Overexpression of a New Chitinase Gene EuCHIT2 Enhances Resistance to Erysiphe cichoracearum DC. in Tobacco Plants
Abstract
:1. Introduction
2. Results
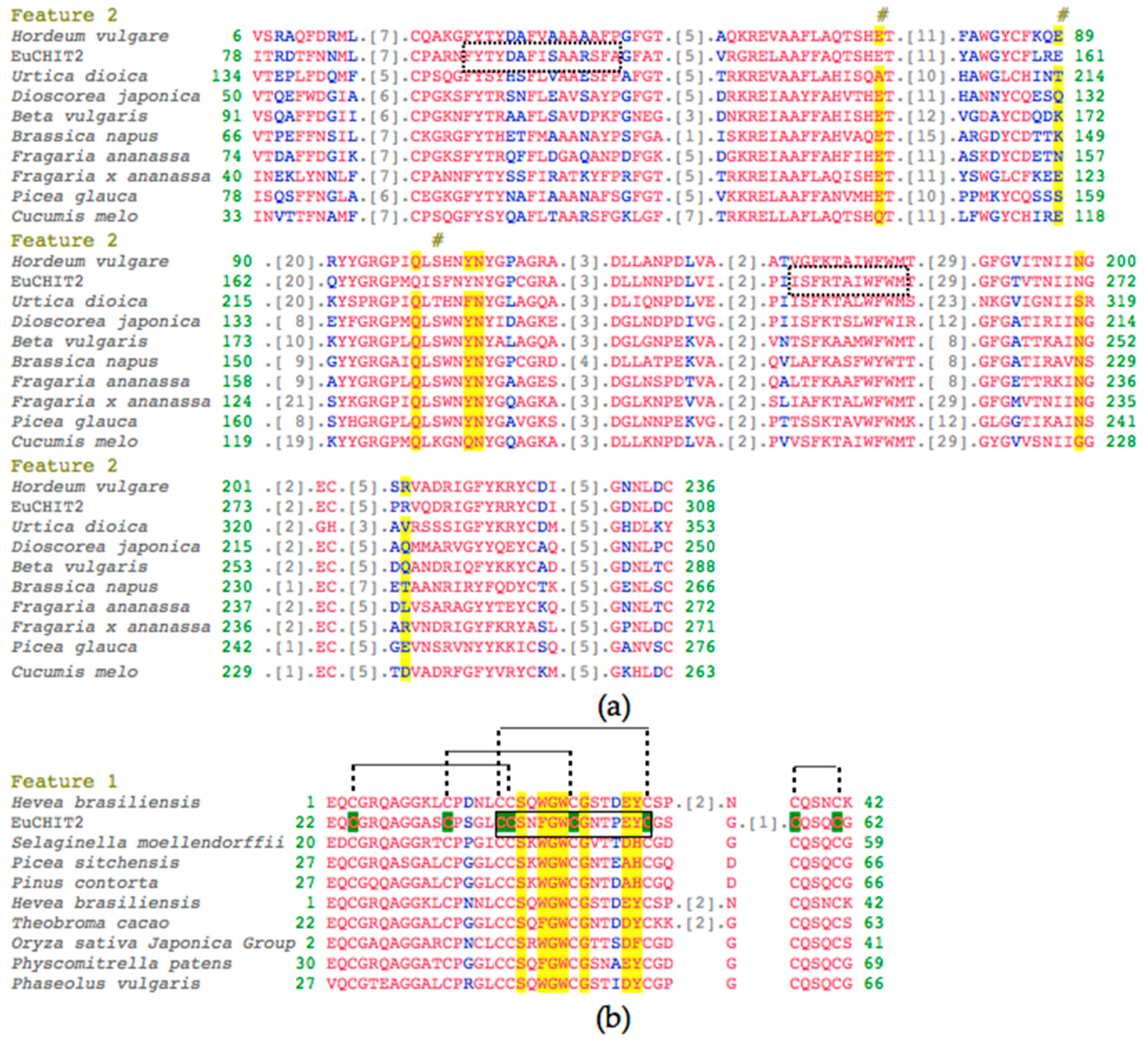
2.1. Cloning and Characterization of EuCHIT2
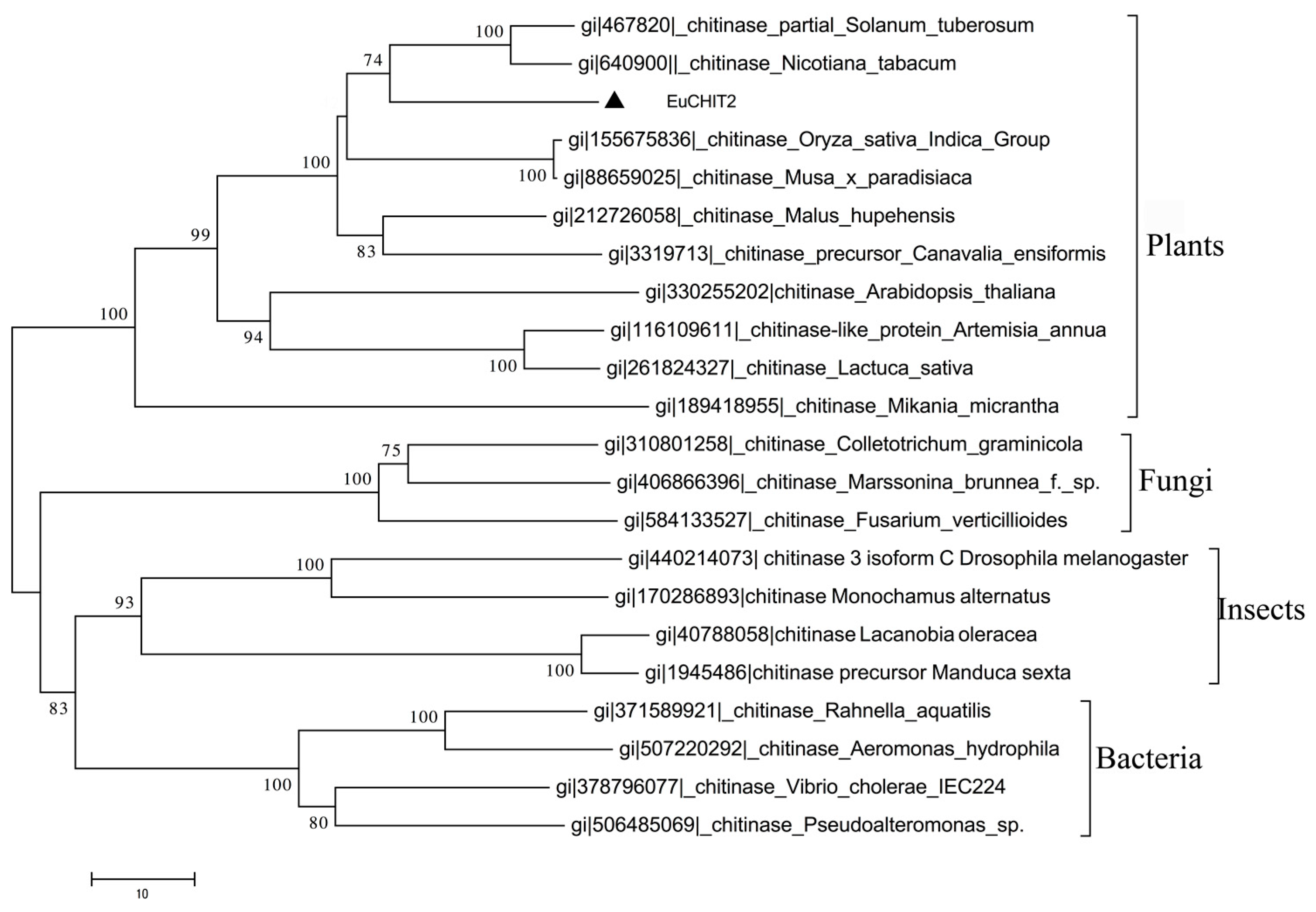
2.2. Phylogenetic Analysis
2.3. Verification of Vectors and Transgenic Tobacco Plants
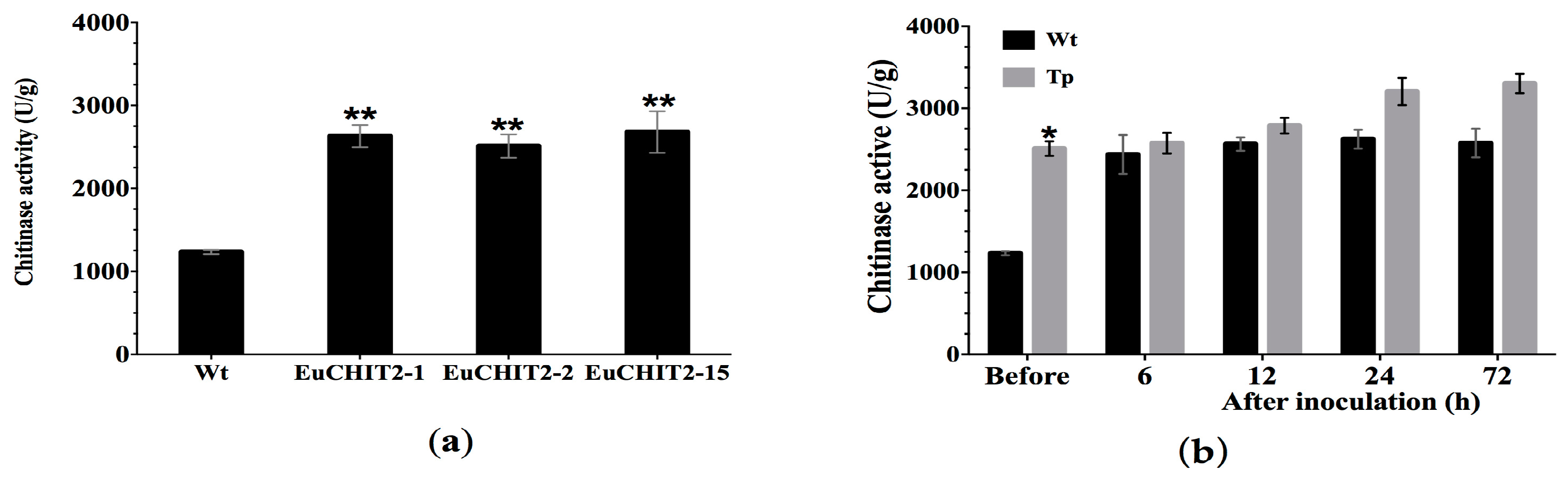
2.4. Chitinase Activity in the Transgenic Tobacco Lines
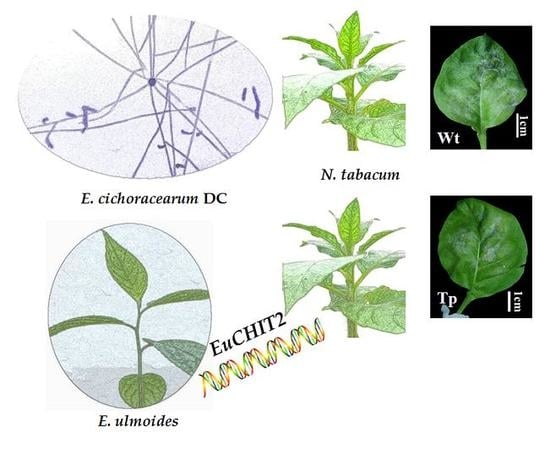
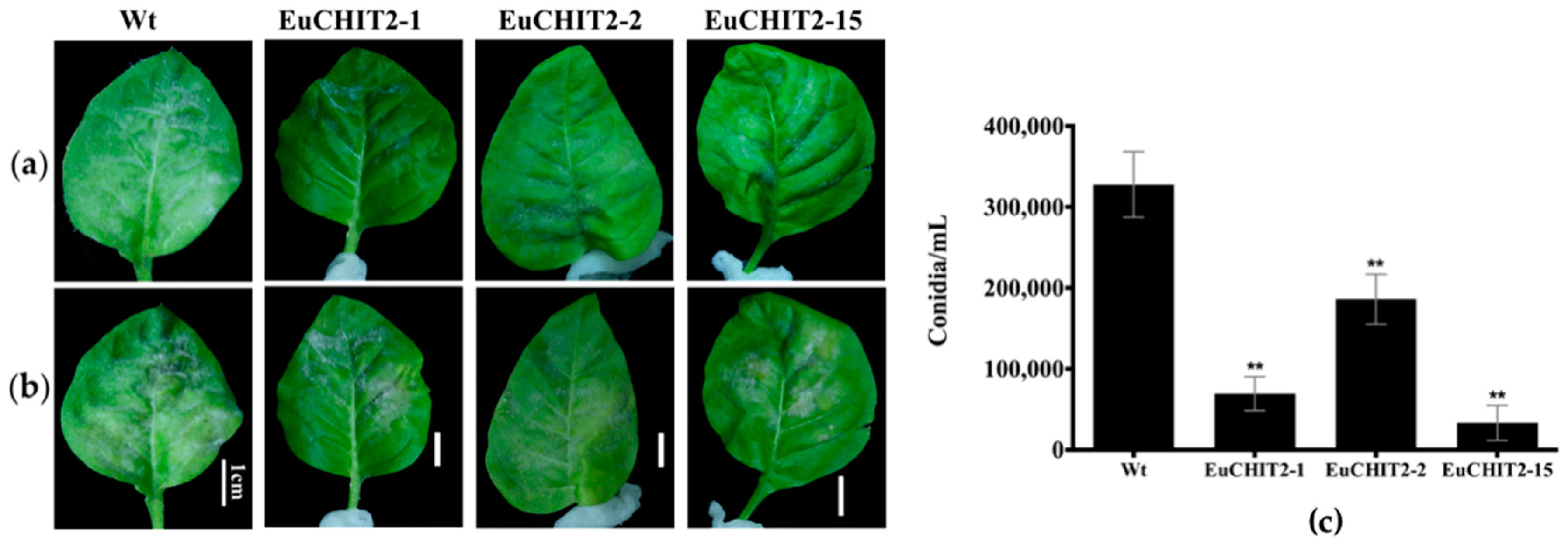
2.5. Disease Resistance Assay with E. cichoracearum DC.
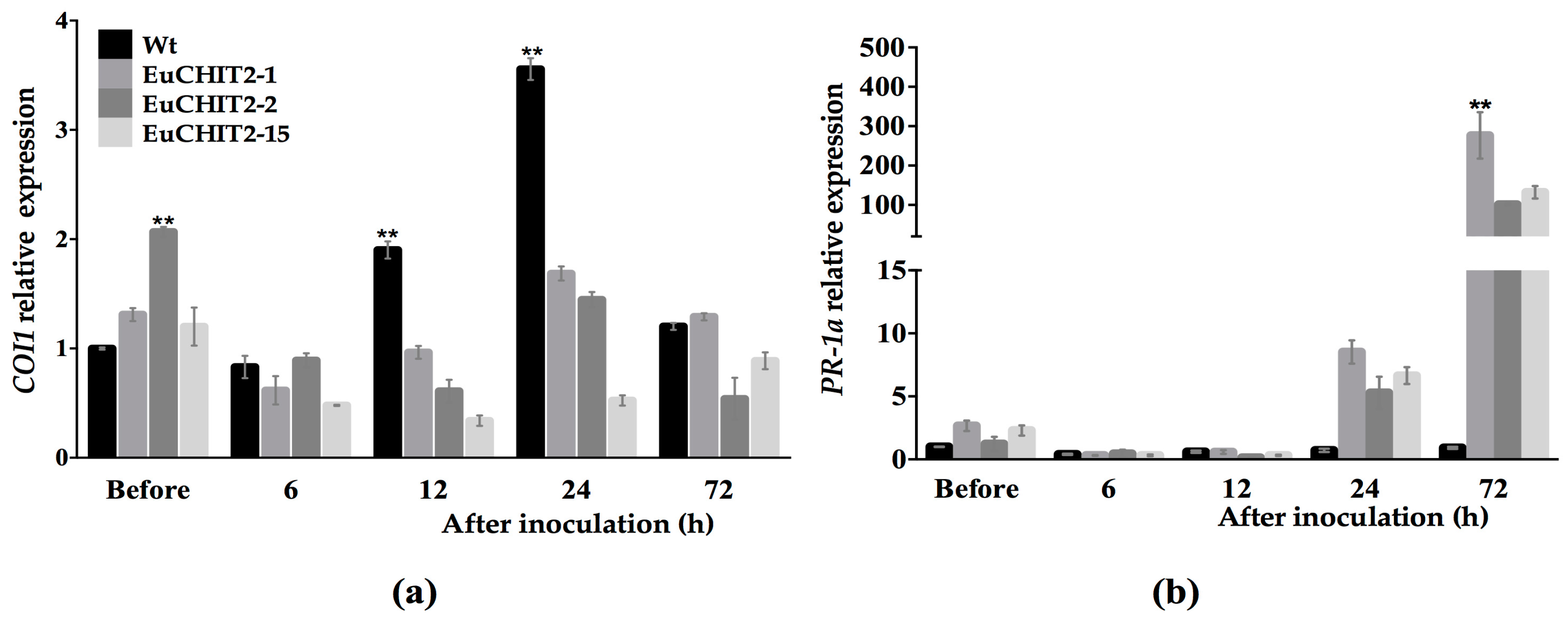
2.6. The Synergistic Effects of EuCHIT2 on E. cichoracearum DC. Resistance-Related Genes Expression
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction Total RNA
4.3. Rapid Amplification of cDNA Ends
4.4. Bioinformatics Analysis of EuCHIT2
4.5. Construction of Plant Overexpression Vector
4.6. Genetic Transformation and Identification of Transgenic Plants
4.7. Analysis of the Chitinase Activity
4.8. Analysis of Pathogen Resistance
4.9. Determination of Physicochemical Indicators
4.10. Analysis of Gene Expression
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| E. ulmoides | Eucommia ulmoides oliver |
| RACE | Rapid amplification of cDNA ends |
| E. cichoracearum DC. | Erysiphe cichoracearum DC. |
| POD | Peroxidase |
| MDA | Malondialdehyde |
| SA | Salicylic acid |
| JA | Jasmonic acid |
| PR-1a | Pathogenesis-related gene 1 |
| COI1 | Coronatine-insensitive 1 |
| GlcNAc | N-acetylglucosamine |
| SAR | Systemic acquired resistance |
| ISR | Induce systemic resistance |
| ORF | Open reading frame |
| aa | Amino acid |
References
- Wang, J.L.; Liao, X.R.; Zhang, H.M.; Du, J.F.; Chen, P.L. Accumulation of chlorogenic acid in cell suspension cultures of Eucommia ulmoides. Plant Cell Tiss. Org. Cult. 2003, 74, 193–195. [Google Scholar] [CrossRef]
