hCG and Its Disruption by Environmental Contaminants during Human Pregnancy
Abstract
:1. Introduction
2. The hCG Molecules
3. Major Roles of hCG during Pregnancy
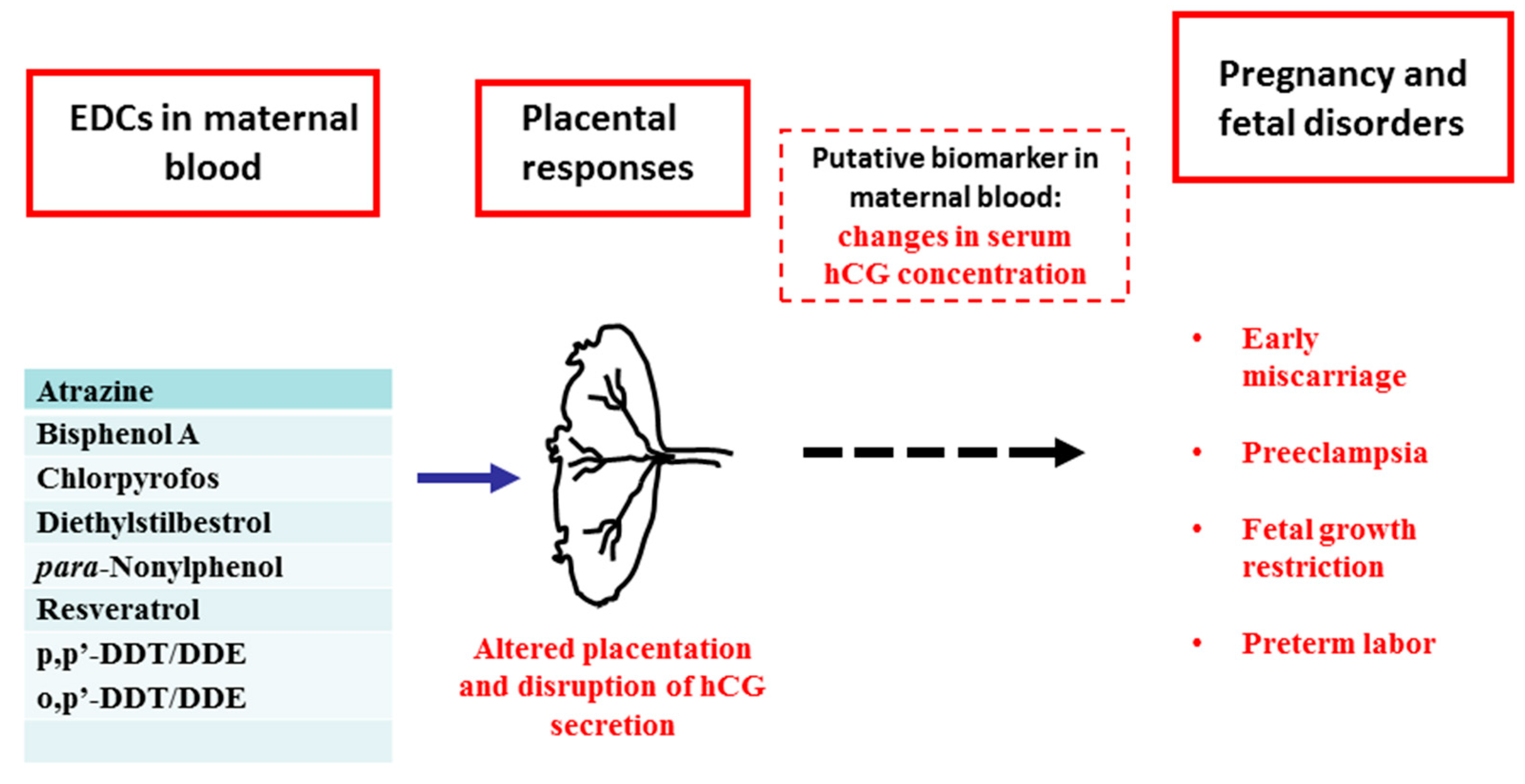
4. Environmental Contaminants in Human Pregnancy: Implications for the Fetus
5. In Vitro Effects of Selected EDCs on Human Placenta
6. EDCs and hCG
7. hCG as a Potential Biomarker of EDCs Action in Human Pregnancy
8. Concluding Remarks
Author Contributions
Conflicts of Interest
References
- Rao, C.V. Multiple non-traditional promising therapies with human chorionic gonadotropin. Austin J. Obstet. Gynecol. 2016, 3, 1066. [Google Scholar]
- Sirikunalai, P.; Wanapirak, C.; Sirichotiyakul, S.; Tongprasert, F.; Srisupundit, K.; Luewan, S.; Traisrisilp, K.; Tongsong, T. Associations between maternal serum free beta human chorionic gonadotropin (β-hCG) levels and adverse pregnancy outcomes. J. Obstet. Gynaecol. 2016, 36, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Palomaki, G.E.; Neveux, L.M.; Haddow, J.E.; Wyatt, P. Hyperglycosylated-hCG (h-hCG) and Down syndrome screening in the first and second trimesters of pregnancy. Prenat. Diagn. 2007, 27, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Bahado-Singh, R.O.; Oz, A.U.; Kingston, J.M.; Shahabi, S.; Hsu, C.D.; Cole, L. The role of hyperglycosylated hCG in trophoblast invasion and the prediction of subsequent pre-eclampsia. Prenat. Diagn. 2002, 22, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Norris, W.; Nevers, T.; Sharma, S.; Kalkunte, S. Review: hCG, preeclampsia and regulatory T cells. Placenta 2011, 32 (Suppl. 2), S182–S185. [Google Scholar] [CrossRef] [PubMed]
- Barjaktarovic, M.; Korevaar, T.I.; Jaddoe, V.W.; de Rijke, Y.B.; Visser, T.J.; Peeters, R.P.; Steegers, E.A. Human chorionic gonadotropin (hCG) concentrations during the late first trimester are associated with fetal growth in a fetal sex-specific manner. Eur. J. Epidemiol. 2017, 32, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Stevens, F.T.; Katzorke, N.; Tempfer, C.; Kreimer, U.; Bizjak, G.I.; Fleisch, M.C.; Fehm, T.N. Gestational Trophoblastic Disorders: An Update in 2015. Geburtshilfe Frauenheilkd 2015, 75, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V. Inclusion of human chorionic gonadotropin in the family of therapeutic glycoproteins. Women Health Open J. 2017, 3, 30–35. [Google Scholar] [CrossRef]
- Fournier, T. Human chorionic gonadotropin: Different glycoforms and biological activity depending on its source of production. Ann. Endocrinol. 2016, 77, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Dufau, M.L. The luteinizing hormone receptor. Annu. Rev. Physiol. 1998, 60, 461–496. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E. Physiology and Pathology of the Female Reproductive Axis. In Williams Textbook of Endocrinology, 12th ed.; Melmed, S., Polonsky, K.S., Larsen, P., Kronenberg, H.M., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2011; pp. 581–660. [Google Scholar]
