A Mini-Review of the NADPH Oxidases in Vascular Dementia: Correlation with NOXs and Risk Factors for VaD
Abstract
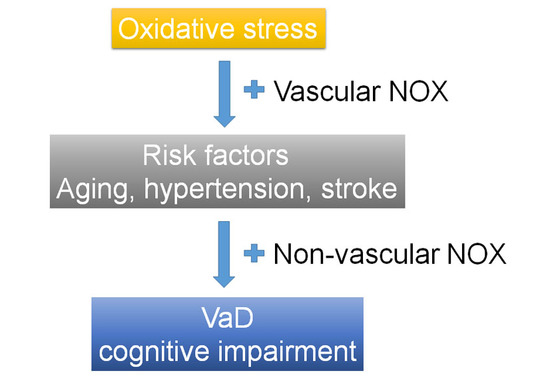
:1. Introduction
2. Association between Vascular Dementia (VaD) and Risk Factors and Oxidative Stress (OS)
3. NADPH Oxidase in Cerebrovascular Disease
3.1. NADPH Oxidase—An Overview
3.2. NOX-Dependent Generation of ROS Involves in Cerebrovascular Impairment
4. NADPH Oxidase in Risk Factors for VaD
4.1. Increased NOX Expression is Associated with Increasing Age
4.2. NOX in Hypertension
4.3. NOX in Stroke
5. Association of NADPH Oxidase in Cognitive Impairment
6. Conclusions
Acknowledgments
Author contributions
Conflicts of Interest
Abbreviations
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| OS | Oxidative stress |
| VaD | Vascular dementia |
| ROS | Reactive oxygen species |
| NOX | NADPH oxidase |
| phox | Phagocytic oxidase |
| DUOX | Dual oxidase |
| NOXO1 | NOX organizer 1 |
| NOXA1 | NOX activator 1 |
| Rac | Ras-related C3 botulinum toxin substrate |
| Ang II | Angiotensin II |
| SAH | Subarachnoid hemorrhage |
| ICH | Intracerebral hemorrhage |
| CA1 | Cornu Ammonis 1 |
| ACE | Angiotensin (Ang)-converting enzyme |
| IL-6 | Interleukin-6 |
| nNOS | Neuronal nitric oxide synthase |
| TBI | Traumatic brain injury |
| TBHQ | Tertiary Butylhydroquinone |
| LTP | Long-term potentiation |
| SAE | Sepsis-associated encephalopathy |
| AD | Alzheimer’s disease |
| KO | Knockout |
| Nrf2 | NF-E2-related factor-2 |
References
- McGuinness, B.; Craig, D.; Bullock, R.; Passmore, P. Statins for the prevention of dementia. Cochrane Database Syst. Rev. 2016, 1, CD003160. [Google Scholar]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J.P.; Scutt, P.; Sprigg, N.; Bath, P.M. Hypercholesterolaemia and vascular dementia. Clin. Sci. 2017, 131, 1561–1578. [Google Scholar] [CrossRef] [PubMed]
- Van Oijen, M.; de Jong, F.J.; Witteman, J.C.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M. Atherosclerosis and risk for dementia. Ann. Neurol. 2007, 61, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lee, W.T.; Park, K.A.; Lee, J.E. Association between risk factors for vascular dementia and adiponectin. Biomed. Res. Int. 2014, 2014, 261672. [Google Scholar] [CrossRef] [PubMed]
- Venkat, P.; Chopp, M.; Chen, J. Models and mechanisms of vascular dementia. Exp. Neurol. 2015, 272, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Craft, S. The role of metabolic disorders in alzheimer disease and vascular dementia: Two roads converged. Arch. Neurol. 2009, 66, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Fillit, H.; Nash, D.T.; Rundek, T.; Zuckerman, A. Cardiovascular risk factors and dementia. Am. J. Geriatr. Pharmacother. 2008, 6, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Honjo, K.; Black, S.E.; Verhoeff, N.P. Alzheimer’s disease, cerebrovascular disease, and the β-amyloid cascade. Can. J. Neurol. Sci. 2012, 39, 712–728. [Google Scholar] [CrossRef] [PubMed]
- Purnell, C.; Gao, S.; Callahan, C.M.; Hendrie, H.C. Cardiovascular risk factors and incident alzheimer disease: A systematic review of the literature. Alzheimer Dis. Assoc. Disord. 2009, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Jialal, I. Oxidative stress and atherosclerosis. Pathophysiology 2006, 13, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Horke, S.; Forstermann, U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 2014, 237, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J. Cerebral hypoperfusion and cognitive impairment: The pathogenic role of vascular oxidative stress. Int. J. Neurosci. 2012, 122, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, B.; Nie, K.; Jia, Y.; Yu, J. Effects of acupuncture on declined cerebral blood flow, impaired mitochondrial respiratory function and oxidative stress in multi-infarct dementia rats. Neurochem. Int. 2014, 65, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Drummond, G.R.; Selemidis, S.; Griendling, K.K.; Sobey, C.G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug. Discov. 2011, 10, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Luca, M.; Luca, A.; Calandra, C. The role of oxidative damage in the pathogenesis and progression of alzheimer’s disease and vascular dementia. Oxid. Med. Cell. Longev. 2015, 2015, 504678. [Google Scholar] [CrossRef] [PubMed]