- Deyama, T.; Nishibe, S.; Nakazawa, Y. Constituents and pharmacological effects of Eucommia and Siberian ginseng. Acta Pharmacol. Sin. 2001, 22, 1057–1070. [Google Scholar] [PubMed]
- Huang, R.H.; Xiang, Y.; Liu, X.Z.; Zhang, Y.; Hu, Z.; Wang, D.C. Two novel antifungal peptides distinct with a five-disulfide motif from the bark of Eucommia ulmoides Oliv. FEBS Lett. 2002, 521, 87–90. [Google Scholar] [CrossRef]
- Broekaert, I.; Lee, H.I.; Kush, A.; Chua, N.H.; Raikhel, N. Wound-induced accumulation of mRNA containing a hevein sequence in laticifers of rubber tree (Hevea brasiliensis). Proc. Natl. Acad. Sci. USA 1990, 87, 7633–7637. [Google Scholar] [CrossRef] [PubMed]
- Tangpakdee, J.; Tanaka, Y.; Shiba, K.I.; Kawahara, S.; Sakurai, K.; Suzuki, Y. Structure and biosynthesis of transpolyisoprene from Eucommia ulmoides. Phytochemistry 1997, 45, 75–80. [Google Scholar] [CrossRef]
- Archer, B.; Audley, B.; Mc Sweeney, G.; Hong, T.C. Studies on composition of latex serum and bottom fraction particles. J. Rubber Res. Inst. Malays 1969, 21, 560–569. [Google Scholar]
- Liu, W.P.; Han, Y.Z.; Zhao, D.G. Cloning of cDNA encoding cinnamyl alcohol dehydrogenase from Eucommia ulmoides Oliv. J. Chin. Agric. Univ. 2003, 8, 27–30. [Google Scholar]
- Zhou, M.B.; Xiao, Y.H.; Zhu, D.X.; Pei, Y.; Zhao, D.G. Molecular cloning and sequence analysis of EAFPS involved into rubber biosynthesis in Eucommia ulmoides Olive. Mol. Plant Breed. 2003, 1, 66–71. [Google Scholar]
- Liu, S.H.; Zhao, D.G.; Han, Y.Z. Isolation of a novel antifungal peptide from the bark of Eucommia ulmoides Oliv. High Technol. Lett. 2008, 14, 216–219. [Google Scholar]
- Cohen-Kupiec, R.; Chet, I. The molecular biology of chitin digestion. Curr. Opin. Biotechnol. 1998, 9, 270–277. [Google Scholar] [CrossRef]