- Filicori, M.; Fazleabas, A.T.; Huhtaniemi, I.; Licht, P.; Rao, Ch.V.; Tesarik, J.; Zygmunt, M. Novel concepts of human chorionic gonadotropin: Reproductive system interactions and potential in the management of infertility. Fertil. Steril. 2005, 84, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Smitz, J. Luteinizing hormone and human chorionic gonadotropin: Origins of difference. Mol. Cell. Endocrinol. 2014, 383, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Casarini, L.; Lispi, M.; Longobardi, S.; Milosa, F.; La Marca, A.; Tagliasacchi, D.; Pignatti, E.; Simoni, M. LH and hCG action on the same receptor results in quantitatively and qualitatively different intracellular signalling. PLoS ONE 2012, 7, e46682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccetti, L.; Yvinec, R.; Klett, D.; Gallay, N.; Combarnous, Y.; Reiter, E.; Simoni, M.; Casarini, L.; Ayoub, M.A. Human Luteinizing Hormone and Chorionic Gonadotropin Display Biased Agonism at the LH and LH/CG Receptors. Sci. Rep. 2017, 7, 940. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.A. hCG, the wonder of today’s science. Reprod. Biol. Endocrinol. 2012, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Fournier, T.; Guibourdenche, J.; Evain-Brion, D. Review: HCGs: Different sources of production, different glycoforms and functions. Placenta 2015, S60–S65. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Smitz, J. Luteinizing hormone and human chorionic gonadotropin: Distinguishing unique physiologic roles. Gynecol. Endocrinol. 2014, 30, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Ticconi, C.; Zicari, A.; Belmonte, A.; Realacci, M.; Rao, Ch.V.; Piccione, E. Pregnancy-Promoting Actions of HCG in Human Myometrium and Fetal Membranes. Placenta 2007, 28, S137–S143. [Google Scholar] [CrossRef] [PubMed]
- Ticconi, C.; Piccione, E.; Belmonte, A.; Rao, Ch.V. HCG—A new kid on the block in prematurity preven. J. Matern.-Fetal Neonatal Med. 2006, 19, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Ambrus, G.; Rao, C.V. Novel regulation of pregnant human myometrial smooth muscle cell gap junctions by human chorionic gonadotropin. Endocrinology 1994, 135, 2772. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, M.; Herr, F.; Keller-Schoenwetter, S.; Kunzi-Rapp, K.; Münstedt, K.; Rao, C.V.; Lang, U.; Preissner, K.T. Characterization of human chorionic gonadotropin as a novel angiogenic factor. J. Clin. Endocrinol. Metab. 2002, 87, 5290–5296. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V.; Li, X.; Toth, P.; Lei, Z.M.; Cook, V.D. Novel expression of functional human chorionic gonadotropin/luteinizing hormone receptor in human umbilical cords. J. Clin. Endocrinol. Metab. 1993, 77, 1706–1714. [Google Scholar] [PubMed]
- Akoum, A.; Metz, C.N.; Morin, M. Marked increase in macrophage migration inhibitory factor synthesis and secretion in human endometrial cells in response to human chorionic gonadotropin hormone. J. Clin. Endocrinol. Metab. 2005, 90, 2904–2910. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.M.; Yang, M.; Li, X.; Takikawa, O.; Rao, C.V. Upregulation of placental indoleamine 2,3-dioxygenase by human chorionic gonadotropin. Biol. Reprod. 2007, 76, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A.; Heinze, K.; Witte, J.; Poloski, E.; Linzke, N.; Woidacki, K.; Zenclussen, A.C. Human chorionic gonadotropin as a central regulator of pregnancy immune tolerance. J. Immunol. 2013, 190, 2650–2658. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A.; Brachwitz, N.; Sohr, S.; Engeland, K.; Langwisch, S.; Dolaptchieva, M.; Alexander, T.; Taran, A.; Malfertheiner, S.F.; Costa, S.D.; et al. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J. Immunol. 2009, 182, 5488–5497. [Google Scholar] [CrossRef] [PubMed]
