Regulation of the Rhythmic Emission of Plant Volatiles by the Circadian Clock
Abstract
:1. Introduction
2. Techniques for the Collection and Detection of Plant Volatiles
3. Plant Volatiles Showing Circadian Rhythmic Emission
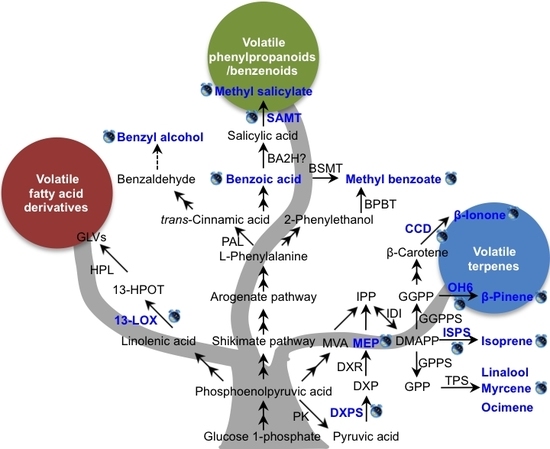
4. Mechanisms of Circadian Rhythmic Emission of Plant Volatiles
5. Potential Impact of Other Factors on Circadian Rhythmic Emission of Plant Volatiles
6. Concluding Remarks and Perspectives
- 1
- Not all plant volatiles show rhythmic emission regulated by the circadian clock. In addition, not all plants and not all plant tissues show such rhythmic emissions. Why are some plant volatiles rhythmically released and others not? Why is the emission of some plant volatiles controlled by endogenous circadian mechanisms while the emission of others is controlled by external factors such as light or temperature?
- 2
- Many studies on the regulation of the rhythmic emission of plant volatiles by the circadian clock focus on downstream pathways or the final step in volatile biosynthesis. Is there a unified mechanism of action for the circadian clock to mediate the rhythmic emission of plant volatiles? What are the upstream signaling events that control circadian clock-mediated rhythmic emission?
- 3
- In most cases, external factors such as insect attack, high temperature, or light readily induce the transient emission (in minor cases, rhythmic emission) of plant volatiles, some of which can protect plants. Why do plants regulate rhythmic emission of plant volatiles through the endogenous circadian clock? What are the benefits for plant growth and development? Besides ecological functions such as interaction with insects, do volatiles have physiological functions in plants?
- 4
- Plants are exposed to many environmental factors/stresses. Are there interactions between these environmental factors and regulation of the endogenous circadian clock? If endogenous circadian clock regulation is relatively independent, how do plants maintain the stability of endogenous circadian clock regulation?
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CCA1 | Circadian clock associated 1 |
| CCD | Carotenoid cleavage dioxygenase |
| DXP | 1-Deoxy-d-xylulose-5-phosphate |
| DXPS | 1-Deoxy-d-xylulose-5-phosphate synthase |
| GLVs | Green leaf volatiles |
| LHY | Late elongated hypocotyl |
| LOX | Lipoxygenase |
| MEP | Methylerythritol phosphate |
| SAMT | Salicylic acid carboxyl methyltransferase |
| ISPS | Isoprene synthase |
| TOC1 | Timing of cab expression 1 |
| VPBs | Volatile phenylpropanoid/benzenoid |
References
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 331, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Pasqua, G.; Monacelli, B.; Manfredini, C.; Loreto, F.; Perez, G. The role of isoprenoid accumulation and oxidation in sealing wounded needles of Mediterranean pines. Plant Sci. 2002, 163, 355–359. [Google Scholar] [CrossRef]
- Loreto, F.; Schnitzler, J.P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Gertsch, J.; Appendino, G. Plant volatiles: Production, function and pharmacology. Nat. Prod. Rep. 2011, 28, 1359–1380. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Fu, X.M.; Watanabe, N.; Su, X.G.; Yang, Z.Y. Recent advances in the emission and functions of plant vegetative volatiles. Molecules 2016, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Raguso, R.A. Wake up and smell the roses: The ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 549–569. [Google Scholar] [CrossRef]