- Hamel, F.; Bellemare, G. Characterization of a class I chitinase gene and of wound-inducible, root and flower-specific chitinase expression in Brassica napus. Biochim. Biophys. Acta 1995, 1263, 212–220. [Google Scholar] [CrossRef]
- Patil, R.S.; Ghormade, V.; Deshpande, M.V. Chitinolytic enzymes: An exploration. Enzyme Microb. Technol. 2000, 26, 473–483. [Google Scholar] [CrossRef]
- Durrant, W.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Rakwal, R.; Yang, G.; Komatsu, S. Chitinase induced by jasmonic acid, methyl jasmonate, ethylene and protein phosphatase inhibitors in rice. Mol. Biol. Rep. 2004, 31, 113. [Google Scholar] [CrossRef] [PubMed]
- Thomma, B.P.; Eggermont, K.; Penninckx, I.A.; Mauch-Mani, B.; Vogelsang, R.; Cammue, B.P.; Broekaert, W.F. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA 1998, 95, 15107–15111. [Google Scholar] [CrossRef] [PubMed]
- Cole, J. Powdery mildew of tobacco (Erysiphe cichoracearum DC.). Ann. Appl. Biol. 1966, 57, 201–209. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced systemic resistance (ISR) in plants: Mechanism of action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Velazhahan, R.; Muthukrishnan, S. Transgenic tobacco plants constitutively overexpressing a rice thaumatin-like protein (PR-5) show enhanced resistance to Alternaria alternata. Biol. Plant. 2003, 47, 347–354. [Google Scholar] [CrossRef]
- Pozo, M.J.; Van Loon, L.; Pieterse, C.M. Jasmonates-signals in plant-microbe interactions. J. Plant Growth Regul. 2004, 23, 211–222. [Google Scholar]
- Collinge, D.B.; Kragh, K.M.; Mikkelsen, J.D.; Nielsen, K.K.; Rasmussen, U.; Vad, K. Plant chitinases. Plant J. 1993, 3, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Brunner, F.; Stintzi, A.; Fritig, B.; Legrand, M. Substrate specificities of tobacco chitinases. Plant J. 1998, 14, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Reyes-López, C.A.; Hernández-Santoyo, A.; Pedraza-Escalona, M.; Mendoza, G.; Hernández-Arana, A.; Rodríguez-Romero, A. Insights into a conformational epitope of Hev b 6.02 (hevein). Biochem. Biophys. Res. Commun. 2004, 314, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Henrissat, B. Classification of chitinases modules. EXS 1999, 87, 137. [Google Scholar] [PubMed]