- Zekry, D.; Epperson, T.K.; Krause, K.H. A role for nox nadph oxidases in alzheimer’s disease and other types of dementia? IUBMB Life 2003, 55, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Lee, K.H.; Kim, J.H.; Seo, J.H.; Kim, H.Y.; Shin, C.Y.; Han, J.S.; Han, S.H.; Kim, Y.S.; Lee, J. Nadph oxidase 1, a novel molecular source of ROS in hippocampal neuronal death in vascular dementia. Antioxid. Redox. Signal. 2014, 21, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Miller, A.A.; Drummond, G.R.; Thrift, A.G.; Arumugam, T.V.; Phan, T.G.; Srikanth, V.K.; Sobey, C.G. Vascular cognitive impairment and alzheimer’s disease: Role of cerebral hypoperfusion and oxidative stress. N-S Arch. Pharmacol. 2012, 385, 953–959. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.T.; Thomas, A. Vascular dementia. Lancet 2015, 386, 1698–1706. [Google Scholar] [CrossRef]
- Cervellati, C.; Romani, A.; Seripa, D.; Cremonini, E.; Bosi, C.; Magon, S.; Passaro, A.; Bergamini, C.M.; Pilotto, A.; Zuliani, G. Oxidative balance, homocysteine, and uric acid levels in older patients with late onset alzheimer’s disease or vascular dementia. J. Neurol. Sci. 2014, 337, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wong, A.; Wang, Z.; Liu, W.; Au, L.; Xiong, Y.; Chu, W.W.; Leung, E.Y.; Chen, S.; Lau, C.; et al. Risk factors for incident dementia after stroke and transient ischemic attack. Alzheimers Dement. 2015, 11, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Wiesmann, M.; Kiliaan, A.J.; Claassen, J.A. Vascular aspects of cognitive impairment and dementia. J. Cereb. Blood Flow. Metab. 2013, 33, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.; Grant, M.M.; Aldred, S. Oxidative stress in vascular dementia and alzheimer’s disease: A common pathology. J. Alzheimers Dis. 2009, 17, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Polidori, M.C.; Mattioli, P.; Aldred, S.; Cecchetti, R.; Stahl, W.; Griffiths, H.; Senin, U.; Sies, H.; Mecocci, P. Plasma antioxidant status, immunoglobulin g oxidation and lipid peroxidation in demented patients: Relevance to alzheimer disease and vascular dementia. Dement. Geriatr. Cogn. Disord. 2004, 18, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Casado, A.; Encarnacion Lopez-Fernandez, M.; Concepcion Casado, M.; de La Torre, R. Lipid peroxidation and antioxidant enzyme activities in vascular and alzheimer dementias. Neurochem. Res. 2008, 33, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Gustaw-Rothenberg, K.; Kowalczuk, K.; Stryjecka-Zimmer, M. Lipids’ peroxidation markers in alzheimer’s disease and vascular dementia. Geriatr. Gerontol. Int. 2010, 10, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Gackowski, D.; Rozalski, R.; Siomek, A.; Dziaman, T.; Nicpon, K.; Klimarczyk, M.; Araszkiewicz, A.; Olinski, R. Oxidative stress and oxidative DNA damage is characteristic for mixed alzheimer disease/vascular dementia. J. Neurol. Sci. 2008, 266, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B., 3rd. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Dias, I.H.; Polidori, M.C.; Griffiths, H.R. Hypercholesterolaemia-induced oxidative stress at the blood-brain barrier. Biochem. Soc. Trans. 2014, 42, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Rubattu, S.; Pagliaro, B.; Pierelli, G.; Santolamazza, C.; Castro, S.D.; Mennuni, S.; Volpe, M. Pathogenesis of target organ damage in hypertension: Role of mitochondrial oxidative stress. Int. J. Mol. Sci. 2014, 16, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.C.; Cosso, R.G.; Alberici, L.C.; Maciel, E.N.; Salerno, A.G.; Dorighello, G.G.; Velho, J.A.; de Faria, E.C.; Vercesi, A.E. Oxidative stress in atherosclerosis-prone mouse is due to low antioxidant capacity of mitochondria. FASEB J. 2005, 19, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Luc, G.; Fruchart, J.C. Oxidation of lipoproteins and atherosclerosis. Am. J. Clin. Nutr. 1991, 53, 206S–209S. [Google Scholar] [PubMed]
- Polidori, M.C.; Frei, B.; Cherubini, A.; Nelles, G.; Rordorf, G.; Keaney, J.F., Jr.; Schwamm, L.; Mecocci, P.; Koroshetz, W.J.; Beal, M.F. Increased plasma levels of lipid hydroperoxides in patients with ischemic stroke. Free Radic. Biol. Med. 1998, 25, 561–567. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating nadph oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Zatti, M. Biochemical aspects of phagocytosis in polymorphonuclear leucocytes. Nadh and nadph oxidation by the granules of resting and phagocytizing cells. Experientia 1964, 20, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Cao, Z.; Xu, X.; van Meir, E.G.; Lambeth, J.D. Homologs of gp91phox: Cloning and tissue expression of nox3, nox4, and nox5. Gene 2001, 269, 131–140. [Google Scholar] [CrossRef]
- Geiszt, M.; Leto, T.L. The Nox family of NAD(P)H oxidases: Host defense and beyond. J. Biol. Chem. 2004, 279, 51715–51718. [Google Scholar] [CrossRef] [PubMed]