- Poloski, E.; Oettel, A.; Ehrentraut, S.; Luley, L.; Costa, S.D.; Zenclussen, A.C.; Schumacher, A. JEG-3 Trophoblast Cells Producing Human Chorionic Gonadotropin Promote Conversion of Human CD4+FOXP3− T Cells into CD4+FOXP3+ Regulatory T Cells and Foster T Cell Suppressive Activity. Biol. Reprod. 2016, 94, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A. Human Chorionic Gonadotropin as a Pivotal Endocrine Immune Regulator Initiating and Preserving Fetal Tolerance. Int. J. Mol. Sci. 2017, 18, 2166. [Google Scholar] [CrossRef] [PubMed]
- Furcron, A.-E.; Romero, R.; Mial, T.N.; Balancio, A.; Panaitescu, B.; Hassan, S.S.; Sahi, A.; Nord, C.; Gomez-Lopez, N. Human Chorionic Gonadotropin Has Anti-Inflammatory Effects at the Maternal-Fetal Interface and Prevents Endotoxin-Induced Preterm Birth, but Causes Dystocia and Fetal Compromise in Mice. Biol. Reprod. 2016, 94, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.A.; Lei, Z.M.; Li, X.; Greenwold, N.; Nakajima, S.T.; Jauniaux, E.; Rao, C.V. Human fetal nongonadal tissues contain human chorionic gonadotropin/luteinizing hormone receptors. J. Clin. Endocrinol. Metab. 2004, 89, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Riccetti, L.; De Pascali, F.; Gilioli, L.; Potì, F.; Giva, L.B.; Marino, M.; Tagliavini, S.; Trenti, T.; Fanelli, F.; Mezzullo, M.; et al. Human LH and hCG stimulate differently the early signalling pathways but result in equal testosterone synthesis in mouse Leydig cells in vitro. Reprod. Biol. Endocrinol. 2017, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Lei, Z.; Rao, C.; Lin, J. Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology 1993, 132, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, J.; Bhattacharyya, S.; Das, C. Regulation of progesterone secretion in human syncytiotrophoblast in culture by human chorionic gonadotropin. J. Steroid Biochem. Mol. Biol. 1992, 42, 425–432. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Chaudhary, J.; Das, C. Antibodies to hCG inhibit progesterone production from human syncytiotrophoblast cells. Placenta 1992, 13, 135–139. [Google Scholar] [CrossRef]
- Berndt, S.; Blacher, S.; Munaut, C.; Detilleux, J.; Perrier d’Hauterive, S.; Huhtaniemi, I.; Evain-Brion, D.; Noël, A.; Fournier, T.; Foidart, J.M. Hyperglycosylated human chorionic gonadotropin stimulates angiogenesis through TGF-receptor activation. FASEB J. 2013, 27, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Mallozzi, M.; Bordi, G.; Garo, C.; Caserta, D. The effect of maternal exposure to endocrine disrupting chemicals on fetal and neonatal development: A review on the major concerns. Birth Defects Res. C Embryo Today 2016, 108, 224–242. [Google Scholar] [CrossRef] [PubMed]
- Tomza-Marciniak, A.; Stępkowska, P.; Kuba, J.; Pilarczyk, B. Effect of bisphenol A on reproductive processes: A review of in vitro, in vivo and epidemiological studies. J. Appl. Toxicol. 2018, 38, 51–80. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.E.; Chandsawangbhuwana, C. 3D models of MBP, a biologically active metabolite of bisphenol A, in human estrogen receptor α and estrogen receptor β. PLoS ONE 2012, 7, e46078. [Google Scholar] [CrossRef] [PubMed]