- Beintema, J.J. Structural features of plant chitinases and chitin-binding proteins. FEBS Lett. 1994, 350, 159. [Google Scholar] [CrossRef]
- Sinha, S.; Gupta, G.; Vijayan, M.; Surolia, A. Subunit assembly of plant lectins. Curr. Opin. Struct. Biol. 2007, 17, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Kezuka, Y.; Kitazaki, K.; Itoh, Y.; Watanabe, J.; Takaha, O.; Watanabe, T.; Nishizawa, Y.; Nonaka, T. Crystallization and preliminary X-ray analysis of plant class I chitinase from rice. Protein Pept. Lett. 2004, 11, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.H.; Cao, B.; Rodriguezromero, A.; Arreguin, B. Hevein: NMR assignment and assessment of solution-state folding for the agglutinin-toxin motif. Biochemistry 1993, 32, 1407–1422. [Google Scholar] [CrossRef] [PubMed]
- Kush, A.; Goyvaerts, E.; Chye, M.L.; Chua, N.H. Laticifer-specific gene expression in Hevea brasiliensis (rubber tree). Proc. Natl. Acad. Sci. USA 1990, 87, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- De Fay, E.; Jacob, J.L. Anatomical organization of the laticiferous system in the bark. Transpl. Int. 1989, 18, 1221–1222. [Google Scholar]
- D’Auzac, J.; Jacob, J.L.; Chrestin, H. The laticiferous cell and latex a model of cytoplasm. In Physiology of Rubber Tree Latex; CRC Pres Inc.: Boca Raton, FL, USA, 1989; pp. 470–482. [Google Scholar]
- Tian, L.; Hu, Z. Study on Tissue Differentiation Eucommia ulmoides. Act. Bot. Bor. Occ. Sin. 1981, 1, 67–75. [Google Scholar]
- Cui, Y.H.; Wang, M.; Sun, K.L. Morphological study of gutta containing cells in Eucommia ulmoides Oliv. Chin. Bull. Bot. 1999, 16, 439–443. [Google Scholar]
