortensen, M.M.; Hoyer, S.; Orntoft, T.F.; Sorensen, K.D.; Dyrskjot, L.; Borre, M. High miR-449b expression in prostate cancer is associated with biochemical recurrence after radical prostatectomy. BMC Cancer 2014, 14, 859. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Peskoe, S.B.; Ribas, J.; Rafiqi, F.; Kudrolli, T.; Meeker, A.K.; de Marzo, A.M.; Platz, E.A.; Lupold, S.E. Investigation of miR-21, miR-141, and miR-221 expression levels in prostate adenocarcinoma for associated risk of recurrence after radical prostatectomy. Prostate 2014, 74, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.H.; Kirste, S.; Fleming, J.L.; Stegmaier, P.; Drendel, V.; Mo, X.; Ling, S.; Fabian, D.; Manring, I.; Jilg, C.A.; et al. A novel miRNA-based predictive model for biochemical failure following post-prostatectomy salvage radiation therapy. PLoS ONE 2015, 10, e0118745. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Chen, Q.B.; Han, Z.D.; Zhang, Y.Q.; He, H.C.; Chen, J.H.; Chen, Y.R.; Yang, S.B.; Wu, Y.D.; Zeng, Y.R.; et al. miR-195 inhibits tumor progression by targeting RPS6KB1 in human prostate cancer. Clin. Cancer Res. 2015, 21, 4922–4934. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, M.; Liu, X. MicroRNA-195 suppresses tumor cell proliferation and metastasis by directly targeting BCOX1 in prostate carcinoma. J. Exp. Clin. Cancer Res. 2015, 34, 91. [Google Scholar] [CrossRef] [PubMed]
- Leite, K.R.; Reis, S.T.; Viana, N.; Morais, D.R.; Moura, C.M.; Silva, I.A.; Pontes, J., Jr.; Katz, B.; Srougi, M. Controlling RECK miR21 promotes tumor cell invasion and is related to biochemical recurrence in prostate cancer. J. Cancer 2015, 6, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Lichner, Z.; Ding, Q.; Samaan, S.; Saleh, C.; Nasser, A.; Al-Haddad, S.; Samuel, J.N.; Fleshner, N.E.; Stephan, C.; Jung, K.; et al. miRNAs dysregulated in association with Gleason grade regulate extracellular matrix, cytoskeleton and androgen receptor pathways. J. Pathol. 2015, 237, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Nam, R.K.; Amemiya, Y.; Benatar, T.; Wallis, C.J.; Stojcic-Bendavid, J.; Bacopulos, S.; Sherman, C.; Sugar, L.; Naeim, M.; Yang, W.; et al. Identification and validation of a five microrna signature predictive of prostate cancer recurrence and metastasis: A cohort study. J. Cancer 2015, 6, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, Z.; Zhang, Y.; He, J.; Wang, F.; Su, P.; Han, J.; Song, Z.; Fei, Y. Prognostic implications of tissue and serum levels of microRNA-128 in human prostate cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 8394–8401. [Google Scholar] [PubMed]
- Khan, A.P.; Poisson, L.M.; Bhat, V.B.; Fermin, D.; Zhao, R.; Kalyana-Sundaram, S.; Michailidis, G.; Nesvizhskii, A.I.; Omenn, G.S.; Chinnaiyan, A.M.; et al. Quantitative proteomic profiling of prostate cancer reveals a role for miR-128 in prostate cancer. Mol. Cell Proteom. 2010, 9, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Huo, N.; Li, M.; Li, Y.; He, Z. let-7a And its target, insulin-like growth factor 1 receptor, are differentially expressed in recurrent prostate cancer. Int. J. Mol. Med. 2015, 36, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.J.; Gordanpour, A.; Bendavid, J.S.; Sugar, L.; Nam, R.K.; Seth, A. mir-182 is associated with growth, migration and invasion in prostate cancer via suppression of FOXO1. J. Cancer 2015, 6, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zeng, Z.C.; Xi, M.; Wan, S.; Hua, W.; Liu, Y.L.; Zhou, Y.L.; Luo, H.W.; Jiang, F.N.; Zhong, W.D. Dysregulated microRNA-224/apelin axis associated with aggressive progression and poor prognosis in patients with prostate cancer. Hum. Pathol. 2015, 46, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Huang, Y.; Niu, X.; Tao, T.; Jiang, L.; Tong, N.; Chen, S.; Liu, N.; Zhu, W.; Chen, M. Hsa-miR-146a-5p modulates androgen-independent prostate cancer cells apoptosis by targeting ROCK1. Prostate 2015, 75, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Bakkar, A.; Alshalalfa, M.; Petersen, L.F.; Abou-Ouf, H.; Al-Mami, A.; Hegazy, S.A.; Feng, F.; Alhajj, R.; Bijian, K.; Alaoui-Jamali, M.A.; et al. microRNA 338-3p exhibits tumor suppressor role and its down-regulation is associated with adverse clinical outcome in prostate cancer patients. Mol. Biol. Rep. 2016, 43, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Bucay, N.; Sekhon, K.; Majid, S.; Yamamura, S.; Shahryari, V.; Tabatabai, Z.L.; Greene, K.; Tanaka, Y.; Dahiya, R.; Deng, G.; et al. Novel tumor suppressor microRNA at frequently deleted chromosomal region 8p21 regulates epidermal growth factor receptor in prostate cancer. Oncotarget 2016, 7, 70388–70403. [Google Scholar] [CrossRef] [PubMed]
- Das, D.K.; Osborne, J.R.; Lin, H.Y.; Park, J.Y.; Ogunwobi, O.O. miR-1207-3p is a novel prognostic biomarker of prostate cancer. Transl. Oncol. 2016, 9, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.H.; Chen, Z.Y.; Luo, H.W.; Liu, Z.Z.; Liang, Y.K.; Chen, G.X.; Jiang, F.N.; Zhong, W.D. BCL9, a coactivator for Wnt/β-catenin transcription, is targeted by miR-30c and is associated with prostate cancer progression. Oncol. Lett. 2016, 11, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Nam, R.K.; Benatar, T.; Wallis, C.J.; Amemiya, Y.; Yang, W.; Garbens, A.; Naeim, M.; Sherman, C.; Sugar, L.; Seth, A. miR-301a regulates E-cadherin expression and is predictive of prostate cancer recurrence. Prostate 2016, 76, 869–884. [Google Scholar] [CrossRef] [PubMed]