- Bouskine, A.; Nebout, M.; Brücker-Davis, F.; Benahmed, M.; Fenichel, P. Low doses of bisphenol A promote human seminoma cell proliferation by activating PKA and PKG via a membrane G-protein-coupled estrogen receptor. Environ. Health Perspect. 2009, 117, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol, A. Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.; Cipelli, R.; Guralnik, J.; Ferrucci, L.; Bandinelli, S.; Corsi, A.M.; Money, C.; McCormack, P.; Melzer, D. Daily bisphenol A excretion and associations with sex hormone concentrations: Results from the InCHIANTI adult population study. Environ. Health Perspect. 2010, 118, 1603–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schönfelder, G.; Wittfoht, W.; Hopp, H.; Talsness, C.E.; Paul, M.; Chahoud, I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 2002, 110, A703–A707. [Google Scholar] [CrossRef] [PubMed]
- Ikezuki, Y.; Tsutsumi, O.; Takai, Y.; Kamei, Y.; Taketani, Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum. Reprod. 2002, 17, 2839–2841. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Eriksson, J.G.; Forsén, T.; Osmond, C. Fetal origins of adult disease: Strength of effects and biological basis. Int. J. Epidemiol. 2002, 3, 1235–1239. [Google Scholar] [CrossRef]
- Barker, D.J. In utero programming of chronic disease. Clin. Sci. 1998, 95, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Magdalena, P.; Quesada, I.; Nadal, Á. Prenatal Exposure to BPA and Offspring Outcomes: The Diabesogenic Behavior of BPA. Dose Response 2015, 3, 1559325815590395. [Google Scholar] [CrossRef] [PubMed]
- Peretz, J.; Vrooman, L.; Ricke, W.A.; Hunt, P.A.; Ehrlich, S.; Hauser, R.; Padmanabhan, V.; Taylor, H.S.; Swan, S.H.; VandeVoort, C.A.; et al. Bisphenol a and reproductive health: Update of experimental and human evidence, 2007–2013. Environ. Health Perspect. 2014, 122, 775–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palanza, P.; Nagel, S.C.; Parmigiani, S.; Vom Saal, F.S. Perinatal exposure to endocrine disruptors: Sex, timing and behavioral endpoints. Curr. Opin. Behav. Sci. 2016, 7, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Mallozzi, M.; Leone, C.; Manurita, F.; Bellati, F.; Caserta, D. Endocrine Disrupting Chemicals and Endometrial Cancer: An Overview of Recent Laboratory Evidence and Epidemiological Studies. Int. J. Environ. Res. Public Health 2017, 22, 14. [Google Scholar] [CrossRef] [PubMed]
- Vähäkangas, K.; Myllynen, P. Drug transporters in the human blood-placental barrier. Br. J. Pharmacol. 2009, 158, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Bechi, N.; Ietta, F.; Romagnoli, R.; Focardi, S.; Corsi, I.; Buffi, C.; Paulesu, L. Estrogen-like response to p-nonylphenol in human first trimester placenta and BeWo choriocarcinoma cells. Toxicol. Sci. 2006, 93, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, C.; Ietta, F.; Avanzati, A.M.; Skarzynski, D.; Paulesu, L. Biological Tools to Study the Effects of Environmental Contaminants at the Feto-Maternal Interface. Dose Response 2015, 13, 1559325815611902. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.S.; Bisphenol, A. an endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Mørck, T.J.; Sorda, G.; Bechi, N.; Rasmussen, B.S.; Nielsen, J.B.; Ietta, F.; Rytting, E.; Mathiesen, L.; Paulesu, L.; Knudsen, L.E. Placental transport and in vitro effects of Bisphenol, A. Reprod. Toxicol. 2010, 30, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Spagnoletti, A.; Paulesu, L.; Mannelli, C.; Ermini, L.; Romagnoli, R.; Cintorino, M.; Ietta, F. Low concentrations of Bisphenol A and para-Nonylphenol affect extravillous pathway of human trophoblast cells. Mol. Cell. Endocrinol. 2015, 412, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, C.; Guan, H.; Langlois, D.; Cernea, M.; Yang, K. Bisphenol A disrupts gene expression in human placental trophoblast cells. Reprod. Toxicol. 2015, 53, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.M.; Justicia, H.; Wray, J.W.; Sonnenschein, C. p-Nonylphenol: An estrogenic xenobiotic released from “modified” polystyrene. Environ. Health Perspect. 1991, 92, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.; Filis, P.; Soffientini, U.; Bellingham, M.; O’Shaughnessy, P.J.; Fowler, P.A. Placental transporter localization and expression in the Human: The importance of species, sex, and gestational age differences. Biol. Reprod. 2017, 96, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Sieppi, E.; Vähäkangas, K.; Rautio, A.; Ietta, F.; Paulesu, L.; Myllynen, P. The xenoestrogens, bisphenol A and para-nonylphenol, decrease the expression of the ABCG2 transporter protein in human term placental explant cultures. Mol. Cell. Endocrinol. 2016, 429, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Inoue, K.; Sakui, N.; Ito, R.; Izumi, S.; Makino, T.; Okanouchi, N.; Nakazawa, H. Stir bar sorptive extraction and thermal desorption-gas chromatography-mass spectrometry for the measurement of 4-nonylphenol and 4-tert-octylphenol in human biological samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 799, 119–125. [Google Scholar] [CrossRef]
- Bechi, N.; Sorda, G.; Spagnoletti, A.; Bhattacharjee, J.; Vieira Ferro, E.A.; de Freitas Barbosa, B.; Frosini, M.; Valoti, M.; Sgaragli, G.; Paulesu, L.; et al. Toxicity assessment on trophoblast cells for some environment polluting chemicals and 17β-estradiol. Toxicol. In Vitro 2013, 27, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, C.; Ietta, F.; Carotenuto, C.; Romagnoli, R.; Szostek, A.Z.; Wasniewski, T.; Skarzynski, D.J.; Paulesu, L. Bisphenol A alters β-hCG and MIF release by human placenta: An in vitro study to understand the role of endometrial cells. Mediators Inflamm. 2014, 635364. [Google Scholar] [CrossRef]
- Ridano, M.E.; Racca, A.C.; Flores-Martin, J.B.; Fretes, R.; Bandeira, C.L.; Reyna, L.; Bevilacqua, E.; Genti-Raimondi, S.; Panzetta-Dutari, G.M. Impact of chlorpyrifos on human villous trophoblasts and chorionic villi. Toxicol. Appl. Pharmacol. 2017, 329, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, A.K.; Augustowska, K.; Gregoraszczuk, E.L. The short- and long-term effects of two isomers of DDT and their metabolite DDE on hormone secretion and survival of human choriocarcinoma JEG-3 cells. Pharmacol. Rep. 2007, 59, 224–232. [Google Scholar] [PubMed]
