Human Chorionic Gonadotropin as a Pivotal Endocrine Immune Regulator Initiating and Preserving Fetal Tolerance
Abstract
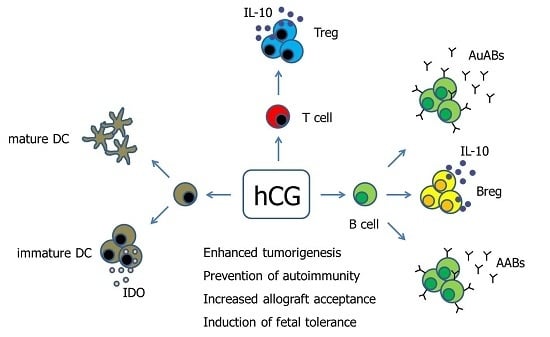
:1. Introduction
2. Human Chorionic Gonadotropin—Inducer of Tolerogenic Dendritic Cells?
3. Human Chorionic Gonadotropin—Supporter of Baby’s Best Friends
4. Human Chorionic Gonadotropin—Friend or Foe for B Cell-Mediated Fetal Tolerance
5. Human Chorionic Gonadotropin—Does the Source and Concentration Matter for Its Immune Regulatory Properties?
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Cole, L.A. hCG, the wonder of today’s science. Reprod. Biol. Endocrinol. 2012, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Kane, N.; Kelly, R.; Saunders, P.T.K.; Critchley, H.O.D. Proliferation of Uterine Natural Killer Cells Is Induced by Human Chorionic Gonadotropin and Mediated via the Mannose Receptor. Endocrinology 2009, 150, 2882–2888. [Google Scholar] [CrossRef] [PubMed]
- Palomino, W.A.; Argandoña, F.; Azúa, R.; Kohen, P.; Devoto, L. Complement C3 and decay-accelerating factor expression levels are modulated by human chorionic gonadotropin in endometrial compartments during the implantation window. Reprod. Sci. 2013, 20, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Kayisli, U.A.; Selam, B.; Guzeloglu-Kayisli, O.; Demir, R.; Arici, A. Human chorionic gonadotropin contributes to maternal immunotolerance and endometrial apoptosis by regulating Fas-Fas ligand system. J. Immunol. 2003, 171, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Khare, P.; Bose, A.; Singh, P.; Singh, S.; Javed, S.; Jain, S.K.; Singh, O.; Pal, R. Gonadotropin and tumorigenesis: Direct and indirect effects on inflammatory and immunosuppressive mediators and invasion. Mol. Carcinogenesis 2017, 56, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Sharma, M.D.; Baban, B.; Harding, H.P.; Zhang, Y.; Ron, D.; Mellor, A.L. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 2005, 22, 633–642. [Google Scholar] [CrossRef] [PubMed]
- He, L.-Z.; Ramakrishna, V.; Connolly, J.E.; Wang, X.-T.; Smith, P.A.; Jones, C.L.; Valkova-Valchanova, M.; Arunakumari, A.; Treml, J.F.; Goldstein, J.; et al. A novel human cancer vaccine elicits cellular responses to the tumor-associated antigen, human chorionic gonadotropin β. Clin. Cancer Res. 2004, 10, 1920–1927. [Google Scholar] [CrossRef] [PubMed]
- Ueno, A.; Cho, S.; Cheng, L.; Wang, J.; Hou, S.; Nakano, H.; Santamaria, P.; Yang, Y. Transient upregulation of indoleamine 2,3-dioxygenase in dendritic cells by human chorionic gonadotropin downregulates autoimmune diabetes. Diabetes 2007, 56, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Fredarow, A.; Tadmor, A.; Raz, T.; Meterani, N.; Addadi, Y.; Nevo, N.; Solomonov, I.; Sagi, I.; Mor, G.; Neeman, M.; et al. Ovarian dendritic cells act as a double-edged pro-ovulatory and anti-inflammatory sword. Mol. Endocrinol. 2014, 28, 1039–1054. [Google Scholar] [CrossRef] [PubMed]