- Nip, H.; Dar, A.A.; Saini, S.; Colden, M.; Varahram, S.; Chowdhary, H.; Yamamura, S.; Mitsui, Y.; Tanaka, Y.; Kato, T.; et al. Oncogenic microRNA-4534 regulates PTEN pathway in prostate cancer. Oncotarget 2016, 7, 68371–68384. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yi, X.M.; Zhang, Z.Y.; Ge, J.P.; Zhou, W.Q. miR-129 predicts prognosis and inhibits cell growth in human prostate carcinoma. Mol. Med. Rep. 2016, 14, 5025–5032. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Qu, S.; Li, X.; Zhong, J.; Chen, X.; Qu, Z.; Wu, D. miR-129 suppresses tumor cell growth and invasion by targeting PAK5 in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2015, 464, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Colden, M.; Dar, A.A.; Saini, S.; Dahiya, P.V.; Shahryari, V.; Yamamura, S.; Tanaka, Y.; Stein, G.; Dahiya, R.; Majid, S. MicroRNA-466 inhibits tumor growth and bone metastasis in prostate cancer by direct regulation of osteogenic transcription factor RUNX2. Cell Death Dis. 2017, 8, e2572. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.Y.; Chen, G.; Zhang, Y.Q.; He, H.C.; Liang, Y.X.; Ye, J.H.; Liang, Y.K.; Mo, R.J.; Lu, J.M.; Zhuo, Y.J.; et al. MicroRNA-30d promotes angiogenesis and tumor growth via MYPT1/c-JUN/VEGFA pathway and predicts aggressive outcome in prostate cancer. Mol. Cancer 2017, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Xuan, H.; Xue, W.; Pan, J.; Sha, J.; Dong, B.; Huang, Y. Downregulation of miR-221, -30d, and -15a contributes to pathogenesis of prostate cancer by targeting BMI-1. Biochemistry 2015, 80, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Leng, J.; Shao, H.; Wang, W. miR-1, A potential predictive biomarker for recurrence in prostate cancer after radical prostatectomy. Am. J. Med. Sci. 2017, 353, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Nakajima, G.; Gavin, E.; Morris, C.G.; Kudo, K.; Hayashi, K.; Ju, J. Systematic analysis of microRNA expression of RNA extracted from fresh frozen and formalin-fixed paraffin-embedded samples. RNA 2007, 13, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Szafranska, A.E.; Davison, T.S.; Shingara, J.; Doleshal, M.; Riggenbach, J.A.; Morrison, C.D.; Jewell, S.; Labourier, E. Accurate molecular characterization of formalin-fixed, paraffin-embedded tissues by microRNA expression profiling. J. Mol. Diagn. 2008, 10, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Leite, K.R.; Canavez, J.M.; Reis, S.T.; Tomiyama, A.H.; Piantino, C.B.; Sanudo, A.; Camara-Lopes, L.H.; Srougi, M. miRNA analysis of prostate cancer by quantitative real time PCR: Comparison between formalin-fixed paraffin embedded and fresh-frozen tissue. Urol. Oncol. 2011, 29, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Tetzlaff, M.T.; Vanbelle, P.; Elder, D.; Feldman, M.; Tobias, J.W.; Sepulveda, A.R.; Xu, X. MicroRNA expression profiling outperforms mRNA expression profiling in formalin-fixed paraffin-embedded tissues. Int. J. Clin. Exp. Pathol. 2009, 2, 519–527. [Google Scholar] [PubMed]
- Jung, M.; Schaefer, A.; Steiner, I.; Kempkensteffen, C.; Stephan, C.; Erbersdobler, A.; Jung, K. Robust microRNA stability in degraded RNA preparations from human tissue and cell samples. Clin. Chem. 2010, 56, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Smyth, P.; Flavin, R.; Cahill, S.; Denning, K.; Aherne, S.; Guenther, S.M.; O’Leary, J.J.; Sheils, O. Comparison of miRNA expression patterns using total RNA extracted from matched samples of formalin-fixed paraffin-embedded (FFPE) cells and snap frozen cells. BMC Biotechnol. 2007, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Peskoe, S.B.; Barber, J.R.; Zheng, Q.; Meeker, A.K.; de Marzo, A.M.; Platz, E.A.; Lupold, S.E. Differential long-term stability of microRNAs and RNU6B snRNA in 12–20 year old archived formalin-fixed paraffin-embedded specimens. BMC Cancer 2017, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Kakimoto, Y.; Tanaka, M.; Kamiguchi, H.; Ochiai, E.; Osawa, M. MicroRNA stability in FFPE tissue samples: Dependence on GC content. PLoS ONE 2016, 11, e0163125. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J.; Helenius, G.; Karlsson, M.; Lubovac, Z.; Andren, O.; Olsson, B.; Klinga-Levan, K. Validation of suitable endogenous control genes for expression studies of miRNA in prostate cancer tissues. Cancer Genet. Cytogenet. 2010, 202, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Bovelstad, H.M.; Nygard, S.; Borgan, O. Survival prediction from clinico-genomic models—A comparative study. BMC Bioinform. 2009, 10, 413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, E.A.; Cooperberg, M.R.; Magi-Galluzzi, C.; Simko, J.P.; Falzarano, S.M.; Maddala, T.; Chan, J.M.; Li, J.; Cowan, J.E.; Tsiatis, A.C.; et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur. Urol. 2014, 66, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Gerszten, R.E.; Wang, T.J. The search for new cardiovascular biomarkers. Nature 2008, 451, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.E.; Dabbs, D.J.; Shuai, Y.; Brufsky, A.M.; Jankowitz, R.; Puhalla, S.L.; Bhargava, R. Prediction of the Oncotype DX recurrence score: Use of pathology-generated equations derived by linear regression analysis. Mod. Pathol. 2013, 26, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Guo, J.; Zhang, X.; Feng, X.; Zhang, H.; Cheng, Z.; Johnson, H.; Persson, J.L.; Chen, L. Use of two gene panels for prostate cancer diagnosis and patient risk stratification. Tumour. Biol 2016, 37, 10115–10122. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.E.; Johnson, M.H.; Yousefi, K.; Davicioni, E.; Netto, G.J.; Marchionni, L.; Fedor, H.L.; Glavaris, S.; Choeurng, V.; Buerki, C.; et al. Tissue-based genomics augments post-prostatectomy risk stratification in a natural history cohort of intermediate- and high-risk men. Eur. Urol. 2016, 69, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, C.C.; Cheng, H.H.; Tewari, M. MicroRNA profiling: Approaches and considerations. Nat. Rev. Genet. 2012, 13, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; McElroy, J.P.; Volinia, S.; Palatini, J.; Warner, S.; Ayers, L.W.; Palanichamy, K.; Chakravarti, A.; Lautenschlaeger, T. Comparison of microRNA deep sequencing of matched formalin-fixed paraffin-embedded and fresh frozen cancer tissues. PLoS ONE 2013, 8, e64393. [Google Scholar]
- Nagy, Z.B.; Wichmann, B.; Kalmar, A.; Bartak, B.K.; Tulassay, Z.; Molnar, B. miRNA isolation from FFPET specimen: A technical comparison of miRNA and total RNA isolation methods. Pathol. Oncol. Res. 2016, 22, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Howe, K. Extraction of miRNAs from formalin-fixed paraffin-embedded (FFPE) tissues. Methods Mol. Biol. 2017, 1509, 17–24. [Google Scholar] [PubMed]
- Doleshal, M.; Magotra, A.A.; Choudhury, B.; Cannon, B.D.; Labourier, E.; Szafranska, A.E. Evaluation and validation of total RNA extraction methods for microRNA expression analyses in formalin-fixed, paraffin-embedded tissues. J. Mol. Diagn. 2008, 10, 203–211. [Google Scholar] [CrossRef] [PubMed]
- ThermoFisher Scientific. TaqMan Advanced miRNA Assays—Superior Performance for miRNA Detection and Quantification. Available online: https://www.thermofisher.com/content/dam/LifeTech/Documents/PDFs/TaqMan-Advanced-miRNA-Performance-White-Paper.pdf (accessed on 20 September 2017).