- Cheng, S.; Fu, X.M.; Mei, X.; Zhou, Y.; Du, B.; Watanabe, N.; Yang, Z.Y. Regulation of biosynthesis and emission of volatile phenylpropanoids/benzenoids in petunia × hybrida flowers by multi-factors of circadian clock, light, and temperature. Plant Physiol. Biochem. 2016, 107, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Farré, E.M. The regulation of plant growth by the circadian clock. Plant Biol. 2012, 14, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Yakir, E.; Hilman, D.; Harir, Y.; Green, R.M. Regulation of output from the plant circadian clock. FEBS J. 2007, 274, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Silk, W.K.; Schurr, U. Environmental effects on spatial and temporal patterns of leaf and root growth. Annu. Rev. Plant Biol. 2009, 60, 279–304. [Google Scholar] [CrossRef] [PubMed]
- Doherty, C.J.; Kay, S.A. Circadian control of global gene expression patterns. Annu. Rev. Genet. 2010, 44, 419–444. [Google Scholar] [CrossRef] [PubMed]
- McClung, C.R. Comes a time. Curr. Opin. Plant Biol. 2008, 11, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Tholl, D.; Röse, U.S.R. Detection and identification of floral scent compounds. In Biology of Floral Scent, Section II Biochemistry and Molecular Biology of Floral Scent; Dudareva, N., Pichersky, E., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 3–25. [Google Scholar]
- Matile, P.; Altenburger, R. Rhythms of fragrance emission in flowers. Planta 1988, 174, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Loughrin, J.H.; Hamilton-Kemp, T.R.; Anderson, R.A.; Hildebrand, D.F. Volatiles from flowers of Nicotiana sylvestris, N. otophora and Malus domestica: Headspace components and day/night changes in their relative concentrations. Phytochemistry 1990, 29, 2473–2477. [Google Scholar] [CrossRef]
- Nielsen, J.K.; Jakobsen, H.B.; Hansen, P.F.K.; Moller, J.; Olsen, C.E. Asynchronous rhythms in the emission of volatiles from Hesperis matronalis flowers. Phytochemistry 1995, 38, 847–851. [Google Scholar] [CrossRef]
- Thomas, B.; Vince-Prue, D. Photoperiodism in Plants. In Photoperiodic Timekeeping; Thomas, B., Vince-Prue, D., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 29–62. [Google Scholar]
- Johnson, C.H.; Knight, M.; Trewavas, A.; Kondo, T. A clockwork green: Circadian programs in photosynthetic organ-isms. In Biological Rhythms and Photoperiodism in Plants; Lumsden, P.J., Millar, A.J., Eds.; BIOS Scientific Publishers: Oxford, UK, 1998; pp. 1–34. [Google Scholar]
- Somers, D.E. The physiology and molecular bases of the plant circadian clock. Plant Physiol. 1999, 121, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, I.T.; Halitschke, R.; Paschold, A.; von Dahl, C.C.; Preston, C.A. Volatile signaling in plant-plant interactions: “Talking trees” in the genomics era. Science 2006, 311, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Fatouros, N.E.; van Loon, J.J.; Hordijk, K.A.; Smid, H.M.; Dicke, M. Herbivore-induced plant volatiles mediate in-flight host discrimination by parasitoids. J. Chem. Ecol. 2005, 31, 2033–2047. [Google Scholar] [CrossRef] [PubMed]
- Paré, P.W.; Tumlinson, J.H. Plant volatiles as a defense against insect herbivores. Plant Physiol. 1999, 121, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Adebesin, F.; Widhalm, J.R.; Boachon, B.; Lefèvre, F.; Pierman, B.; Lynch, J.H.; Alam, I.; Junqueira, B.; Benke, R.; Ray, S.; et al. Emission of volatile organic compounds from petunia flowers is facilitated by an ABC transporter. Science 2017, 356, 1386–1388. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.A.; Nemchenko, A.; Borrego, E.; Murray, I.; Sobhy, I.S.; Bosak, L.; DeBlasio, S.; Erb, M.; Robert, C.A.; Vaughn, K.A.; et al. The maize lipoxygenase, ZmLOX10, mediates green leaf volatile, jasmonate and herbivore-induced plant volatile production for defense against insect attack. Plant J. 2013, 74, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, F.P. The evolution of floral scent and insect chemical communication. Ecol. Lett. 2010, 13, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Raguso, R.A.; Levin, R.A.; Foose, S.E.; Holmberg, M.W.; McDade, L.A. Fragrance chemistry, nocturnal rhythms and pollination ‘syndromes’ in Nicotiana. Phytochemistry 2003, 63, 265–284. [Google Scholar] [CrossRef]