- Peng, F.; Ji, W.; Zhu, F.; Peng, D.; Yang, M.; Liu, R.; Pu, Y.; Yin, L. A study on phthalate metabolites, bisphenol A and nonylphenol in the urine of Chinese women with unexplained recurrent spontaneous abortion. Environ. Res. 2016, 150, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Lathi, R.B.; Liebert, C.A.; Brookfield, K.F.; Taylor, J.A.; vom Saal, F.S.; Fujimoto, V.Y.; Baker, V.L. Conjugated bisphenol A in maternal serum in relation to miscarriage risk. Fertil. Steril. 2014, 102, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Cantonwine, D.E.; Ferguson, K.K.; Mukherjee, B.; McElrath, T.F.; Meeker, J.D. Urinary Bisphenol A levels during pregnancy and risk of preterm birth. Environ. Health Perspect. 2015, 123, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Behnia, F.; Peltier, M.; Getahun, D.; Watson, C.; Saade, G.; Menon, R. High bisphenol A (BPA) concentration in the maternal, but not fetal, compartment increases the risk of spontaneous preterm delivery. J. Matern. Fetal Neonatal Med. 2016, 29, 3583–3589. [Google Scholar] [CrossRef] [PubMed]
- Troisi, J.; Mikelson, C.; Richards, S.; Symes, S.; Adair, D.; Zullo, F.; Guida, M. Placental concentrations of bisphenol A and birth weight from births in the Southeastern, U.S. Placenta 2014, 35, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, F.; Dubois, M.F.; Aris, A. Maternal, placental and fetal exposure to bisphenol A in women with and without preeclampsia. Hypertens. Pregnancy 2014, 33, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhou, Q.; Feng, L.; Xiong, Y.; Li, X. Maternal serum bisphenol A levels and risk of pre-eclampsia: A nested case-control study. Eur. J. Public Health 2017, 27, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.K.; McElrath, T.F.; Cantonwine, D.E.; Mukherjee, B.; Meeker, J.D. Phthalate metabolites and bisphenol-A in association with circulating angiogenic biomarkers across pregnancy. Placenta 2015, 36, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.K.; Cantonwine, D.E.; McElrath, T.F.; Mukherjee, B.; Meeker, J.D. Repeated measures analysis of associations between urinary bisphenol-A concentrations and biomarkers of inflammation and oxidative stress in pregnancy. Reprod. Toxicol. 2016, 66, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Albaladejo, E.; Fernandes, D.; Lacorte, S.; Porte, C. Comparative toxicity, oxidative stress and endocrine disruption potential of plasticizers in JEG-3 human placental cells. Toxicol. In Vitro 2017, 38, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, S.; Williams, P.L.; Missmer, S.A.; Flaws, J.A.; Berry, K.F.; Calafat, A.M.; Ye, X.; Petrozza, J.C.; Wright, D.; Hauser, R. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ. Health Perspect. 2012, 120, 978–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braunstein, G.D.; Karow, W.G.; Gentry, W.C.; Rasor, J.; Wadw, M.E. First trimester chorionic gonadotrophin measurements as an aid in the diagnosis of early pregnancy disorders. Am. J. Obstet. Gynecol. 1978, 131, 25–32. [Google Scholar] [CrossRef]