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.; et al. The need for transparency and good practices in the qPCR literature. Nat. Methods 2013, 10, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; McShane, L.M.; Sauerbrei, W.; Taube, S.E. Reporting recommendations for tumor marker prognostic studies (REMARK): Explanation and elaboration. PLoS Med 2012, 9, e1001216. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.; et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. Clin. Chem. 2015, 61, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Peduzzi, P.; Concato, J.; Feinstein, A.R.; Holford, T.R. Importance of events per independent variable in proportional hazards regression analysis II. Accuracy and precision of regression estimates. J. Clin. Epidemiol. 1995, 48, 1503–1510. [Google Scholar] [CrossRef]
- Nadiminty, N.; Tummala, R.; Lou, W.; Zhu, Y.; Zhang, J.; Chen, X.; eVere White, R.W.; Kung, H.J.; Evans, C.P.; Gao, A.C. MicroRNA let-7c suppresses androgen receptor expression and activity via regulation of Myc expression in prostate cancer cells. J. Biol Chem. 2012, 287, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Ozen, M.; Creighton, C.J.; Ozdemir, M.; Ittmann, M. Widespread deregulation of microRNA expression in human prostate cancer. Oncogene 2008, 27, 1788–1793. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Takayama, K.; Katayama, S.; Urano, T.; Horie-Inoue, K.; Ikeda, K.; Takahashi, S.; Kawazu, C.; Hasegawa, A.; Ouchi, Y.; et al. miR-148a is an androgen-responsive microRNA that promotes LNCaP prostate cell growth by repressing its target CAND1 expression. Prostate Cancer Prostatic. Dis. 2010, 13, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Waltering, K.K.; Porkka, K.P.; Jalava, S.E.; Urbanucci, A.; Kohonen, P.J.; Latonen, L.M.; Kallioniemi, O.P.; Jenster, G.; Visakorpi, T. Androgen regulation of micro-RNAs in prostate cancer. Prostate 2011, 71, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Coarfa, C.; Fiskus, W.; Eedunuri, V.K.; Rajapakshe, K.; Foley, C.; Chew, S.A.; Shah, S.S.; Geng, C.; Shou, J.; Mohamed, J.S.; et al. Comprehensive proteomic profiling identifies the androgen receptor axis and other signaling pathways as targets of microRNAs suppressed in metastatic prostate cancer. Oncogene 2016, 35, 2345–2356. [Google Scholar] [CrossRef] [PubMed]
- Kneitz, B.; Krebs, M.; Kalogirou, C.; Schubert, M.; Joniau, S.; van, P.H.; Lerut, E.; Kneitz, S.; Scholz, C.J.; Strobel, P.; et al. Survival in patients with high-risk prostate cancer is predicted by miR-221, which regulates proliferation, apoptosis, and invasion of prostate cancer cells by inhibiting IRF2 and SOCS3. Cancer Res. 2014, 74, 2591–2603. [Google Scholar] [CrossRef] [PubMed]
- Gui, B.; Hsieh, C.L.; Kantoff, P.W.; Kibel, A.S.; Jia, L. Androgen receptor-mediated downregulation of microRNA-221 and -222 in castration-resistant prostate cancer. PLoS ONE 2017, 12, e0184166. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Kojima, S.; Nishikawa, R.; Kurozumi, A.; Kato, M.; Enokida, H.; Matsushita, R.; Yamazaki, K.; Ishida, Y.; Nakagawa, M.; et al. MicroRNA expression signature of castration-resistant prostate cancer: The microRNA-221/222 cluster functions as a tumour suppressor and disease progression marker. Br. J. Cancer 2015, 113, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wang, Q.; Balk, S.; Brown, M.; Lee, G.S.; Kantoff, P. The role of microRNA-221 and microRNA-222 in androgen-independent prostate cancer cell lines. Cancer Res. 2009, 69, 3356–3363. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Yang, M.; Chen, S.; Balk, S.; Pomerantz, M.; Hsieh, C.L.; Brown, M.; Lee, G. M.; Kantoff, P.W. The altered expression of miR-221/-222 and miR-23b/-27b is associated with the development of human castration resistant prostate cancer. Prostate 2012, 72, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wang, X.; He, H.H.; Sweeney, C.J.; Liu, S.X.; Brown, M.; Balk, S.; Lee, G.S.; Kantoff, P.W. miR-221 promotes the development of androgen independence in prostate cancer cells via downregulation of HECTD2 and RAB1A. Oncogene 2014, 33, 2790–2800. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Making 2006, 26, 565–574. [Google Scholar] [CrossRef] [PubMed]

| Factors | Comments | References |
|---|---|---|
| Use of different PSA cutoffs combined with or without other criteria for estimation of biochemical recurrence | [27,31,37,42,43,44] |
| Lack of metrological traceability between different PSA assays because of biological (PSA heterogeneity) and methodological reasons (use of different antibodies with different epitope specificities and affinities; different technical principles) | [45,46] |
| Age and ethnic disparities; adverse tumor characteristics (TNM classification, Gleason score or ISUP grade groups; risk classification of patients); surgical complications (positive margins) | [5,6,32,33,34,47,48] |
| The selected follow-up period after surgery decisively determines the total number of observed events of biochemical recurrence | [41,47] |
| 1. Discovery and selection of potential miRNAs |
|
| 2. Validation by clinical assessment |
|
| 3. Validation by clinical usability |
|
| No. | Reference, year | Study Details in the Marker Development Phases 1 | Sample | Methodology 2 | Significant miRNAs 3 | Statistical Methods and Results | Assessment of the Presented Clinical Findings |
|---|---|---|---|---|---|---|---|
| 1 | Tong et al., 2009 [22] | Discovery: 20 early BCR pat. (<2 years after RP) vs. 20 non-BCR pat. (>10 years after RP). Validation: 11 early BCR vs. 11 non-BCR. BCR: PSA criterion not defined. | FFPE | Discovery: microarray, Validation: RT-qPCR (TaqMan) by analysis of 6 miRs; RM: synthetic RNA. | miR-135b-5p ↑ miR-194-5p ↑ | Ratio of BCR to non-BCR: 1.6 for miR-135b and 1.4 for miR-194, but p > 0.050) with MW-test. | Aberrant expression of miR-135b and miR-194 may only reflect a tendency for early disease relapse. Low sample size. |
| 2 | Schaefer et al., 2010 [23] | Discovery: 24 matched normal and malignant tissue samples and literature data. Validation with two independent cohorts: 1) 76 pat., median follow-up of 50 months after RP, 12 BCR. 2) 79 pat., median follow-up of 50 months, 14 BCRs. BCR: PSA >0.1 ng/mL, confirmed by at least one subsequent increasing value. | Fresh-frozen tissue | Discovery: Agilent microarray. Validation: RT-qPCR (TaqMan) by analysis of 15 dysregulated miRs; RM: miR-130b-3p. | miR-96-5p ↑ | (1). KMA of RFS: log-rank test, p = 0.039. (2). CoxM: HR = 3.20, p = 0.023, independent factor for BCR in the combined cohorts. | Increased miR-96 can be considered as a BCR predictor in combination with the Gleason score. |
| 3 | Spahn et al., 2010 [24] | Discovery: 4 pairs of primary carcinoma and metastasis tissues vs. 4 BPH tissues. Validation of clinical utility: 92 high-risk patients with PSA >20 µg/L and positive lymph node status in >50% median follow-up of 74 months. BCR: PSA ≥0.2 ng/mL on 2 consecutive follow-up visits. | FFPE | Discovery: in-house microarray analysis Validation: RT-qPCR (TaqMan) by analysis of 4 out of 14 dysregulated miRs in a limited sample size and later of miR-221 in the high-risk cohort; RM: RNU6B. | miR-221-3p ↓ | (1). KMA of RFS: log-rank test, p < 0.01. (2). CoxM: HR = 0.525, p = 0.032, combined with Gleason score and tumor stage, calculated relative to clinical recurrence (local or distant metastatic disease) but not BCR. | miR-221 downregulation was linked to clinical recurrence in a high-risk PCa cohort as independent factor. |