- Nie, Q.; Gao, G.L.; Fan, Q.j.; Qiao, G.; Wen, X.P.; Liu, T.; Peng, Z.J.; Cai, Y.Q. Isolation and characterizationof a catalase gene “HuCAT3” from pitaya (Hylocereus undatus) and its expression under abiotic stress. Gene 2015, 563, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Mellersh, D.G.; Foulds, I.V.; Higgins, V.J.; Heath, M.C. H2O2 plays different roles in determining penetration failure in three diverse plant–fungal interactions. Plant J. 2002, 29, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Kästner, B.; Tenhaken, R.; Kauss, H. Chitinase in cucumber hypocotyls is induced by germinating fungal spores and by fungal elicitor in synergism with inducers of acquired resistance. Plant J. 1998, 13, 447–454. [Google Scholar] [CrossRef]
- Vad, K.; de Neergaard, E.; Madriz-Ordeñana, K.; Mikkelsen, J.D.; Collinge, D.B. Accumulation of defence-related transcripts and cloning of a chitinase mRNA from pea leaves (Pisum sativum L.) inoculated with Ascochyta pisi Lib. Plant Sci. 1993, 92, 69–79. [Google Scholar] [CrossRef]
- Garthoff, S.M.; Neuhaus, J.M.; Boller, T.; Kemmerling, B.; Kogel, K.H. Expression of β-1,3-glucanase and chitinase in healthy, stem-rust-affected and elicitor-treated near-isogenic wheat lines showing Sr5- or Sr24-specified race-specific rust resistance. Planta 1997, 201, 235–244. [Google Scholar] [CrossRef]
- Mohr, U.; LANGE, J.; Boller, T.; Wiemken, A.; LANGE, R.V. Plant defence genes are induced in the pathogenic interaction between bean roots and Fusarium solani, but not in the symbiotic interaction with the arbuscular mycorrhizal fungus Glomus mosseae. New Phytol. 1998, 138, 589–598. [Google Scholar] [CrossRef]