- Goetzl, L.; Krantz, D.; Simpson, J.L.; Silver, R.K.; Zachary, J.M.; Pergament, E.; Platt, L.D.; Mahoney, M.J.; Wapner, R.J. Pregnancy-associated plasma protein A. free beta-hCG, nucal translucency, and risk of pregnancy loss. Obstet. Gynecol. 2004, 104, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Tuuli, M.G.; Odibo, A.O. The role of serum markers and uterine artery Doppler in identifying at-risk pregnancies. Clin. Perinatol. 2011, 38, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Peter Bonde, J.P.; Meulengracht Flachs, E.; Rimborg, S.; Glazer, C.H.; Giwercman, A.; Høst Ramlau-Hansen, C.; Sørig Hougaard, K.; Bjerre Høyer, B.; Keglberg Hærvig, K.; Bondo Petersen, S.; et al. The epidemiologic evidence linking prenatal and postnatal exposure to endocrine disrupting chemicals with male reproductive disorders: A systematic review and meta-analysis. Hum. Reprod. Update 2017, 23, 104–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Padula, A.; Sirota, M.; Woodruff, T.J. Environmental influences on reproductive health: The importance of chemical exposures. Fertil. Steril. 2016, 106, 905–929. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, J.; Binder, A.M.; McElrath, T.F.; Michels, K.B. First-Trimester Urine Concentrations of Phthalate Metabolites and Phenols and Placenta miRNA Expression in a Cohort of U.S. Women. Environ. Health Perspect. 2016, 124, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Vrooman, L.A.; Xin, F.; Bartolomei, M.S. Morphologic and molecular changes in the placenta: What we can learn from environmental exposures. Fertil. Steril. 2016, 106, 930–940. [Google Scholar] [CrossRef] [PubMed]
| hCG Secretion | Reference | |
|---|---|---|
| Atrazine | Decrease in BeWo cells at 10 pM–1 nM | [64] |
| Increase in BeWo cells at of 0.1–1 mM | [64] | |
| BPA | Decrease in BeWo cells at 30 μM | [56] |
| Increase in primary trophoblast cells from human placenta term at 0.44, 1.1, 2.2, 4.4 or 8.8 µM | [58] | |
| Increase in placental explants from first trimester placenta at 1 or 0.5 nM | [65] | |
| Chlorpyrifos | Increase in primary trophoblast cells from human placenta at term at 50 or 100 μM | [66] |
| DES | Decrease in BeWo cells at 0.1 pM, 10 nM or 0.1 µM | [64] |
| p-NP | Increase in BeWo cells at 0.1 pM | [64] |
| Decrease in BeWo cells at 10 pM–1 nM | [64] | |
| Increase in placental explants from first trimester placenta at 1 nM | [53] | |
| Resveratrol | Decrease in BeWo cells at 0.1–1 pM | [64] |
| p,p′-DDT/DDE | Decrease in JEG3 cells at 1, 10, 100 ng/mL, 1 µg/mL after 24 h | [67] |
| o,p′-DDT/DDE | Increase in JEG3 cells at 1, 10, 100 ng/mL, 1 µg/mL after 72 h | [67] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulesu, L.; Rao, C.V.; Ietta, F.; Pietropolli, A.; Ticconi, C. hCG and Its Disruption by Environmental Contaminants during Human Pregnancy. Int. J. Mol. Sci. 2018, 19, 914. https://doi.org/10.3390/ijms19030914
Paulesu L, Rao CV, Ietta F, Pietropolli A, Ticconi C. hCG and Its Disruption by Environmental Contaminants during Human Pregnancy. International Journal of Molecular Sciences. 2018; 19(3):914. https://doi.org/10.3390/ijms19030914
Chicago/Turabian StylePaulesu, Luana, Ch.V. Rao, Francesca Ietta, Adalgisa Pietropolli, and Carlo Ticconi. 2018. "hCG and Its Disruption by Environmental Contaminants during Human Pregnancy" International Journal of Molecular Sciences 19, no. 3: 914. https://doi.org/10.3390/ijms19030914
APA StylePaulesu, L., Rao, C. V., Ietta, F., Pietropolli, A., & Ticconi, C. (2018). hCG and Its Disruption by Environmental Contaminants during Human Pregnancy. International Journal of Molecular Sciences, 19(3), 914. https://doi.org/10.3390/ijms19030914