| 4 | Fendler et al., 2011 [25] | Discovery: 10 BCR pat. (<1 year after RP) vs. 10 BCR pat. (>1–4 years) vs. 10 non-BCR pat. (within 3 years). Validation: 24 BCR pat. (<1 year) vs. 22 non-BCR pat. (within 2 years). BCR: PSA >0.1 ng/mL confirmed by at least one subsequent increasing value. | FFPE | Discovery: TaqMan array. Validation: RT-qPCR (TaqMan) of out of 65 dysregulated miRs; RM: RNU44. | miR-10b-5p ↑ | (1). KMA of RF of only miR-10b: log-rank test, p = 0.023). (2). ROC of RFS: AUC = 0.72. (3). CoxM: HR = 2.10, p = 0.033. | miR-10b remained the only predictor variable of BCR in a multivariate Cox regression model. |
| 5 | Leite et al., 2011 [52] | Discovery: 14 selected miRNAs based on miR-based prediction of Target genes (TargetScan). Validation: 21 BCR vs. 28 non-BCR, follow-up <10 years. BCR: postoperative PSA ≥0.2 µg/L. | Fresh-frozen tissue | 14 miRs were analyzed by RT-qPCR (TaqMan); RNU43. | miR-100-5p ↑ miR-145-5p ↑ miR-191-5p ↑ let-7c-5p ↑ | (1). KMA of RFS for the 4 miRs: log rank test, p < 0.05. (2). CoxU for the 4 miRs: HRs at least with p < 0.05. (3). CoxM: miR-100 (HR: 3.68, p = 0.009), independent factor in addition with tumor volume. | High levels of miR-100, miR-145, miR-191, and let-7c were related to BCR; miR-100 with highest impact in multivariate model. |
| 6 | Long et al., 2011 [53] | Discovery: 29 BCR pat. median 19 months after RP) vs. 41 non-BCR pat. (median 83 months). Validation: independent cohort (13 BCR pat. vs. 27 non-BCR pat. BCR: two detectable PSA >0.2 ng/mL. | FFPE | Integrated DASL assays (Illumina) for mRNAs and miRNAs; RM: quantile normalization. | 10 mRNAs miR-647 ↓ miR-519 ↑ | Use of the combined mRNA-miRNA panel; KMA and CoxM: at least p < 0.05 of BCR prediction in discovery and validation sets. | Prediction model of the mRNA-miRNA combined with clinicopathological data outperformed the model based on only clinicopathological data. |
| 7 | Barron et al., 2012 [54] | 18 PCa pat. after RP with BCR (<2 years) matched with 18 pat. without BCR (>3 years) according to pT3, similar Gleason score, and preoperative PSA. BCR: PSA criterion not defined. | FFPE | RT-qPCR (TaqMan); RM: RNU48. | miR-200a-3p ↓ | Student’s t-test p = 0.057 | Unclear BCR prediction evidence of miR-200a underexpression although miR-200a overexpression reduced PCa cell growth. |
| 8 | Hudson et al., 2012 [55] | Discovery: miR-1 and miR-133a were selected based on a previous study [56]. Validation: 99 PCa samples and data from another study [57], unclear consideration of clinical factors and number of BCRs. BCR: postoperative PSA ≥0.2 µg/L on two occasions. | Fresh-frozen tissue | RT-qPCR (TaqMan); RM: U6. | miR-1-3p ↓ | (1). KMA for RFS: log-rank test, p = 0.008. (2). CoxM: HR = 0.29 of high vs. low miR-1 in a model adjusted with clinicopathological factors. | Reduced miR-1 was considered a potential BCR risk factor. |
| 9 | Kang et al., 2012 [58] | Intention to confirm miR-96, miR-145, and miR-221 as potential BCR predictors as shown in previous studies [22,23,24]. Validation: 73 PCa pat., 14 BCRs, mean follow-up of 19.4 months. BCR: PSA ≥0.2 µg/L at 2 consecutive follow-up visits. | FFPE | RT-qPCR (TaqMan); RM: RNU6. | miR-96-5p-5p (-) miR-145-5p (-) miR- 221-3p (-) | KMA, CoxU and CoxM: no significant BCR prediction with all three miRs. | None of the 3 miRs could be confirmed as BCR predictors; however, the follow-up period was <2 years. |
| 10 | Kobayashi et al., 2012 [59] | Discovery: Unfounded selection of miR-30d as one of 3 miRs with a >2-fold increased expression in PCa cell lines. Validation: 56 PCa pat. after RP with 10 BCR events. BCR: continuously elevated PSA >0.2 µg/L. | Fresh-frozen tissue | Discovery: microarray (Toray, Japan). Validation: RT-qPCR (TaqMan); RM: RNU6B. | miR-30d-5p ↑ | (1). No association with all standard clinicopathological factors but with BCR. (2). CoxM: in a model adjusted with all standard clinicopathological factors only the combination of high miR-30d and reduced level of its target SOCS remained as the only significant BCR predictor (HR: 4.447, p = 0.004). | miR-30d-overexpression and low SOCS expression seems to be a relevant orthogonal marker combination of early BCR prediction. |
| 11 | Li et al., 2012 [60] | Discovery: miR-21 was found an oncogenic miR in a previous cell line study [61]. Validation: 116 BCR pat. vs. 52 non-BCR pat., with 78 low and 90 high miR-21 expressions. BCR: postoperative PSA of ≥0.2 µg/L. | FFPE | Immuno-reactivity of miR-21 by locked nucleic acid in situ hybridization (Exiqon); RM: not defined. | miR-21-5p ↑ | (1). KMA: increased miR-21 with shorter RFS, log rank test, p = 0.001. (2). CoxM: HR: 2.059, p = 0.029 as independent BCR predictor together with PSA in a model adjusted with standard clinicopathological factors. | High miR-21 expression was associated with poor BCR-free survival and can predict the risk of BCR. |
| 12 | Majid et al., 2012 [62] | Discovery: downregulated miR-23b were found in PCa cell lines. Validation: 151 PCa tissues samples to confirm low expression of miR-23b in malignant vs. non-malignant tissue samples; 105 samples used for BCR prediction, number of BCR not given. BCR: PSA criterion not defined. | Fresh-frozen tissue | Discovery: microarray of cell lines. Validation: RT-qPCR (TaqMan); RM: U6. | miR-23b-3p ↓ | (1). KMA of RFS: log-rank test p < 0.002. (2). Multiple regression analysis (but not CoxM) showed miR-23b as an independent BCR predictor (p < 0.02). | Low miR-23b expression was obviously associated with a short RFS; however, corresponding multivariate Cox regression analyses were not performed. |
| 13 | Saini et al., 2012 [63] | Differential expression of paired malignant to non-malignant miR-708 expression in 22 BCR pat. vs. 70 non-BCR pat. BCR: PSA level not defined. | FFPE | RT-qPCR; RM: RNU48. | miR-708-5p ↓ | Only the statement that 18 of the 22 BCR pat. had reduced miR-708 expression. | Clinical evidence of low miR-708 expression as BCR predictor was not statistically presented. |
| 14 | Amank-wah et al., 2013 [64] | Selection of miR-21, miR-221, and miR-222 as potential predictors of BCR based on literature data and the possible relationship between obesity and recurrence. Validation: 28 recurrent vs. 37 non-recurrent PCa. Recurrence criterion in this study: postoperative PSA ≥0.2 µg/L or clinical metastasis or cancer specific death. | FFPE | RT-qPCR (TaqMan); RM: RNU6B. | miR-21-5p ↓ miR-221-3p (-) miR-222-3p (-) | (1). KMA of RFS: significant log rank test only for miR-21, p = 0.0001. (2). CoxM: low miR-21 in age-adjusted model predicted recurrence in obese (HR: 5.40, p = 0.031), but not in non-obese patients. | miR-21 was only associated with PCa recurrence in obese patients, but no evidence was provided in multivariate models with all standard clinicopathological variables. |
| 15 | Avgeris et al., 2013 [65] | Intention to confirm decreased miR-145 as potential BCR predictor as shown in previous studies. Validation: 62 PCa pat. with follow-ups >40 months, 32 BCRs. BCR: 2 consecutive measurements of PSA ≥0.2 µg/L. | Fresh-frozen tissue | RT-qPCR (SYBR-Green); RM: SNORD48. | miR-145-5p ↓ | (1). KMA for RFS: log-rank test p = 0.027. (2). CoxM: low miR-145 remained as the only significant unfavorable BCR predictor (HR: 4.467, p < 0.02). | Low miR-145 expression outperformed the BCR prediction through standard clinicopathological factors. |
| 16 | He et al., 2013 [66] | Discovery: 4 pairs of primary PCa and adjacent benign tissue. Validation: 104 PCa pat. with 27 BCRs but follow-up time not indicated. BCR: PSA level not defined. | Fresh-frozen tissue | Discovery: Microarray (Agilent). Validation: RT-qPCR (GeneCopoeia) and MIRCURY hybridization (Exiqon); RM: RNU6B and miR-130b-3p. | miR-374b-5p ↓ | (1). KMA for RFS: log-rank test, p = 0.005. (2). CoxM: miR-374b (HR = 0.38, p = 0.018) remained as an independent BCR predictor together with the Gleason score. | Low miR-374b was identified as an independent BCR predictor, specifically in Chinese patients. |