- Fainaru, O.; Hantisteanu, S.; Rotfarb, N.; Michaeli, M.; Hallak, M.; Ellenbogen, A. CD11c+HLADR+ dendritic cells are present in human ovarian follicular fluid, and their maturity correlates with serum estradiol levels in response to gonadotropins. Fertil. Steril. 2012, 97, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Pencovich, N.; Hantisteanu, S.; Hallak, M.; Fainaru, O. Gonadotropin stimulation in mice leads to ovarian accumulation of immature myeloid cells and altered expression of proangiogenic genes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 179, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Segerer, S.E.; Müller, N.; van Den Brandt, J.; Kapp, M.; Dietl, J.; Reichardt, H.M.; Rieger, L.; Kämmerer, U. Impact of Female Sex Hormones on the Maturation and Function of Human Dendritic Cells. Am. J. Reprod. Immunol. 2009, 62, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Inaba, M.; Sugiura, K.; Nakajima, T.; Ito, T.; Nakamura, K.; Kanzaki, H.; Ikehara, S. Analyses of dendritic cell subsets in pregnancy. Am. J. Reprod. Immunol. 2003, 50, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Versnel, M.A.; Leijten, L.M.E.; van Helden-Meeuwsen, C.G.; Fekkes, D.; Leenen, P.J.M.; Khan, N.A.; Benner, R.; Kiekens, R.C.M. Chorionic gonadotropin induces dendritic cells to express a tolerogenic phenotype. J. Leukoc. Biol. 2008, 83, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A.; Heinze, K.; Witte, J.; Poloski, E.; Linzke, N.; Woidacki, K.; Zenclussen, A.C. Human Chorionic Gonadotropin as a Central Regulator of Pregnancy Immune Tolerance. J. Immunol. 2013, 190, 2650–2658. [Google Scholar] [CrossRef] [PubMed]
- Dauven, D.; Ehrentraut, S.; Langwisch, S.; Zenclussen, A.C.; Schumacher, A. Immune Modulatory Effects of Human Chorionic Gonadotropin on Dendritic Cells Supporting Fetal Survival in Murine Pregnancy. Front. Endocrinol. 2016, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Teles, A.; Zenclussen, A.C.; Schumacher, A. Regulatory T Cells are Baby’s Best Friends. Am. J. Reprod. Immunol. 2013, 69, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Teles, A.; Schumacher, A.; Kühnle, M.-C.; Linzke, N.; Thuere, C.; Reichardt, P.; Tadokoro, C.E.; Hämmerling, G.J.; Zenclussen, A.C. Control of Uterine Microenvironment by Foxp3+ Cells Facilitates Embryo Implantation. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Shima, T.; Sasaki, Y.; Itoh, M.; Nakashima, A.; Ishii, N.; Sugamura, K.; Saito, S. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J. Reprod. Immunol. 2010, 85, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Diao, L.-H.; Li, G.-G.; Zhu, Y.-C.; Tu, W.-W.; Huang, C.-Y.; Lian, R.-C.; Chen, X.; Li, Y.-Y.; Zhang, T.; Huang, Y.; et al. Human chorionic gonadotropin potentially affects pregnancy outcome in women with recurrent implantation failure by regulating the homing preference of regulatory T cells. Am. J. Reprod. Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zenclussen, A.C.; Gerlof, K.; Zenclussen, M.L.; Sollwedel, A.; Bertoja, A.Z.; Ritter, T.; Kotsch, K.; Leber, J.; Volk, H.-D. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: Adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am. J. Pathol. 2005, 166, 811–822. [Google Scholar] [CrossRef]