- Feys, B.J.; Benedetti, C.E.; Penfold, C.N.; Turner, J.G. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 1994, 6, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.X.; Feys, B.F.; James, S.; Rostro, M.N.; Turner, J.G. COI1: An Arabidopsis Gene Required for Jasmonate-Regulated Defense and Fertility. Science 1998, 280, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Chen, C.; Chen, Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol. Biol. 2003, 51, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Braun, U. A monograph of the Erysiphales (powdery mildews). Nova Hedwig. 1987, 89, 1–700. [Google Scholar]
- Zhao, D.; Li, Y.; Zhao, Y.; Zhao, D.; Lv, L.; Liu, S.; Song, L.; Dong, X.; Feng, Y. Transcriptome data assembly and gene function annotation of female and male plants in Eucommia ulmoides. J. Mt. Agric. Biol. 2015, 1, 1–12. [Google Scholar]
- Barate, A.K.; Lee, H.Y.; Jeong, H.W.; Truong, L.Q.; Joo, H.G.; Hahn, T.W. An improved multiplex PCR for diagnosis and differentiation of Mycoplasma hyopneumoniae and Mycoplasma hyorhinis. Korean J. Vet. Res. 2012, 52, 39–43. [Google Scholar]
- Bauer, A.M.; Anderson, J.B.; Cherukuri, P.F.; Scott, C.D.; Geer, L.Y.; Gwadz, M.; He, S.; Hurwitz, D.I.; Jackson, J.D.; Ke, Z. CDD: A Conserved Domain Database for protein classification. Nucleic Acids Res. 2005, 33, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.J.; Zhao, D.; Zhang, Y.; Zhao, D.G. Selectable marker-free co-expression of Nicotiana. rustica CN and Nicotiana. tabacum HAK1 genes improves resistance to tobacco mosaic virus in tobacco. Funct. Plant Biol. 2015, 42, 802–815. [Google Scholar] [CrossRef]
- Bevan, M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984, 12, 8711–8721. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Nelson, R.; Sherwood, J. Enhanced recovery of transformants of Agrobacterium tumefaciens after freeze-thaw transformation and drug selection. Biotechniques 1994, 16, 664–668. [Google Scholar] [PubMed]
- Horsch, R.; Fry, J.; Hoffmann, N.; Eichholtz, D.; Rogers, S.A.; Fraley, R. A simple and general method for transferring genes into plants. Science 1985, 227, 1229–1231. [Google Scholar]