| 17 | Larne et al., 2013 [67] | Discovery: based on microarry data of Martens-Uzunova et al. [68] of 50 primary PCa and 11 normal adjacent tissue samples, BCR events not given. Validation for BCR: 52 PCa pat. of cohort 2, number of BCRs not indicated. BCR: consecutive PSA levels >0.2 µg/L or one single >1 µg/L. | FFPE | Discovery: Microarray (Agilent). Validation: RT-qPCR (Exiqon). RM: geometric mean of RNU47, RNU48, RNU66. | miR-96-5p ↑ miR-145-5p ↓ miR-183-5p ↑ miR-221-5p ↓ | Ratio of (miR-96 x miR-183/miR145 x miR-221) was constructed to discriminate between malignant and non-malignant prostate tissue but also predict aggressiveness, metastasis, overall survival, and BCR risk; internal and external validation was performed. | This ratio termed as miQ (miRNA index quote) might be very useful as indicated; however, its use for BCR prediction remains unclear despite the significant KMA, as the relationship and benefit to other clinicopathological variables were not shown. |
| 18 | Lichner et al., 2013 [69] | Discovery: 27 BCR pat. (<3 years) vs. 14 non-BCR pat. (>3 years). Validation: independent cohorts with 35 and 29 corresponding patients. BCR: PSA criterion not defined. | FFPE | Discovery: TaqMan array card A + B. Validation: RT-qPCR (TaqMan); RM: RNU48. | miR-152-3p ↓ miR-331-3p ↑ | (1). Differential expression of 25 miRs between the 2 BCR groups; 16 miRs significantly discriminated (ROC analysis) between them. (2). Three developed logistic regression models with 2–3 miRs correctly classified with >90%. | miR-331-3p and miR-152 were most useful both in the discovery and validation set and could predict BCR risk at the time of prostatectomy. |
| 19 | Majid et al., 2013 [70] | Intention: to validate miR-34b expression as a BCR prediction tool and identify its functional role. Validation: 74 pairs of matched tissue samples, 17 BCRs, follow-up period not given. BCR: first postoperative PSA >0.1 µg/L) after at least one undetectable PSA (<0.04 µg/) after RP. | Fresh-frozen tissue | RT-qPCR (TaqMan); RM: not defined. | miR-34b-3p ↓ | KMA: low expression was associated with shorter RFS (log-rank test, p = 0.02). | Low miR-34b might have prognostic value in BCR prediction but that was not assessed by multivariate analysis. |
| 20 | Schubert et al., 2013 [71] | Discovery: 13 high-risk PCa cases and 6 BPH. Validation: 2 independent, two-centric cohorts of 98 and 92 high-risk PCa pat., mean follow-ups >6.5 years but BCR events not reported. BCR: PSA ≥0.2 µg/L on 2 consecutive follow-up visits. | FFPE | Discovery: microarray analysis. Validation: RT-qPCR (TaqMan); RM: RNU6B. | let-7b-5p ↓ | Specific miR signatures of high-risk PCa patients with different clinical outcomes were identified. CoxM: let-7b was validated in the 2 validation cohorts as independent BCR predictor (HR: 0.44 and 0.30, p ≤ 0.05) together with the Gleason score. | Low let-7b expression was successfully validated as a predictor of BCR and clinical failure (local or distant metastasis) in high-risk PCa patients. |
| 21 | Sun et al., 2013 [72] | Intention to examine the clinical significance of miR-126 as it is known as a regulator in other tumors. Validation: 128 PCa tissue samples, follow-up from 3 to 10 years, BCRs not indicated. BCR: PSA ≥0.2 µg/L on 2 consecutive follow-up visits. | Fresh-frozen tissue | RT-qPCR (TaqMan); RM: RNU6B. | miR-126-3p ↓ | (1). KMA of RFS: log-rank test, p < 0.001. (2). CoxM: low miR (HR = 3.68, p = 0.01). | miR-126 expression, tumor stage and lymph node status were identified as independent BCR predictors. |
| 22 | Avgeris et al., 2014 [73] | Discovery: Based on the reduced miR-378 expression in PCa tissue [68], the regulatory role of this miR on kallikrein 2 and 4 as PCa elements was predicted in silico. Validation: 62 PCa tissue samples, median follow-up <5 years with 32 BCRs. BCR: PSA ≥0.2 µg/L by 2 consecutive measurements. | Fresh-frozen tissue | RT-qPCR; RM: SNORD48. | miR-378a-3p ↓ | (1). KMA of RFS: reduced miR-378 discriminated Gleason 3 + 4 and 4 + 3 in patients with worse RFS (log-rank test, p < 0.001). (2). CoxM: only in high and very-high-risk PCa pat. was the loss of miR-378 an independent BCR predictor together with the Gleason score but not in the whole cohort. | Loss of miR-378 expression showed a limited capability of BCR prediction only in high-risk PCa pat. |
| 23 | Casanova-Salas et al., 2014 [49] | Discovery: differential miR expression in 50 PCa tissue vs. 10 normal tissue samples. Validation: analytical validation in the discovery set, clinical validation in independent samples from 122 BCR vs. 151 non-BCR pat., mean follow-up time 7.7 years. BCR: PSA ≥0.4 µg/L during follow-up. | Fresh frozen tissue; FFPE | Discovery: microarray (Applied) Validation: RT-qPCR (TaqMan); RM: RNU44 and RNU48. | miR-182-5p ↑ miR-187-3p ↓ | (1). miR-182/-87 as the most dysregulated miRs were further analyzed. (2). KMA: high miR-182 predicted shorter RFS, also within the Gleason score groups. (3). CoxM: miR-182 was an independent factor, combined with the Gleason score especially for Gleason score 7. | miR-182 in combination with the Gleason score showed a promising capability for BCR prediction but not for clinical progression. |
| 24 | Karatas et al., 2014 [74] | Discovery: 20 BCR vs. 20 non-BCR pat. Validation: independent 21 BCR vs. 21 non-BCR pat., mean follow-up <5 years. BCR: PSA ≥0.2 µg/L by 2 on 2 consecutive follow-up visits. | Fresh-frozen tissue | Discovery: microarray (Agilent). Validation: RT-qPCR (TaqMan) of selected miRs; RM: RNU43. | miR-1-3p ↓ miR-133b ↓ | (1). Reduced expression of both miRs in BCR samples (Student’s t-test, p < 0.05. (2). ROC analysis: miR-1 with AUC 0.661; miR-133b with AUC 0.692, but PSA 0.950. | miR-1 and miR-133b predicted between BCR and non-BCR pat.; however, PSA clearly outperformed their BCR prediction. Multivariate analysis was missing. |
| 25 | Katz et al., 2014 [75] | Discovery: identification of miRNAs as potential modulators of epithelial-mesenchymal transition based on literature search. Validation: 51 PCa pat., mean follow-up 5.3 years with 17 BCRs. BCR: PSA ≥0.02 µg/L. | Fresh-frozen tissue | RT-qPCR (TaqMan); RM: RNU48. | miR-200b-3p ↓ | KMA of RFS: low miR-200b resultet in shorter RFS (log rank test, p = 0.049). Multivariate analysis was not performed. | Functional significance of miR-200b for epithelial-mesenchymal transition verified but not for BCR compared with standard clinicopathological factors. |
| 26 | Li et al., 2014 [76] | Intention to identify the role of miR-133b as a tumor suppressor as shown in other cancers. Validation: 135 PCa tissue samples, follow-up <5 years with 71 BCRs. BCR: postoperative PSA ≥0.2 µg/L on 2 consecutive follow-up visits. | Fresh-frozen tissue | MIRCURY hybridization (Exiqon); RM: not defined. | miR-133b ↑ | (1). KMA of RFS: log-rank test, p = 0.032. (2). CoxM: HR = 1.775, p = 0.045. | Increased miR-133b expression, Gleason score, pre-operative PSA, and tumor margin status were identified as independent BCR predictors. Downregulated RB1CC1 protein as target of miR-133b acted as poor BCR predictor accordingly. |
| 27 | Lin et al., 2014 [77] | Discovery: Based on a previous microarray study [66] and studies in other tumors, miR-224 was identified as potential candidate. Validation: 114 PCa samples, follow-up from 0.2 to 14 years, BCRs not indicated. BCR: PSA ≥0.2 µg/L on two occasions. | FFPE | RT-qPCR (GeneCopoeia) and MIRCURY hybridization (Exiqon); RM: RNU6B. | miR-224-5p ↓ | (1). KMA of RFS: low expression with shorter RFS, log rank test, p = 0.017. (2). CoxM: HR = 0.25, p = 0.010. | Reduced miR-224 expression, tumor stage and the Gleason score were identified as independent BCR predictors. Upregulated TRIB1 protein as target of miR-224 corresponded as poor BCR predictor. |