- Pendyala, S.; Natarajan, V. Redox regulation of Nox proteins. Respir. Physiol. Neurobiol. 2010, 174, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.W.; Wang, J.; Zhang, Q.; Wang, R.; Dhandapani, K.M.; Vadlamudi, R.K.; Brann, D.W. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Rittinger, K. Regulation of NOXO1 activity through reversible interactions with p22 and NOXA1. PLoS ONE 2010, 5, e10478. [Google Scholar] [CrossRef] [PubMed]
- Leto, T.L.; Morand, S.; Hurt, D.; Ueyama, T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid. Redox. Signal. 2009, 11, 2607–2619. [Google Scholar] [CrossRef] [PubMed]
- Streeter, J.; Thiel, W.; Brieger, K.; Miller, F.J., Jr. Opportunity Nox: The future of NADPH oxidases as therapeutic targets in cardiovascular disease. Cardiovasc. Ther. 2013, 31, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Dinh Cat, A.; Montezano, A.C.; Burger, D.; Touyz, R.M. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid. Redox. Signal. 2013, 19, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.P.; Jandeleit-Dahm, K.A. The role of NADPH oxidase in vascular disease—Hypertension, atherosclerosis & stroke. Curr. Pharm. Des. 2015, 21, 5933–5944. [Google Scholar] [PubMed]
- Miller, A.A.; Drummond, G.R.; Sobey, C.G. Novel isoforms of NADPH-oxidase in cerebral vascular control. Pharmacol. Ther. 2006, 111, 928–948. [Google Scholar] [CrossRef] [PubMed]
- Paravicini, T.M.; Chrissobolis, S.; Drummond, G.R.; Sobey, C.G. Increased NADPH-oxidase activity and Nox4 expression during chronic hypertension is associated with enhanced cerebral vasodilatation to NADPH in vivo. Stroke 2004, 35, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.A.; Drummond, G.R.; Schmidt, H.H.; Sobey, C.G. NADPH oxidase activity and function are profoundly greater in cerebral versus systemic arteries. Circ. Res. 2005, 97, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Ago, T.; Kitazono, T.; Kuroda, J.; Kumai, Y.; Kamouchi, M.; Ooboshi, H.; Wakisaka, M.; Kawahara, T.; Rokutan, K.; Ibayashi, S.; et al. NAD(P)H oxidases in rat basilar arterial endothelial cells. Stroke 2005, 36, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Kazama, K.; Anrather, J.; Zhou, P.; Girouard, H.; Frys, K.; Milner, T.A.; Iadecola, C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ. Res. 2004, 95, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Chrissobolis, S.; Faraci, F.M. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol. Med. 2008, 14, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Erdos, B.; Snipes, J.A.; Tulbert, C.D.; Katakam, P.; Miller, A.W.; Busija, D.W. Rosuvastatin improves cerebrovascular function in zucker obese rats by inhibiting NAD(P)H oxidase-dependent superoxide production. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kobayashi, T.; Wachi, H.; Seyama, Y.; Kamata, K. Vascular NAD(P)H oxidase mediates endothelial dysfunction in basilar arteries from Otsuka long-evans tokushima fatty (OLETF) rats. Atherosclerosis 2007, 192, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Mayhan, W.G.; Arrick, D.M.; Sharpe, G.M.; Patel, K.P.; Sun, H. Inhibition of NAD(P)H oxidase alleviates impaired NOS-dependent responses of pial arterioles in type 1 diabetes mellitus. Microcirculation 2006, 13, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Mayhan, W.G.; Arrick, D.M.; Sharpe, G.M.; Sun, H. Age-related alterations in reactivity of cerebral arterioles: Role of oxidative stress. Microcirculation 2008, 15, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Vallet, P.; Charnay, Y.; Steger, K.; Ogier-Denis, E.; Kovari, E.; Herrmann, F.; Michel, J.P.; Szanto, I. Neuronal expression of the NADPH oxidase nox4, and its regulation in mouse experimental brain ischemia. Neuroscience 2005, 132, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.K.; Lee, J.H.; Kim, K.Y.; Kim, C.D.; Lee, W.S.; Rhim, B.Y.; Hong, K.W. Impairment of autoregulatory vasodilation by NAD(P)H oxidase-dependent superoxide generation during acute stage of subarachnoid hemorrhage in rat pial artery. J. Cereb. Blood Flow. Metab. 2002, 22, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.P.; Di Marco, E.; Kennedy, K.; Chew, P.; Okabe, J.; El-Osta, A.; Calkin, A.C.; Biessen, E.A.; Touyz, R.M.; Cooper, M.E.; et al. Reactive oxygen species can provide atheroprotection via NOX4-dependent inhibition of inflammation and vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Farkas, E.; Luiten, P.G. Cerebral microvascular pathology in aging and alzheimer’s disease. Prog. Neurobiol. 2001, 64, 575–611. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Coull, A.J.; Silver, L.E.; Fairhead, J.F.; Giles, M.F.; Lovelock, C.E.; Redgrave, J.N.; Bull, L.M.; Welch, S.J.; Cuthbertson, F.C.; et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (oxford vascular study). Lancet 2005, 366, 1773–1783. [Google Scholar] [CrossRef]