- Qin, L.J.; Zhao, D.; Zhao, D.G. Overexpression of NrCN improved TMV resistance in selection marker-free tobacco generated by Gene-Deletor system. Plant Mol. Biol. Rep. 2015, 33, 1619–1633. [Google Scholar] [CrossRef]
- Zeng, X.F.; Li, L.; Li, J.R.; Zhao, D.G. Constitutive expression of McCHIT1-PAT enhances resistance to rice blast and herbicide, but does not affect grain yield in transgenic glutinous rice. Biotech. Appl. Biochem. 2016, 63, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Mauch, F.; Hadwiger, L.A.; Boller, T. Ethylene: Symptom, Not Signal for the Induction of Chitinase and beta-1,3-Glucanase in Pea Pods by Pathogens and Elicitors. Plant Physiol. 1984, 76, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Shimahara, K. Preparation of crustacean chitin. Method Enzymol. 1988, 161, 417–424. [Google Scholar]
- Miclot, A.S.; Merdinoglu, S.W.; Duchêne, E.; Merdinoglu, D.; Mestre, P. A standardised method for the quantitative analysis of resistance to grapevine powdery mildew. Eur. J. Plant Pathol. 2012, 133, 483–495. [Google Scholar] [CrossRef]
- Moyer, M.M.; Gadoury, D.M.; Davidson, L.C.; Dry, I.B.; Magarey, P.A.; Wilcox, W.F.; Seem, R.C. Effects of acute low-temperature events on development of Erysiphe necator and susceptibility of Vitis vinifera. Phytopathology 2010, 100, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Doukhanina, E.V.; Chen, S.; van der Zalm, E.; Godzik, A.; Reed, J.; Dickman, M.B. Identification and functional characterization of the BAG protein family in Arabidopsis thaliana. J. Biol. Chem. 2006, 281, 18793–18801. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Hu, X.; Ma, J.; Hettenhausen, C.; Wang, L.; Sun, G.; Wu, J.; Wu, J. Requirement of ABA signalling-mediated stomatal closure for resistance of wild tobacco to Alternaria alternata. Plant Pathol. 2014, 63, 1070–1077. [Google Scholar] [CrossRef]