| 28 | Ling et al., 2014 [78] | Discovery: Based on previous studies [79,80] that miR-30c acts as potential candidate. Validation: 103 pairs of tumor tissues and adjacent benign tissues, median 3.7 years after RP with 25 BCRs. BCR: postoperative PSA ≥0.2 µg/L. | Fresh-frozen tissue | RT-qPCR (GeneCopoeia); RM: RNU6B. | miR-30c-5p ↓ | (1). KMA: low expression with shorter RFS, log rank test, p = 0.023. (2). CoxM: HR = 0.34, p = 0.002. | Reduced miR-30c expression, tumor stage and the Gleason score were identified as independent BCR predictors. |
| 29 | Melbø-Jørgensen et al., 2014 [81] | Discovery: 14 PCa pat. with BCR within 24 months vs. 16 non-BCR. Validation: 535 PCa tissue samples, median follow-up 7.4 years with 170 BCRs. BCR: PSA ≥0.4 µg/L. | FFPE | Discovery: microarray. Validation: RT-qPCR, in situ hybridization (Exiqon); RM: miR-23b-3p. | 4 up- and 3 downregulated miRs in the discovery step. Only miR-21-5p↑ was significantly validated. | (1). Higher miR-21 expression in tumor stroma than in tumor epithelial cells. (2). KMA of shorter RFS: log rank tests of high miR-21 in tumor stroma and Gleason score 6, p = 0.006 and p = 0.023. (3). CoxM for BCR: HR = 2.40, p = 0.037 for high stromal miR-21 in patients with Gleason 6, but only p = 0.08 for total cohort. | Upregulation of miR-21 was associated with BCR only in tumor stroma and only in low risk patients. Detection needs a more complicated and less convenient method than the in situ hybridization method. |
| 30 | Mortensen et al., 2014 [82] | Discovery: 22 BCR vs. 14 non-BCR pat. Validation: Independent 163 PCa cases, median follow up 5.5 years, 96 BCRs. BCR: postoperative PSA >0.2 µg/L on 2 consecutive follow-up visits. | FFPE | Discovery: TaqMan card A + B analysis, miR-449b ↑: 2.8 times higher in BCR than in non-BCR compared to other 31 dysregulated miRs. Validation: RT-qPCR (TaqMan); RM: MammU6. | miR-449b-5p ↑ | (1). KMA of RFS: log rank test, p = 0.026. (2). CoxM: HR = 1.90, p = 0.003. 3. Overall prediction accuracy: Harrell’s C index combined with clinical factors was 0.71. | High miR-449b expression was combined with tumor stage, Gleason score, preoperative PSA an independent BCR predictor. |
| 31 | Zheng et al., 2014 [83] | Discovery: Previous studies found dysregulated miR-21, miR-141, and miR-221 in PCa tissue. Validation: 59 BCR vs. matched paired 59 non-BCR pat. Recurrence: BCR with postoperative PSA >0.2 µg/L or local or distant metastasis or cancer-specific death. | FFPE | RT-qPCR (TaqMan); RM: RNU6. | miR-21-5p ↓ miR-141-3p ↓ miR-221-3p ↓ | (1). Wilcoxon test with reduced miR levels in BCR vs. non-BCR pat., p < 0.02 for the 3 miRs. (2). CoxM: only miR-221 remained as an independent BCR predictor after multivariable adjustment. | Localized PCa pat. with lower miR-221 expression may have a greater risk for cancer recurrence after surgery. |
| 32 | Bell et al., 2015 [84] | 43 PCa pat. after RP and salvage radiation therapy radiation therapy, 19 with early BCR after RP <3 years and 24 with late BCR >3 years, median follow-up of 6.9 years. Recurrence: BCR as PSA ≥0.2 µg/L on 2 consecutive follow-up visits and clinical recurrence as local, regional and systemic recurrence. | FFPE | Nanostring microarray with 800 miRNA probes; RM: geometric mean approach. | Different miRNA signatures for different objectives. miR-4516 ↑ miR-601 ↑ | (1). CoxM for first BCR after RP: 88 miRNA signature combined with D'Amico and Stephenson scores; all single miRs and in combination with significant HRs. (2). CoxM for first BCR after salvage radiation: significant 9 miRNA signature. (3). miR-4516 and miR-601 combined with the Gleason score and lymph node status significantly improved the prediction of BCR after salvage radiation compared to only clinical factors (AUC of 0.83 vs. 0.66). | The developed models with the 88-miRNA signature and the two-miRNA signatures (miR-4516 and miR-601) combined with clinicopathological factors underline the impact of miRNAs to improve the predictive BCR capability of tools based on only clinicopathological factors. Valuable additional bioinformatic data. |
| 33 | Cai et al., 2015 [85] | Discovery: miR-195 was selected as a potential BCR marker according to the Taylor data set. Validation: use of the data of Taylor et al. [57], 61 BCR pat. vs. 137 non-BCR pat. with mean follow-up 4 years. BCR: PSA ≥0.2 µg/L on two occasions according to Taylor et al. [57]. | FFPE | Microarray | miR-195-5p ↓ | (1). MW test: lower level miR-195 in BCR vs. non-BCR pat. (p < 0.05). (2). KMA: shorter RFS in low miR-195 vs. high miR-195, p = 0.022. 3. CoxM: miR-195 and the Gleason score remained independent BCR predictors. | Decreased expression of miR-195 predicted BCR. |
| 34 | Guo et al., 2015 [86] | Discovery/background: miR-195 was examined based on re-analysis of the Taylor data set [57] with decreased miR-195 in PCa tissue. Validation: 31 BCR vs. 109 non-BCR pat., follow-up time not given. BCR: PSA criterion not indicated. | Fresh-frozen tissue | RT-qPCR (TaqMan); RM: RNU6. | miR-195-5p ↓ | (1). Association of low miR-195 expression with recurrence (Chi-square, 0.002). (2). CoxU, -M: HR = 5.98 and 5.96, p < 0.001 and 0.031. miR-195, the Gleason score and lymph node status remained as independent factors in the multivariate model. | miR-195 improved the BCR prediction in a model combined with conventional clinicopathological factors. |
| 35 | Leite et al., 2015 [87] | Discovery: 13 BCR vs. 40 non-BCR pat. Validation: 51 of the discovery group and additional 37 BCR and 39 non-BCR pat. with follow-ups up to 10 years. BCR: postoperative PSA >0.2 µg/L. | Fresh-frozen tissue | Discovery: microarray (Affymetrix). Validation: RT-qPCR (TaqMan); RM: RNU43. | miR-21-3p ↑ of the 31 dysregulated miRs identified in discovery were further validated. | (1). Student’s t-test: mean expression in BCR group 7.20 vs. 2.21 in non-BCR group, p = 0.014. (2). KMA: high miR-21 resulted in shorter BCR-free survival (p = 0.003). (3). CoxM: HR = 2.5 for miR-21 was the sole independent BCR predictor in a model with all standard clinicopathological factors. | High level of miR-21 seems to be associated with BCR. However, detailed data of the multivariate model were not shown. |
| 36 | Lichner et al., 2015 [88] | Discovery: 45 PCa patients, 15 of each with a Gleason grade of 3, 4 or 5. Validation 1: independent 60 PCa after RP to validate relationship between miRNAs and Gleason grade. Validation 2: 23 high risk BCR pat. (≤2 years) vs. 37 low risk BCR pat. BCR: PSA criterion not indicated. | FFPE | Discovery: TaqMan miRNA array cards A + B. Validation: RT-qPCR (TaqMan); RNU6, RNU44 and RNU48. | miR-29c-3p ↓ miR-141-3p ↓ miR-148a-3p ↓ miR-34a-5p ↓ | (1). Indicated miRs showed a decreased expression with increasing Gleason grade. (2). MW test: high-risk vs. low-risk BCR pat. for miR-29c, miR-141, miR-148a, p < 0.05 and also BCR vs. non-BCR regardless of the time of BCR. | Identification of Gleason grade-dependent of miRNAs that were related to BCR. Not evaluated by multivariate analysis. Detailed bioinformatic information based on experimental work. |
| 37 | Nam et. al 2015 [89] | Discovery: 18 PCa pat. with metastasis and 13 non-BCR within 5 years after RP. Validation: 491 PCa patients (167 with BCR and 25 with metastasis), median follow-up 8.7 years. BCR: PSA >0.2 µg/L on 2 consecutive follow-up visits. | FFPE | Discovery: Next-generation miRNA sequencing. Validation: RT-qPCR (Qiagen); RM: miR-28-5p. | Out of 33 potential candidates, 5 miRs were selected for validation: miR-301a-3p ↑ + miR-652-3p ↑ + miR-454-3p ↑ + miR-223-3p ↓ + miR-139-5p ↓ | (1). This 5-miR panel predicted metastasis with ROC-AUC of 95.3% in the discovery set. (2). CoxU,-M: HR = 3.9 and 2.6, always p = 0.0001 for this miR panel in the validation set. The miR panel remained an independent factor in the multivariate model together with the Gleason score, tumor stage, and PSA. | This 5-miR signature could be used as a potential new and promising prognostic factor combined with known clinicopathological factors to improve the clinical management of patients after RP. Until now, it is one of the most convincing studies. |