- Hoballah, M.E.; Stuurman, J.; Turlings, T.C.; Guerin, P.M.; Connetable, S.; Kuhlemeier, C. The composition and timing of flower odour emission by wild Petunia axillaris coincide with the antennal perception and nocturnal activity of the pollinator Manduca sexta. Planta 2005, 222, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, C.; Parra, L.; Quiroz, A.; Isaacs, R. Variation in highbush blueberry floral volatile profiles as a function of pollination status, cultivar, time of day and flower part: Implications for flower visitation by bees. Ann. Bot. 2011, 107, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Kolosova, N.; Gorenstein, N.; Kish, C.M.; Dudareva, N. Regulation of circadian methyl benzoate emission in diurnally and nocturnally emitting plants. Plant Cell 2001, 13, 2333–2347. [Google Scholar] [CrossRef] [PubMed]
- Loughrin, J.H.; Hamilton-Kemp, T.R.; Andersen, R.A.; Hildebrand, D.F. Circadian rhythm of volatile emission from flowers of Nicotiana sylvestris and N. suaveolens. Physiol. Plant. 1991, 83, 492–496. [Google Scholar] [CrossRef]
- Wilkinson, M.J.; Owen, S.M.; Possell, M.; Hartwell, J.; Gould, P.; Hall, A.; Vickers, C.; Nicholas Hewitt, C. Circadian control of isoprene emissions from oil palm (Elaeis guineensis). Plant J. 2006, 47, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Loivamaki, M.; Louis, S.; Cinege, G.; Zimmer, I.; Fischbach, R.J.; Schnitzler, J.P. Circadian rhythms of isoprene biosynthesis in grey poplar leaves. Plant Physiol. 2007, 143, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; Underwood, B.A.; Auldridge, M.; Loucas, H.M.; Shibuya, K.; Schmelz, E.; Clark, D.G.; Klee, H.J. Circadian regulation of the PhCCD1 carotenoid cleavage dioxygenase controls emission of beta-ionone, a fragrance volatile of petunia flowers. Plant Physiol. 2004, 136, 3504–3514. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N. (E)-β-Ocimene and myrcene synthase genes of floral scent biosynthesis in Snapdragon: Function and expression of three terpene synthase genes of a new terpene synthase subfamily. Plant Cell 2003, 15, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Pott, M.B.; Effmert, U.; Piechulla, B. Transcriptional and post-translational regulation of S-adenosyl-l-methionine: Salicylic acid carboxyl methyltransferase (SAMT) during Stephanotis floribunda flower development. J. Plant Physiol. 2003, 160, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Xu, R.; Jia, J.W.; Pang, J.; Matsuda, S.P.; Chen, X.Y. Cloning and functional characterization of a beta-pinene synthase from Artemisia annua that shows a circadian pattern of expression. Plant Physiol. 2002, 130, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Altenburger, R.; Matile, P. Further observations on rhythmic emission of fragarance in flowers. Planta 1990, 180, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Andersson, S.; Orlova, I.; Gatto, N.; Reichelt, M.; Rhodes, D.; Boland, W.; Gershenzon, J. The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proc. Natl. Acad. Sci. USA 2005, 18, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Nemchenko, A.; Kunze, S.; Feussner, I.; Kolomiets, M. Duplicate maize 13-lipoxygenase genes are differentially regulated by circadian rhythm, cold stress, wounding, pathogen infection, and hormonal treatments. J. Exp. Bot. 2006, 57, 3767–3779. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Pichersky, E. (Eds.) Biology of Floral Scent; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Hendel-Rahmanim, K.; Masci, T.; Vainstein, A.; Weiss, D. Diurnal regulation of scent emission in rose flowers. Planta 2007, 226, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Bruügemann, N.; Schnitzler, J. Relationship of isopentenyl diphosphate (IDP) isomerase activity to isoprene emission of oak leaves. Tree Physiol. 2002, 22, 1011–1018. [Google Scholar] [CrossRef]