- Darrasse-Jèze, G.; Darasse-Jèze, G.; Klatzmann, D.; Charlotte, F.; Salomon, B.L.; Cohen, J.L. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol. Lett. 2006, 102, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Darrasse-Jeze, G.; Bergot, A.-S.; Courau, T.; Churlaud, G.; Valdivia, K.; Strominger, J.L.; Ruocco, M.G.; Chaouat, G.; Klatzmann, D. Self-Specific Memory Regulatory T Cells Protect Embryos at Implantation in Mice. J. Immunol. 2013, 191, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Kang, X.M.; Zhao, A.M. Regulation of CD4⁺FOXP3⁺ T cells by CCL20/CCR6 axis in early unexplained recurrent miscarriage patients. Genet. Mol. Res. 2015, 14, 9145–9154. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Wang, J.; Kang, Y.; Tai, P.; Wen, J.; Zou, Q.; Li, G.; Ouyang, H.; Xia, G.; Wang, B. Progesterone increases systemic and local uterine proportions of CD4+CD25+ Treg cells during midterm pregnancy in mice. Endocrinology 2010, 151, 5477–5488. [Google Scholar] [CrossRef] [PubMed]
- Mjösberg, J.; Svensson, J.; Johansson, E.; Hellström, L.; Casas, R.; Jenmalm, M.C.; Boij, R.; Matthiesen, L.; Jönsson, J.-I.; Berg, G.; et al. Systemic reduction of functionally suppressive CD4dimCD25highFoxp3+ Tregs in human second trimester pregnancy is induced by progesterone and 17β-estradiol. J. Immunol. 2009, 183, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Prieto, G.A.; Rosenstein, Y. Oestradiol potentiates the suppressive function of human CD4 CD25 regulatory T cells by promoting their proliferation. Immunology 2006, 118, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Tai, P.; Wang, J.; Jin, H.; Song, X.; Yan, J.; Kang, Y.; Zhao, L.; An, X.; Du, X.; Chen, X.; et al. Induction of regulatory T cells by physiological level estrogen. J. Cell. Physiol. 2008, 214, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.; Hammarström, L.; Smith, C.I.; Brundin, J. In vitro induction of human suppressor T cells by a chorionic gonadotropin preparation. J. Reprod. Immunol. 1981, 3, 75–84. [Google Scholar] [CrossRef]
- Fuchs, T.; Hammarström, L.; Smith, C.I.; Brundin, J. In vitro induction of murine suppressor T-cells by human chorionic gonadotropin. Acta Obstet. Gynecol. Scand. 1980, 59, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.; Hammarström, L.; Smith, C.I.; Brundin, J. Sex-dependent induction of human suppressor T cells by chorionic gonadotropin. J. Reprod. Immunol. 1982, 4, 185–190. [Google Scholar] [CrossRef]
- Kirpatovskiĭ, I.D.; Suskova, V.S.; Sutiusheva, L.V.; Suzdal’tseva, A.A.; Rakhmanova, G.A.; Emets, V.I.; Kosova, I.P. Izbiratel’noe vliianie khorionicheskogo gonadotropina na subpopuliatsii limfotsitov. Biulleten Eksp. Biol. Med. 1989, 108, 71–74. [Google Scholar]
- Schumacher, A.; Brachwitz, N.; Sohr, S.; Engeland, K.; Langwisch, S.; Dolaptchieva, M.; Alexander, T.; Taran, A.; Malfertheiner, S.F.; Costa, S.-D.; et al. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J. Immunol. 2009, 182, 5488–5497. [Google Scholar] [CrossRef] [PubMed]
- Poloski, E.; Oettel, A.; Ehrentraut, S.; Luley, L.; Costa, S.D.; Zenclussen, A.C.; Schumacher, A. JEG-3 Trophoblast Cells Producing Human Chorionic Gonadotropin Promote Conversion of Human CD4+FOXP3− T Cells into CD4+FOXP3+ Regulatory T Cells and Foster T Cell Suppressive Activity. Biol. Reprod. 2016, 95. [Google Scholar] [CrossRef] [PubMed]