| 38 | Sun et al., 2015 [90] | Discovery: previous study [91] on regulatory role of miR-128 in PCa cell invasion resulted in the aim of this study with a focus on the prognostic role of miR-128. Validation: 128 PCa pat., follow-up after RP between 3 and 10 years, number of BCRs not given. BCR: PSA ≥0.2 µg/L on 2 consecutive follow-up visits. | Fresh-frozen tissue | RT-qPCR; RM: RNU6B. | miR-128-3p ↓ | (1). KMA and CoxU: low level of miR-128 predicted a shorter RFS, log rank test, p < 0.001. (2). CoxM: HR = 3.96, p < 0.01, remained with tumor stage and lymph node status as independent factors in the model. | Decreased expression of miR-128 was proved to be an independent predictor of the BCR-free survival. |
| 39 | Tian et al., 2015 [92] | Based on the significance of stem cells in cancerogenesis, 6 miRs previously reported as differentially expressed miRs in PCa stem cells were tested as BCR predictors.: 32 BCR (within <4 years) vs. 36 non-BCR (≥4 years) pat. BCR: PSA >0.2 µg/L on 2 consecutive follow-up visits. | Fresh-frozen tissue | RT-qPCR (TaqMan); RNU43. | let-7a-5p ↓ | Only let7a was significantly downregulated in BCR pat. No further statistical evaluation in combination with clinicopathological variables. | Let-7a may be functionally involved in PCa cancerogenesis; however, its role as a BCR predictor remains an unsolved question in this study. |
| 40 | Wallis et al., 2015 [93] | Discovery: based on a previous Study, i.e., 22 [49], miR-182 was examined to obtain more information on the functional role of this miR. Validation: intended as external validation of [49] with 50 BCR and 50 non-BCR pat., median follow-up 5 years. BCR: PSA increase of ≥0.2 µg/L on at least 2 consecutive follow-up visits. | Fresh-frozen tissue | RT-qPCR (Qiagen); RNU6B. | miR-182-5p (-) | miR-182 was not associated with BCR according to the interpretation of the data by the authors; the used statistical methods (univariate and multivariate logistic regression) did not consider the follow-up time frame. | This study should not be considered as external validation of Study 22 [49]. |
| 41 | Wan et al., 2015 [94] | Discovery: based on previous studies of the authors [77,79] with decreased miR-224 as potential modulator of its target apelin. Validation: 20 matched pairs of PCa for miR-224/apelin axis and 104 PCa data of the Taylor data set [57]. BCR: PSA threshold not reported, probably postoperative PSA ≥0.2 µg/L on two occasions according to Taylor et al. [57]. | Fresh-frozen tissue | Discovery: microarray. Validation: microarray and RT-qPCR (GeneCopoeia); RM: RNU6B. | miR-224-5p ↓, combined with its increased target APLN mRNA | (1). KMA of RFS: low miR-224 + high APLN vs. high miR-224 + low APLN 224 with shorter BCR-free survival, log-rank test, p = 0.031. (2). CoxM: miR-224 and APLN mRNA could not be confirmed as independent BCR predictors (p > 0.3). | The association of the dysregulated miR-224/APLN axis to tumorigenesis, but their significance as prognostic markers of BCR could not be validated. |
| 42 | Xu et al., 2015 [95] | Study of the role of miR-146-5p as a modulator of apoptosis in PCa cells by targeting ROCK1 based on the re-analysis the Taylor data set with 98 pat. [57]. BCR: PSA ≥0.2 µg/L on two occasions according to Taylor et al. [57]. | Fresh-frozen tissue | Microarray (Agilent). | miR-146a-5p ↓ | KMA of RFS: pat. with low level of miR-146a had shorter RFS than pat. with high level, log rank test, p < 0.048. | Low level of miR-146a represented a high BCR risk but multivariate analysis was not performed. The BCR analysis was obviously only intended to support the results of cell line experiments. |
| 43 | Bakkar et al., 2016 [96] | Discovery: in ERG differentially expressed PCa samples, miR-338-3p was identified as one of 11 differentially expressed miRs. Validation: miR-338-3p expression in 25 matched non-malignant vs. malignant PCa samples and RFS validation of this miR in the Taylor data set [57]. BCR: PSA ≥0.2 µg/L according to Taylor et al. [57]. | FFPE | Discovery: microarray/RT-qPCR (TaqMan), RM: RNU48. Validation: microarray (Agilent) according to Taylor et al. [57]. | miR-338-3p ↓ | KMA of RFS: log-rank test, HR = 0.78, p = 0.02. | Less informative data regarding the usefulness of this miR for BCR prediction. |
| 44 | Bucay et al., 2016 [97] | Discovery/background: Based on the frequently genomic loss of chromosome 8p21 region in PCa and its association with the corresponding miR cluster, miR-3622b was examined as relevant cancer. Validation: 35 BCR vs. 57 non-BCR pat., follow-up up to ten years. BCR: PSA criterion not indicated. | FFPE | RT-qPCR (TaqMan); RM: RNU48. | miR-3622b-3p ↓ | KMA: low miR-3622 expression predicted a shorter RFS, log rank test, p = 0.0321. | Low miR expression resulted in reduced BCR-free survival probability. Lack of evidence as independent factor because of the missing adjustment to standard clinical factors strongly limits the clinical significance. |
| 45 | Das et al., 2016 [98] | No background was given why miR-1207-3p was selected as a potential BCR marker. Study of RP specimens in 155 BCR vs. 249 non-BCR pat. BCR: PSA criterion not indicated. | FFPE | RT-qPCR (SYBR Green); RM: RNU6. | miR-1207-3p ↑ | (1). miR expression higher in BCR pat. in comparison to non-BCR pat. (t-test, p < 0.0001). (2). CoxM: HR = 2.5, p < 0.001, adjusted for age and tumor stage. | PCa patients with a high miR-1207-3p expression had a high-risk of BCR. |
| 46 | Kristensen et al., 2016 [50] | Discovery: Training cohort 1 with RP specimens of localized PCa from 57 BCR vs. 69 non-BCR pat., mean follow-up 3 years. Validation: using 2 cohorts, own cohort 2 with 50 BCR vs. 60 non-BCR pat, mean follow-up 3.3; external cohort 3 of a publicly data set with 25 BCR vs. 74 non-BCR pat., follow-up 6 years. BCR: postoperative PSA >0.2 µg/L. | cohort 1 & 2: FFPE cohort 3: fresh-frozen tissue | For cohort 1 and 2: RT-qPCR platforms with different panels (Exiqon); RM: miR-151a-5p. Cohort 3: Microarray (Agilent). | RFS classifier: miR-185-5p ↑ miR-221-3p ↓ miR-326 ↓ | (1). Development of a 3-BCR classifier from 11 individual miRs that remained significant in a multivariate model with standard clinicopathological factors. (2). KMA for RFS: log rank test, p < 0.050 in all 3 cohorts. (3). CoxM: Addition of the classifier to a multivariate model with clinicopathological factors increased the predictive accuracy. | This classifier (miR-185-5p + miR-221-3p + miR-326) was validated in two independent cohorts in an extensive manner and resulted in a benefit if included in a standard model with only clinicopathological factors. |
| 47 | Ling et al., 2016 [99] | Part of the study on the role of miR-30c and its target BCL9 in PCa progression and their combined use for BCR prediction: 18 BCR pat. vs. 80 non-BCR pat., median follow-up 3.8 years. These 98 pat. were identical to 98 pat. of 103 pat. included in a previous study about miR-30c [78]. BCR: postoperative PSA >0.2 µg/L. | Fresh-frozen tissue | RT-qPCR (GeneCopoeia); RM: RNU6B. | miR-30c-5p ↓ combined with its target BCL9 | CoxU and CoxM: HR = 5.79 and 5.08, p = 0.023 and 0.048 for miR-30c/BCL9 status. This score remained an independent factor in the multivariate mode, together with the Gleason score. | The combined analysis of miR-30c and BCL9 may be a valuable tool for BCR prediction. The benefit of this score compared with miR-30c expression as shown in the previous study of the authors was not explained. |