- Ali, S.S.; Young, J.W.; Wallace, C.K.; Gresack, J.; Jeste, D.V.; Geyer, M.A.; Dugan, L.L.; Risbrough, V.B. Initial evidence linking synaptic superoxide production with poor short-term memory in aged mice. Brain Res. 2011, 1368, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Faraci, F.M. Reactive oxygen species: Influence on cerebral vascular tone. J. Appl. Physiol. 2006, 100, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Park, L.; Koizumi, K.; El Jamal, S.; Zhou, P.; Previti, M.L.; Van Nostrand, W.E.; Carlson, G.; Iadecola, C. Age-dependent neurovascular dysfunction and damage in a mouse model of cerebral amyloid angiopathy. Stroke 2014, 45, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Park, L.; Anrather, J.; Girouard, H.; Zhou, P.; Iadecola, C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J. Cereb. Blood Flow. Metab. 2007, 27, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Kurz, S.; Munzel, T.; Tarpey, M.; Freeman, B.A.; Griendling, K.K.; Harrison, D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Investig. 1996, 97, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Chapman, A.; Boyd, R.; Wang, H.D. ERK activation contributes to regulation of spontaneous contractile tone via superoxide anion in isolated rat aorta of angiotensin II-induced hypertension. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2997–3005. [Google Scholar] [CrossRef] [PubMed]
- Nakane, H.; Miller, F.J., Jr.; Faraci, F.M.; Toyoda, K.; Heistad, D.D. Gene transfer of endothelial nitric oxide synthase reduces angiotensin II-induced endothelial dysfunction. Hypertension 2000, 35, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Faraci, F.M.; Lamping, K.G.; Modrick, M.L.; Ryan, M.J.; Sigmund, C.D.; Didion, S.P. Cerebral vascular effects of angiotensin II: New insights from genetic models. J. Cereb. Blood Flow. Metab. 2006, 26, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Alwahdani, A.; Iida, S.; Lund, D.D.; Faraci, F.M.; Heistad, D.D. Vascular effects of the human extracellular superoxide dismutase R213G variant. Circulation 2005, 112, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Feldberg, W.; Myers, R.D.; Veale, W.L. Perfusion from cerebral ventricle to cisterna magna in the unanaesthetized cat. Effect of calcium on body temperature. J. Physiol. 1970, 207, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Chrissobolis, S.; Banfi, B.; Sobey, C.G.; Faraci, F.M. Role of Nox isoforms in angiotensin II-induced oxidative stress and endothelial dysfunction in brain. J. Appl. Physiol. 2012, 113, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Chabrashvili, T.; Kitiyakara, C.; Blau, J.; Karber, A.; Aslam, S.; Welch, W.J.; Wilcox, C.S. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R117–124. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, K.; Yamada, H.; Iwata, K.; Jin, D.; Katsuyama, M.; Matsuki, M.; Takai, S.; Yamanishi, K.; Miyazaki, M.; Matsubara, H.; et al. Nox1 is involved in angiotensin II-mediated hypertension: A study in Nox1-deficient mice. Circulation 2005, 112, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Minieri, C.A.; Ollerenshaw, J.D.; Alexander, R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994, 74, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Chen, X.; Tabet, F.; Yao, G.; He, G.; Quinn, M.T.; Pagano, P.J.; Schiffrin, E.L. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: Regulation by angiotensin II. Circ. Res. 2002, 90, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M.; Zafari, A.M.; Fukui, T.; Ishizaka, N.; Griendling, K.K. P22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J. Biol. Chem. 1996, 271, 23317–23321. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Sadowski, J.; Guzik, B.; Jopek, A.; Kapelak, B.; Przybylowski, P.; Wierzbicki, K.; Korbut, R.; Harrison, D.G.; Channon, K.M. Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Zafari, A.M.; Ushio-Fukai, M.; Akers, M.; Yin, Q.; Shah, A.; Harrison, D.G.; Taylor, W.R.; Griendling, K.K. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension 1998, 32, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Konior, A.; Schramm, A.; Czesnikiewicz-Guzik, M.; Guzik, T.J. NADPH oxidases in vascular pathology. Antioxid. Redox. Signal. 2014, 20, 2794–2814. [Google Scholar] [CrossRef] [PubMed]