- Ling, P.; Zhang, X.; Wang, J.; Xiao, M.; Zhou, M.; Huang, L.K.; Gang, N.; Wang, P.; Yang, Z.; Ji, L. Transcriptional Profiles of Drought-Related Genes in Modulating Metabolic Processes and Antioxidant Defenses in Lolium multiflorum. Front. Plant Sci. 2016, 7, 519. [Google Scholar]
- Feeney, B.C. A Simple Guide to IBM SPSS Statistics for Version 20.0; Wadsworth Publishing: Belmont, CA, USA, 2012. [Google Scholar]

| Use | Name | Sequence (5′–3′) |
|---|---|---|
| RACE | EuCHIT2-GSP1 | CAGGCCATAGCTAGTGCTGATGTTG |
| EuCHIT2-GSP2 | CCCGCCCGGAACTTCTACACCTACG | |
| Full-length | EuCHIT2-XbaI-F | GCTCTAGAGCAAATGAGGTTTACCTTGTCCACTCTCC |
| EuCHIT2-EcoRI-R | CGGAATTCCGGACTACTGGGCCTGAACTAAAAGCC | |
| Verification | psH-35s-F | TCGTCAACATGGTGGAGCACGAC |
| TEuCHIT2-R | CGAATCCGGCGAAAGATCTG | |
| Internal control | Actin-F | TGGTTAAGGCTGGATTTGCT |
| Actin-R | TGCATCCTTTGACCCATAC | |
| Expression Analysis | PR-1a-F | ACAGCTCGTGCAGATGTAGGT |
| PR-1a-R | GCTAGGTTTTCGCCGTATTG | |
| COI1-F | GTTGTAGCCAGTGAGGGAAATA | |
| COI1-R | TTGCCCAGCAAGAGAATAGTAG |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Zhao, Y.; Ran, X.; Guo, L.; Zhao, D.-G. Overexpression of a New Chitinase Gene EuCHIT2 Enhances Resistance to Erysiphe cichoracearum DC. in Tobacco Plants. Int. J. Mol. Sci. 2017, 18, 2361. https://doi.org/10.3390/ijms18112361
Dong X, Zhao Y, Ran X, Guo L, Zhao D-G. Overexpression of a New Chitinase Gene EuCHIT2 Enhances Resistance to Erysiphe cichoracearum DC. in Tobacco Plants. International Journal of Molecular Sciences. 2017; 18(11):2361. https://doi.org/10.3390/ijms18112361
Chicago/Turabian StyleDong, Xuan, Yichen Zhao, Xin Ran, Linxia Guo, and De-Gang Zhao. 2017. "Overexpression of a New Chitinase Gene EuCHIT2 Enhances Resistance to Erysiphe cichoracearum DC. in Tobacco Plants" International Journal of Molecular Sciences 18, no. 11: 2361. https://doi.org/10.3390/ijms18112361