| 48 | Nam et al., 2016 [100] | Based on a previous study about a 5-miR signature for BCR prediction [89], a more detailed study was performed using the single miR-301a: 585 PCa pat. (197 with BCR and 32 with metastasis vs, 388 non-BCR), median follow-up 8.4 years. BCR: PSA ≥0.2 µg/L on 2 consecutive follow-up visits that are at least 3 months apart. | FFPE | RT-qPCR (Qiagen); RM: miR-28-5p. | miR-301a-3p ↑ | (1). No associations of miR-301a expression with conventional prognostic factors. (2). CoxU and CoxM: High level of miR-301a: HR = 1.55 and 1.42, p = 0.003 and p = 0.019. miR-301a remained an independent factor in the multivariate model, together with all conventional factors. | miR-301a may serve as a useful single BCR biomarker in combination with clinicopathological data. Illuminating mechanistic experiments regarding the role of miR-301a, but the authors did not comment whether this single miR could replace the 5-miR-signature recommend in their previous paper [89]. |
| 49 | Nip et al., 2016 [101] | Discovery/background: based on a previous study on PCa cell lines that miR-4534 was upregulated [62]. Validation: 84 malignant vs. non-malignant matched PCa tissue samples, 34 BCR vs. 37 non-BCR., follow-up not given. BCR: PSA criterion not indicated. | Fresh-frozen tissue | RT-qPCR (TaqMan); RM: not defined. | miR-4534 ↑ | KMA: high miR-4534 expression predicted a shorter RFS, log rank test, p < 0.01. | High miR-4534 expression was related to higher BCR risk. Lack of evidence of the miR as an independent factor because of the missing adjustment to standard clinical factors. |
| 50 | Xu et al., 2016 [102] | Discovery/background: miR-129 was examined in this study based on the role of miR-129 in other cancers [103]. Validation: 29 BCR vs. 89 non-BCR pat. BCR: PSA ≥0.2 µg/L following surgical treatment. | FFPE | RT-qPCR (Takara); RM: RNU6. | miR-129-5p ↓ | (1). KMA: low miR-129 expression predicted a shorter RFS, log rank test, p < 0.001. (2). CoxU and CoxM: HR = 5.63 and 2.69, p < 0.001 in each case. miR-129 retained with the Gleason score, tumor and lymph node status as independent factors in the multivariate model. | Downregulation of miR-129 was associated with poor BCR-free survival. |
| 51 | Colden et al., 2017 [104] | Discovery/background: miR-466 was examined based on a previous study its downregulation PCa cell lines [62]. Validation: 92 PCa pat. from two sources, 34 BCR vs. 37 non-BCR pat., follow-up up to 12 years. BCR: first postoperative PSA >0.1 µg/L. | FFPE | RT-qPCR (TaqMan); RM: not defined. | miR-466 ↓ | (1). Association of down-regulated miR-466 with the Gleason score, tumor stage (p < 0.0001). (2). KMA: low miR-466 expression predicted a shorter RFS, log rank test, p = 0.01. (3). Missing multivariate analysis. | Low expression of miR-466 can predict BCR. |
| 52 | Lin et al., 2017 [105] | Discovery/background: miR-30d was examined based on controversial expression and functional data [59,106]. Validation: with the Taylor data set [57] and TCGA data with 27 and 59 BCR and 80 and 365 non-BCR, respectively, follow-up up to 14 years. BCR: PSA ≥0.2 µg/L on two occasions after RP according to Taylor et al. [57]. | Fresh-frozen tissue | Microarray (Agilent), see Taylor et al. [57]. | Model with miR-30d-5p ↑ + MYPT1 ↓ | (1). Upregulation of miR-30d and downregulation of its target MYPT1. (2). KMA: Combination of both (miR-30dhigh/MYPT1low) predicted better shorter RFS than markers alone (p = 0.003). (3). CoxM: HR = 5.13, p = 0.026, remained as an independent factor with tumor stage in the Taylor data set but not in the TCGA data set. | miR-30d/MYPT1 combination was identified as an independent factor to predict BCR of PCa patients, but controversial results in two data sets were shown. |
| 53 | Wei et al., 2017 [107] | Discovery/background: miR-1 was examined based on a previous study with miR-1 downregulation in recurrent cases [74]. Validation: 27 BCR vs. 51 non-BCR pat. of clinically localized PCa, follow-up within 4 years after RP. Recurrence definition: BCR with PSA <0.2 µg/L, local and systemic recurrence and cancer-related death. | FFPE | RT-qPCR (TaqMan); RM: RNU43. | miR-1-3p ↓ | (1). Downregulated miR-1 in recurrent pat., (t test, p < 0.001). (2). ROC for recurrence: AUC = 0.885, p < 0.001. (3). CoxU and CoxM: HR = 1.53 and 1.86, p = 0.024 and p = 0.011. | miR-1 can function as an independent recurrence predictor together with standard clinicopathological variables. |
| miRNA | Studies, n | Study Nos. (Table 3) | References |
|---|---|---|---|
| miR-221-3p | 6 | ↓: 3, 31, 46 a; (-): 9, 14 | [24,50,58,64,83] |
| miR-21-5p | 4 | ↑: 11, 29; ↓:14, 31 | [60,64,81,83] |
| miR-145-5p | 4 | ↑: 5; ↓: 15, 17; (-): 9 | [52,58,65,67] |
| miR-1-3p | 3 | ↓: 8, 24, 53 | [55,74,107] |
| miR-96-5p | 3 | ↑: 2, 17; (-): 9 | [23,58,67] |
| miR-30c-5p | 2 | ↓: 28, 47 | [78,99] |
| miR-30d-5p | 2 | ↑: 10, 52 | [59,105] |
| miR-133b | 2 | ↑: 26; ↓:24 | [74,76] |
| miR-141-3p | 2 | ↓: 31, 36 | [83,88] |
| miR-185-5p | 2 | ↑: 46 a | [50] |
| miR-195-5p | 2 | ↓: 33, 34 | [85,86] |
| miR-224-5p | 2 | ↓: 27, 41 | [77,94] |
| miR-301a-3p | 2 | ↑: 37, 48 | [89,100] |
| miR-326 | 2 | ↑: 46 a | [50] |
| miR-182-5p | 2 | ↑: 23; (-): 40 | [49,93] |
| Characteristics | Studies, n (%) |
|---|---|
| 1. PSA cutoff for biochemical recurrence | |
| ≥0.1 µg/L | 4 (7) |
| ≥0.2 µg/L | 35 (66) |
| ≥0.4 µg/L | 2 (4) |
| Not specified | 12 (23) |
| 2. Preoperative PSA level | |
| <10 µg/L | 3 (6) |
| >10 µg/L | 43 (81) |
| Not specified | 7 (13) |
| 3. Tumor characteristics | |
| pT classification/clinical stage | |
| Specified | 50 (94) |
| Not specified | 3 (6) |
| Gleason score | |
| Specified | 52 (98) |
| Not specified | 1 (2) |
| Resection margin status | |
| Specified | 21 (40) |
| Not specified | 32 (60) |
| Lymph node status/Metastasis | |
| Specified | 16 (30) |
| Not specified | 37 (70) |
| 4. Study design features | |
| According to MIQE, REMARK, STARD 1 guidelines | |
| Yes | 2 (4) |
| No | 51 (96) |
| Type of study | |
| Retrospective | 53 (100) |
| Multi-institutional study (n ≥ 2) | 9 (17) |
| Studies with functional miR data | |
| Yes | 30 (57) |
| No | 23 (43) |
| Sample size (patients/study) | |
| <50 | 7 (13) |
| 50–100 | 23 (44) |
| >100–150 | 15 (28) |
| >150 | 8 (15) |
| Events of biochemical recurrence (n/study) | |
| 10–20 | 11 (21) |
| 20–30 | 15 (28) |
| >30 | 17 (32) |
| Not specified | 10 (19) |
| Follow-up time (mean/median years) | |
| <5 | 18 (34) |
| >5 | 25 (47) |
| Not specified | 9 (19) |
| Statistical analysis | |
| Only univariate | 18 (34) |
| Multivariate | 35 (66) |
| Studies with internal/external validation | |
| Yes | 8 (15) |
| No | 45 (85) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Stephan, C.; Weickmann, S.; Jung, M.; Kristiansen, G.; Jung, K. Tissue-Based MicroRNAs as Predictors of Biochemical Recurrence after Radical Prostatectomy: What Can We Learn from Past Studies? Int. J. Mol. Sci. 2017, 18, 2023. https://doi.org/10.3390/ijms18102023
Zhao Z, Stephan C, Weickmann S, Jung M, Kristiansen G, Jung K. Tissue-Based MicroRNAs as Predictors of Biochemical Recurrence after Radical Prostatectomy: What Can We Learn from Past Studies? International Journal of Molecular Sciences. 2017; 18(10):2023. https://doi.org/10.3390/ijms18102023
Chicago/Turabian StyleZhao, Zhongwei, Carsten Stephan, Sabine Weickmann, Monika Jung, Glen Kristiansen, and Klaus Jung. 2017. "Tissue-Based MicroRNAs as Predictors of Biochemical Recurrence after Radical Prostatectomy: What Can We Learn from Past Studies?" International Journal of Molecular Sciences 18, no. 10: 2023. https://doi.org/10.3390/ijms18102023