- Girouard, H.; Park, L.; Anrather, J.; Zhou, P.; Iadecola, C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through Nox-2-derived radicals. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Davisson, R.L. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008, 7, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Didion, S.P.; Faraci, F.M. Angiotensin II produces superoxide-mediated impairment of endothelial function in cerebral arterioles. Stroke 2003, 34, 2038–2042. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Takac, I.; Schroder, K. No superoxide—No stress?: Nox4, the good NADPH oxidase! Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1255–1257. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Zhang, M.; Benkhoff, S.; Mieth, A.; Pliquett, R.; Kosowski, J.; Kruse, C.; Luedike, P.; Michaelis, U.R.; Weissmann, N.; et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012, 110, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Kleinschnitz, C.; Grund, H.; Wingler, K.; Armitage, M.E.; Jones, E.; Mittal, M.; Barit, D.; Schwarz, T.; Geis, C.; Kraft, P.; et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010, 8, e1000479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Wu, J.; Duan, X.; Tian, X.; Shen, H.; Sun, Q.; Chen, G. NADPH oxidase: A potential target for treatment of stroke. Oxid. Med. Cell. Longev. 2016, 2016, 5026984. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yoshioka, H.; Kim, G.S.; Jung, J.E.; Okami, N.; Sakata, H.; Maier, C.M.; Narasimhan, P.; Goeders, C.E.; Chan, P.H. Oxidative stress in ischemic brain damage: Mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid. Redox. Signal. 2011, 14, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood Flow. Metab. 2001, 21, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Song, Y.S.; Chan, P.H. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J. Cereb. Blood Flow. Metab. 2009, 29, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.G.; Raz, L.; Wang, R.; Han, D.; De Sevilla, L.; Yang, F.; Vadlamudi, R.K.; Brann, D.W. Estrogen attenuates ischemic oxidative damage via an ERα-mediated inhibition of NADPH oxidase activation. J. Neurosci. 2009, 29, 13823–13836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.G.; Laird, M.D.; Han, D.; Nguyen, K.; Scott, E.; Dong, Y.; Dhandapani, K.M.; Brann, D.W. Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS ONE 2012, 7, e34504. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Kim, J.H.; Lee, K.H.; Kim, H.Y.; Kim, Y.S.; Choi, W.S.; Lee, J. Role of neuronal NADPH oxidase 1 in the peri-infarct regions after stroke. PLoS ONE 2015, 10, e0116814. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Niizuma, K.; Katsu, M.; Okami, N.; Sakata, H.; Kim, G.S.; Narasimhan, P.; Chan, P.H. NADPH oxidase mediates striatal neuronal injury after transient global cerebral ischemia. J. Cereb. Blood Flow. Metab. 2011, 31, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wei, X.; Kong, L.; Liu, X.; Cheng, L.; Yan, S.; Zhang, X.; Chen, L. Nod2 is involved in the inflammatory response after cerebral ischemia-reperfusion injury and triggers NADPH oxidase 2-derived reactive oxygen species. Int. J. Biol. Sci. 2015, 11, 525–535. [Google Scholar] [CrossRef] [PubMed]
- De Silva, T.M.; Brait, V.H.; Drummond, G.R.; Sobey, C.G.; Miller, A.A. Nox2 oxidase activity accounts for the oxidative stress and vasomotor dysfunction in mouse cerebral arteries following ischemic stroke. PLoS ONE 2011, 6, e28393. [Google Scholar] [CrossRef] [PubMed]
- Radermacher, K.A.; Wingler, K.; Langhauser, F.; Altenhofer, S.; Kleikers, P.; Hermans, J.J.; Hrabe de Angelis, M.; Kleinschnitz, C.; Schmidt, H.H. Neuroprotection after stroke by targeting NOX4 as a source of oxidative stress. Antioxid. Redox. Signal. 2013, 18, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Raz, L.; Zhang, Q.G.; Zhou, C.F.; Han, D.; Gulati, P.; Yang, L.C.; Yang, F.; Wang, R.M.; Brann, D.W. Role of Rac1 gtpase in NADPH oxidase activation and cognitive impairment following cerebral ischemia in the rat. PLoS ONE 2010, 5, e12606. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.X.; Wang, X.R.; Yan, C.Q.; He, T.; Yang, J.W.; Zeng, X.H.; Xu, Q.; Zhu, W.; Du, S.Q.; Liu, C.Z. Acupuncture elicits neuroprotective effect by inhibiting NAPDH oxidase-mediated reactive oxygen species production in cerebral ischaemia. Sci. Rep. 2015, 5, 17981. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.F.; Kataoka, K.; Toyama, K.; Sueta, D.; Koibuchi, N.; Yamamoto, E.; Yata, K.; Tomimoto, H.; Ogawa, H.; Kim-Mitsuyama, S. Attenuation of brain damage and cognitive impairment by direct renin inhibition in mice with chronic cerebral hypoperfusion. Hypertension 2011, 58, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Tsukuda, K.; Mogi, M.; Li, J.M.; Iwanami, J.; Min, L.J.; Sakata, A.; Fujita, T.; Iwai, M.; Horiuchi, M. Amelioration of cognitive impairment in the type-2 diabetic mouse by the angiotensin II type-1 receptor blocker candesartan. Hypertension 2007, 50, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, R.P.; Colohan, A.R.; Zhang, J.H. Neuroprotective effect of hyperbaric oxygen in a rat model of subarachnoid hemorrhage. Acta Neurochir. Suppl. 2006, 96, 188–193. [Google Scholar] [PubMed]