- Alabadi, D.; Oyama, T.; Yanovsky, M.J.; Harmon, F.G.; Mas, P.; Kay, S.A. Reciprocal regulation between TOC1 and LHY/ CCA1 within the Arabidopsis circadian clock. Science 2001, 293, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Yon, F.; Kessler, D.; Joo, Y.; Llorca, L.C.; Kim, S.G.; Baldwin, I.T. Fitness consequences of altering floral circadian oscillations for Nicotiana attenuate. J. Integr. Plant Biol. 2017, 59, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Fenske, M.P.; Hewett Hazelton, K.D.; Hempton, A.K.; Sung Shim, J.; Yamamoto, B.M.; Riffell, J.A.; Imaizumi, T. Circadian clock gene LATE ELONGATED HYPOCOTYL directly regulates the timing of floral scent emission in Petunia. Proc. Natl. Acad. Sci. USA 2015, 112, 9775–9780. [Google Scholar] [CrossRef] [PubMed]
- Yon, F.; Joo, Y.; Llorca, L.C.; Rothe, E.; Baldwin, I.T.; Kim, S.G. Silencing Nicotiana attenuata LHY and ZTL alters circadian rhythms in flowers. New Phytol. 2016, 209, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.; Köpke, S.; Kunert, M.; Volpe, V.; David, A.; Brand, P.; Dabrowska, P.; Maffei, M.E.; Boland, W. Effects of feeding Spodoptera littoralis on lima bean leaves: IV. Diurnal and nocturnal damage differentially initiate plant volatile emission. Plant Physiol. 2008, 146, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Van Moerkercke, A.; Haring, M.A.; Schuurink, R.C. The transcription factor EMISSION OF BENZENOIDS II activates the MYB ODORANT1 promoter at a MYB binding site specific for fragrant petunias. Plant J. 2011, 67, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Can’ani, A.; Mühlemann, J.K.; Ravid, J.; Masci, T.; Klempien, A.; Nguyen, T.T.H.; Dudareva, N.; Pichersky, E.; Vainstein, A. Petunia × hybrida floral scent production is negatively affected by high-temperature growth conditions. Plant Cell Environ. 2015, 38, 1333–1346. [Google Scholar]


| Volatile | Plant | Emission Level | Ref. |
|---|---|---|---|
| Volatile phenylpropanoids/benzenoids | |||
| l-Nitro-2-phenylethane | Stephanotis floribunda | - | [17] |
| Methyl benzoate | Antirrhinum majus | 0.6–1.9 μg/g FW | [36] |
| Nicotiana suaveolens | - | [36] | |
| Petunia × hybrida | - | [36] | |
| Nicotiana sylvestris; Nicotiana suaveolens | - | [37] | |
| Benzyl alcohol | Nicotiana sylvestris; Nicotiana suaveolens | - | [37] |
| Methyl salicylate | Nicotiana suaveolens | - | [37] |
| Stephanotis floribunda | - | [42] | |
| Volatile terpenes | |||
| Isoprene | Elaeis guineensis | - | [38] |
| Populus × canescens | nearly 100–1600 nmol/g FW·s | [39] | |
| β-Pinene | Artemisia annua | - | [43] |
| β-Ionone | Petunia × hybrida | nearly 22–25 pg/g FW·h | [40] |
| (E)-β-Ocimene | Antirrhinum majus | nearly 0.2–3 μg/flower·h | [41] |
| Myrcene | Antirrhinum majus | nearly 0.05–0.8 μg/flower·h | [41] |
| Linalool | Hoya carfiosa | - | [44] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, L.; Wang, X.; Kang, M.; Dong, F.; Yang, Z. Regulation of the Rhythmic Emission of Plant Volatiles by the Circadian Clock. Int. J. Mol. Sci. 2017, 18, 2408. https://doi.org/10.3390/ijms18112408
Zeng L, Wang X, Kang M, Dong F, Yang Z. Regulation of the Rhythmic Emission of Plant Volatiles by the Circadian Clock. International Journal of Molecular Sciences. 2017; 18(11):2408. https://doi.org/10.3390/ijms18112408
Chicago/Turabian StyleZeng, Lanting, Xiaoqin Wang, Ming Kang, Fang Dong, and Ziyin Yang. 2017. "Regulation of the Rhythmic Emission of Plant Volatiles by the Circadian Clock" International Journal of Molecular Sciences 18, no. 11: 2408. https://doi.org/10.3390/ijms18112408