- Haxhinasto, S.; Mathis, D.; Benoist, C. The Akt-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 2008, 205, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Shirshev, S.V.; Orlova, E.G.; Zamorina, S.A.; Nekrasova, I.V. Influence of reproductive hormones on the induction of CD4+CD25brightFoxp3+ regulatory T cells. Dokl. Biol. Sci. 2011, 440, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Koldehoff, M.; Katzorke, T.; Wisbrun, N.C.; Propping, D.; Wohlers, S.; Bielfeld, P.; Steckel, N.K.; Beelen, D.W.; Elmaagacli, A.H. Modulating impact of human chorionic gonadotropin hormone on the maturation and function of hematopoietic cells. J. Leukoc. Biol. 2011, 90, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Furcron, A.-E.; Romero, R.; Mial, T.N.; Balancio, A.; Panaitescu, B.; Hassan, S.S.; Sahi, A.; Nord, C.; Gomez-Lopez, N. Human Chorionic Gonadotropin Has Anti-Inflammatory Effects at the Maternal-Fetal Interface and Prevents Endotoxin-Induced Preterm Birth, but Causes Dystocia and Fetal Compromise in Mice. Biol. Reprod. 2016, 94, 136. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Liu, F.; Zhai, J.; Liu, X.; Zhang, Q.; Zhang, B. Alteration of Th17 and Foxp3+ regulatory T cells in patients with unexplained recurrent spontaneous abortion before and after the therapy of hCG combined with immunoglobulin. Exp. Ther. Med. 2017, 14, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.S.; Schumacher, A. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology 2016, 148, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Zamorina, S.A.; Shirshev, S.V. Oligopeptides of Chorionic Gonadotropin β-Subunit in Induction of T Cell Differentiation into Treg and Th17. Bull. Exp. Biol. Med. 2015, 160, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.M.; Mithal, A.; Bieber, M.M.; Herzenberg, L.A.; Teng, N.N. Human CD5+ B lymphocytes (B-1 cells) decrease in peripheral blood during pregnancy. J. Reprod. Immunol. 1995, 28, 53–60. [Google Scholar] [CrossRef]
- Ait-Azzouzene, D.; Gendron, M.C.; Houdayer, M.; Langkopf, A.; Burki, K.; Nemazee, D.; Kanellopoulos-Langevin, C. Maternal B lymphocytes specific for paternal histocompatibility antigens are partially deleted during pregnancy. J. Immunol. 1998, 161, 2677–2683. [Google Scholar] [PubMed]
- Canellada, A.; Farber, A.; Zenclussen, A.C.; Gentile, T.; Dokmetjian, J.; Keil, A.; Blois, S.; Miranda, S.; Berod, L.; Gutierrez, G.; et al. Interleukin regulation of asymmetric antibody synthesized by isolated placental B cells. Am. J. Reprod. Immunol. 2002, 48, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.S.; Witvliet, M.D.; Steffensen, R.; Haasnoot, G.W.; Goulmy, E.; Christiansen, O.B.; Claas, F. The presence of HLA-antibodies in recurrent miscarriage patients is associated with a reduced chance of a live birth. J. Reprod. Immunol. 2010, 87, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Khare, P.; Singh, O.; Jain, S.K.; Javed, S.; Pal, R. Inhibitory effect of antibodies against human chorionic gonadotropin on the growth of colorectal tumour cells. Indian J. Biochem. Biophys. 2012, 49, 92–96. [Google Scholar] [PubMed]
- Talwar, G.P.; Singh, O.M.; Gupta, S.K.; Hasnain, S.E.; Pal, R.; Majumbar, S.S.; Vrati, S.; Mukhopadhay, A.; Srinivasan, J.; Deshmukh, U.; et al. The HSD-hCG vaccine prevents pregnancy in women: Feasibility study of a reversible safe contraceptive vaccine. Am. J. Reprod. Immunol. 1997, 37, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Jensen, F.; Wallukat, G.; Herse, F.; Budner, O.; El-Mousleh, T.; Costa, S.-D.; Dechend, R.; Zenclussen, A.C. CD19+CD5+ Cells as Indicators of Preeclampsia. Hypertension 2012, 59, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Kalkunte, S.; Nevers, T.; Norris, W.; Banerjee, P.; Fazleabas, A.; Kuhn, C.; Jeschke, U.; Sharma, S. Presence of non-functional hCG in preeclampsia and rescue of normal pregnancy by recombinant hCG. Placenta 2010, 31, 126–133. [Google Scholar]