- Ostrowski, R.P.; Tang, J.; Zhang, J.H. Hyperbaric oxygen suppresses NADPH oxidase in a rat subarachnoid hemorrhage model. Stroke 2006, 37, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, S.; Yu, S.; Chen, Y.; Li, L.; Zhang, H.; Zhao, Y. C1q/tumor necrosis factor-related protein 3 inhibits oxidative stress during intracerebral hemorrhage via PKA signaling. Brain Res. 2017, 1657, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.E.; Suh, Y.S.; Lee, M.S.; Kim, K.Y.; Lee, J.H.; Lee, H.S.; Hong, K.W.; Kim, C.D. Vascular NAD(P)H oxidase triggers delayed cerebral vasospasm after subarachnoid hemorrhage in rats. Stroke 2002, 33, 2687–2691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.; Feng, D.; Shen, H.; Tian, X.; Li, H.; Wang, Z.; Chen, G. Involvement of Nox2 and Nox4 NADPH oxidases in early brain injury after subarachnoid hemorrhage. Free Radic. Res. 2017, 51, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, Y.; Ding, R.; Fu, Z.; Yang, S.; Deng, X.; Zeng, J. P2X7R blockade prevents NLRP3 inflammasome activation and brain injury in a rat model of intracerebral hemorrhage: Involvement of peroxynitrite. J. Neuroinflamm. 2015, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Toyama, K.; Koibuchi, N.; Uekawa, K.; Hasegawa, Y.; Kataoka, K.; Katayama, T.; Sueta, D.; Ma, M.J.; Nakagawa, T.; Yasuda, O.; et al. Apoptosis signal-regulating kinase 1 is a novel target molecule for cognitive impairment induced by chronic cerebral hypoperfusion. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.; Ramesh, V.; Gozal, D. Adverse cognitive effects of high-fat diet in a murine model of sleep apnea are mediated by NADPH oxidase activity. Neuroscience 2012, 227, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Dayyat, E.A.; Zhang, S.X.; Wang, Y.; Cheng, Z.J.; Gozal, D. Exogenous erythropoietin administration attenuates intermittent hypoxia-induced cognitive deficits in a murine model of sleep apnea. BMC Neurosci. 2012, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.; Dayyat, E.A.; Zhang, S.X.; Wang, Y.; Gozal, D. Intermittent hypoxia-induced cognitive deficits are mediated by nadph oxidase activity in a murine model of sleep apnea. PLoS ONE 2011, 6, e19847. [Google Scholar] [CrossRef] [PubMed]
- Hui-guo, L.; Kui, L.; Yan-ning, Z.; Yong-jian, X. Apocynin attenuate spatial learning deficits and oxidative responses to intermittent hypoxia. Sleep Med. 2010, 11, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Han, B.H.; Zhou, M.L.; Johnson, A.W.; Singh, I.; Liao, F.; Vellimana, A.K.; Nelson, J.W.; Milner, E.; Cirrito, J.R.; Basak, J.; et al. Contribution of reactive oxygen species to cerebral amyloid angiopathy, vasomotor dysfunction, and microhemorrhage in aged Tg2576 mice. Proc. Natl. Acad. Sci. USA 2015, 112, E881–E890. [Google Scholar] [CrossRef] [PubMed]
- Bruce-Keller, A.J.; Gupta, S.; Knight, A.G.; Beckett, T.L.; McMullen, J.M.; Davis, P.R.; Murphy, M.P.; Van Eldik, L.J.; St Clair, D.; Keller, J.N. Cognitive impairment in humanized APPxPS1 mice is linked to Aβ1-42 and NOX activation. Neurobiol. Dis. 2011, 44, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Park, L.; Zhou, P.; Pitstick, R.; Capone, C.; Anrather, J.; Norris, E.H.; Younkin, L.; Younkin, S.; Carlson, G.; McEwen, B.S.; et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc. Natl. Acad. Sci. USA 2008, 105, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Dugan, L.L.; Ali, S.S.; Shekhtman, G.; Roberts, A.J.; Lucero, J.; Quick, K.L.; Behrens, M.M. IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS ONE 2009, 4, e5518. [Google Scholar] [CrossRef] [PubMed]
- Chandran, R.; Kim, T.; Mehta, S.L.; Udho, E.; Chanana, V.; Cengiz, P.; Kim, H.; Kim, C.; Vemuganti, R. A combination antioxidant therapy to inhibit NOX2 and activate Nrf2 decreases secondary brain damage and improves functional recovery after traumatic brain injury. J. Cereb. Blood Flow. Metab. 2017, 271678X17738701. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.P.; Rodrigues, F.S.; Della-Pace, I.D.; Mota, B.C.; Oliveira, S.M.; de Campos Velho Gewehr, C.; Bobinski, F.; de Oliveira, C.V.; Brum, J.S.; Oliveira, M.S.; et al. HOE-140, an antagonist of B2 receptor, protects against memory deficits and brain damage induced by moderate lateral fluid percussion injury in mice. Psychopharmacology 2014, 231, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.P.; Rodrigues, F.S.; Della-Pace, I.D.; Mota, B.C.; Oliveira, S.M.; Velho Gewehr Cde, C.; Bobinski, F.; de Oliveira, C.V.; Brum, J.S.; Oliveira, M.S.; et al. The effect of NADPH-oxidase inhibitor apocynin on cognitive impairment induced by moderate lateral fluid percussion injury: Role of inflammatory and oxidative brain damage. Neurochem. Int. 2013, 63, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.R.; Zhang, H.; Zhao, H.T.; Ji, M.H.; Li, H.H.; Wu, J.; Li, K.Y.; Yang, J.J. Amelioration of oxidative stress-induced phenotype loss of parvalbumin interneurons might contribute to the beneficial effects of environmental enrichment in a rat model of post-traumatic stress disorder. Behav. Brain Res. 2016, 312, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, M.; de Iure, A.; Giampa, C.; Chiasserini, D.; Tozzi, A.; Orvietani, P.L.; Ghiglieri, V.; Tantucci, M.; Durante, V.; Quiroga-Varela, A.; et al. Persistent activation of microglia and NADPH drive hippocampal dysfunction in experimental multiple sclerosis. Sci. Rep. 2016, 6, 20926. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Yang, J.; Liu, L.; Ye, Y.; Wang, X.; Hu, J.; Chen, B.; Zhuge, Q. Elevated dopamine induces minimal hepatic encephalopathy by activation of astrocytic nadph oxidase and astrocytic protein tyrosine nitration. Int. J. Biochem. Cell. Biol. 2014, 55, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.H.; Qiu, L.L.; Tang, H.; Ju, L.S.; Sun, X.R.; Zhang, H.; Jia, M.; Zuo, Z.Y.; Shen, J.C.; Yang, J.J. Sepsis-induced selective parvalbumin interneuron phenotype loss and cognitive impairments may be mediated by NADPH oxidase 2 activation in mice. J. Neuroinflamm. 2015, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, M.S.; D’Avila, J.C.; Trevelin, S.C.; Reis, P.A.; Kinjo, E.R.; Lopes, L.R.; Castro-Faria-Neto, H.C.; Cunha, F.Q.; Britto, L.R.; Bozza, F.A. The role of Nox2-derived ros in the development of cognitive impairment after sepsis. J. Neuroinflamm. 2014, 11, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Won, S.J.; Yoo, B.H.; Kauppinen, T.M.; Choi, B.Y.; Kim, J.H.; Jang, B.G.; Lee, M.W.; Sohn, M.; Liu, J.; Swanson, R.A.; et al. Recurrent/moderate hypoglycemia induces hippocampal dendritic injury, microglial activation, and cognitive impairment in diabetic rats. J. Neuroinflamm. 2012, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Singh, N. Pharmacological inhibition of inducible nitric oxide synthase (iNOS) and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, convalesce behavior and biochemistry of hypertension induced vascular dementia in rats. Pharmacol. Biochem. Behav. 2013, 103, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Scheff, S.W. NADPH-oxidase activation and cognition in alzheimer disease progression. Free Radic. Biol. Med. 2011, 51, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Bruce-Keller, A.J.; Gupta, S.; Parrino, T.E.; Knight, A.G.; Ebenezer, P.J.; Weidner, A.M.; LeVine, H.; Keller, J.N.; Markesbery, W.R. NOX activity is increased in mild cognitive impairment. Antioxid. Redox. Signal. 2010, 12, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Iwanami, J.; Min, L.J.; Tsukuda, K.; Nakaoka, H.; Bai, H.Y.; Shan, B.S.; Kan-No, H.; Kukida, M.; Chisaka, T.; et al. Deficiency of angiotensin-converting enzyme 2 causes deterioration of cognitive function. NPJ Aging Mech. Dis. 2016, 2, 16024. [Google Scholar] [CrossRef] [PubMed]
- Inaba, S.; Iwai, M.; Furuno, M.; Tomono, Y.; Kanno, H.; Senba, I.; Okayama, H.; Mogi, M.; Higaki, J.; Horiuchi, M. Continuous activation of renin-angiotensin system impairs cognitive function in renin/angiotensinogen transgenic mice. Hypertension 2009, 53, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Singh, N. Pitavastatin and 4′-hydroxy-3′-methoxyacetophenone (HMAP) reduce cognitive dysfunction in vascular dementia during experimental diabetes. Curr. Neurovasc. Res. 2010, 7, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, D.M.; Zheng, Y.L.; Hu, B.; Cheng, W.; Zhang, Z.F. Purple sweet potato color attenuates domoic acid-induced cognitive deficits by promoting estrogen receptor-α-mediated mitochondrial biogenesis signaling in mice. Free Radic. Biol. Med. 2012, 52, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.; Zhang, S.X.; Ramesh, V.; Hakim, F.; Kaushal, N.; Wang, Y.; Gozal, D. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. Am. J. Respir. Crit. Care. Med. 2011, 184, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.L.; Luo, D.; Zhang, H.; Shi, Y.S.; Li, Y.J.; Wu, D.; Chen, J.; Ji, M.H.; Yang, J.J. Nox-2-mediated phenotype loss of hippocampal parvalbumin interneurons might contribute to postoperative cognitive decline in aging mice. Front. Aging Neurosci. 2016, 8, 234. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.L.; Ji, M.H.; Zhang, H.; Yang, J.J.; Sun, X.R.; Tang, H.; Wang, J.; Liu, W.X.; Yang, J.J. NADPH oxidase 2-derived reactive oxygen species in the hippocampus might contribute to microglial activation in postoperative cognitive dysfunction in aged mice. Brain Behav. Immun. 