- Guzman-Genuino, R.M.; Diener, K.R. Regulatory B Cells in Pregnancy: Lessons from Autoimmunity, Graft Tolerance, and Cancer. Front. Immunol. 2017, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Rolle, L.; Memarzadeh Tehran, M.; Morell-García, A.; Raeva, Y.; Schumacher, A.; Hartig, R.; Costa, S.-D.; Jensen, F.; Zenclussen, A.C. Cutting Edge: IL-10-Producing Regulatory B Cells in Early Human Pregnancy. Am. J. Reprod. Immunol. 2013, 70, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Fettke, F.; Schumacher, A.; Canellada, A.; Toledo, N.; Bekeredjian-Ding, I.; Bondt, A.; Wuhrer, M.; Costa, S.-D.; Zenclussen, A.C. Maternal and Fetal Mechanisms of B Cell Regulation during Pregnancy: Human Chorionic Gonadotropin Stimulates B Cells to Produce IL-10 While α-Fetoprotein Drives Them into Apoptosis. Front. Immunol. 2016, 7, 495. [Google Scholar] [CrossRef] [PubMed]
- Hammarström, L.; Fuchs, T.; Smith, C.I. The immunodepressive effect of human glucoproteins and their possible role in the nonrejection process during pregnancy. Acta Obstet. Gynecol. Scand. 1979, 58, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Cocchiara, R.; Lorico, A.; Cefalù, E.; Cittadini, E.; Geraci, D. Modulation of lymphocyte response by hormones. Acta Eur. Fertil. 1983, 14, 197–201. [Google Scholar] [PubMed]
- Zenclussen, A.C.; Gentile, T.; Kortebani, G.; Mazzolli, A.; Margni, R. Asymmetric antibodies and pregnancy. Am. J. Reprod. Immunol. 2001, 45, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Allahbadia, G. Recombinant or urinary human chorionic gonadotropin in ovulation induction? J. Obstet. Gynaecol. India 2011, 61, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Van Dorsselaer, A.; Carapito, C.; Delalande, F.; Schaeffer-Reiss, C.; Thierse, D.; Diemer, H.; McNair, D.S.; Krewski, D.; Cashman, N.R. Detection of prion protein in urine-derived injectable fertility products by a targeted proteomic approach. PLoS ONE 2011, 6, e17815. [Google Scholar] [CrossRef] [PubMed]
- Shirshev, S.V. Molecular mechanisms of immunomodulating effect of chorionic gonadotropin on T- and B-lymphocytes of intact spleen. Biochem. Mosc. 1997, 62, 514–522. [Google Scholar]
- Shirshev, S.V.; Orlova, E.G.; Zamorina, S.A.; Nekrasova, I.V. Hormonal regulation of thymic-stage differentiation of IL-17-producing and T-regulatory lymphocytes. Dokl. Biol. Sci. Proc. Acad. Sci. USSR Biol. Sci. Sect. 2014, 454, 65–68. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schumacher, A. Human Chorionic Gonadotropin as a Pivotal Endocrine Immune Regulator Initiating and Preserving Fetal Tolerance. Int. J. Mol. Sci. 2017, 18, 2166. https://doi.org/10.3390/ijms18102166
Schumacher A. Human Chorionic Gonadotropin as a Pivotal Endocrine Immune Regulator Initiating and Preserving Fetal Tolerance. International Journal of Molecular Sciences. 2017; 18(10):2166. https://doi.org/10.3390/ijms18102166
Chicago/Turabian StyleSchumacher, Anne. 2017. "Human Chorionic Gonadotropin as a Pivotal Endocrine Immune Regulator Initiating and Preserving Fetal Tolerance" International Journal of Molecular Sciences 18, no. 10: 2166. https://doi.org/10.3390/ijms18102166