2016, 51, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, X.R.; Wang, J.; Zhang, Z.Z.; Zhao, H.T.; Li, H.H.; Ji, M.H.; Li, K.Y.; Yang, J.J. Reactive oxygen species-mediated loss of phenotype of parvalbumin interneurons contributes to long-term cognitive impairments after repeated neonatal ketamine exposures. Neurotox. Res. 2016, 30, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Satomoto, M.; Adachi, Y.U.; Kinoshita, H.; Makita, K. Inhibiting nadph oxidase protects against long-term memory impairment induced by neonatal sevoflurane exposure in mice. Br. J. Anaesth. 2016, 117, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Kishida, K.T.; Hoeffer, C.A.; Hu, D.; Pao, M.; Holland, S.M.; Klann, E. Synaptic plasticity deficits and mild memory impairments in mouse models of chronic granulomatous disease. Mol. Cell. Biol. 2006, 26, 5908–5920. [Google Scholar] [CrossRef] [PubMed]
- Walton, J.C.; Selvakumar, B.; Weil, Z.M.; Snyder, S.H.; Nelson, R.J. Neuronal nitric oxide synthase and NADPH oxidase interact to affect cognitive, affective, and social behaviors in mice. Behav. Brain Res. 2013, 256, 320–327. [Google Scholar] [CrossRef] [PubMed]

| Stroke Types | Ischemic Stroke | Subarachnoid Hemorrhage | Intracerebral Hemorrhage |
|---|---|---|---|
| NOX isoforms | NOX1, NOX2, NOX4 | NOX2, NOX4 | NOX2 |
| Region Cell types | Cortex, hippocampus, cerebral arteries Neurons, astrocytes, microglia | Cortex, striatum neurons, astrocytes | Striatum Microglia |
| Disease model | Increase NOX isoforms | Control of NOX | Mechanism of action | Reference |
|---|---|---|---|---|
| Two-vessel occlusion rat | gp91phox, p47phox, or p67phox | Apocynin 1 | Oxidative stress | [98] |
| NOXs activity, NOX1 | Apocynin, shRNA NOX1 AAV | Oxidative stress | [18] | |
| bilateral common carotid artery stenosis mice | NOXs activity | Apocynin | Oxidative stress | [107] |
| p67phox | Tempol 2 | Oxidative stress | [99] | |
| cerebral ischemic reperfusion rat | NOXs activity | Rac GTPase inhibitor (NSC23766) | Oxidative stress | [97] |
| Obstructive sleep apnea, Long-term exposure to intermittent hypoxia (LTIH) mice | NOX2 (gp91phox), p47phox, p22phox | gp91phox(-/-), erythropoietin, Apocynin | Lipid peroxidation and oxidative DNA damage | [108,109,110,111] |
| Aged Tg2576 Mice | NOXs activity | Apocynin | Oxidative stress and cerebrovascular dysfunction | [112] |
| Aged APP and PS1 knock-in mice | NOXs activity NOX4 | Young age | Deposition of Aβ1-42 | [113] |
| Tg2576 and NOX2(-/-) mice | NOX2 | NOX peptide Inhibitor gp91ds-tat NOX2(-/-) | ROS generation | [114] |
| gp91phox(-/-) and IL-6(-/-) aged mice | NOXs activity, NOX2 | Apocynin, gp91phox(-/-), IL-6(-/-) | Oxidative stress | [115] |
| ACE2KO mice | p22phox, p40phox, p67phox, and gp91phox | angiotensin (Ang)-converting enzyme (ACE), Tempol | Oxidative stress | [128] |
| Renin/angiotensinogen transgenic mice | p47phox and Nox4 | Tempol | Oxidative stress | [129] |
| TBI | NOX2 | Apocynin, NOX2(-/-) | ROS formation | [116] |
| Moderate lateral fluid percussion injury mice | NOXs activity | bradykinin receptors B2 antagonist (HOE-140), Apocynin | ROS formation | [117,118] |
| Post-traumatic stress disorder | NOX2 | Environmental enrichment | Oxidative stress | [119] |
| Encephalomyelitis model of multiple sclerosis | NOX2 | Minocycline 3, apocynin | hippocampal synaptic plasticity deficit | [120] |
| Sepsis-associated encephalopathy | NOX2 | Apocynin, gp91phox(-/-) | Inflammation and oxidative stress | [122,123] |
| Minimal hepatic encephalopathy rats | p47phox | Apocynin | ROS formation | [121] |
| Recurrent/moderate hypoglycemia rat | NOXs activity | Apocynin | Oxidative damage | [124] |
| Streptozotocin diabetes induced vascular dementia in rats | NOXs activity | NOX inhibitor, 4′-hydroxy-3′-methoxyacetophenone | Oxidative stress | [130] |
| Endoplasmic reticulum stress, domoic acid-treated mice | p47phox and gp91phox | estrogen receptor-α | ROS formation, ER stress | [131] |
| Sleep fragmentation in mice | gp91phox | gp91phox(-/-) mice | Lipid peroxidation and oxidative DNA damage | [132] |
| Postoperative aging mice | NOX2 | Apocynin, | Oxidative stress | [133,134] |
| Ketamine treated rats | NOX2 | Apocynin | Oxidative damage | [135] |
| Sevoflurane exposure mice | p22 phox | Apocynin | Increasing superoxide concentrations | [136] |
| Chronic granulomatous disease mice | gp91phox and p47phox | diphenylene iodonium or apocynin gp91phox and p47phox KO | LTP Blocking | [137] |
| p47phox and nNOS double KO mice | p47phox | p47phox(-/-)and nNOS(-/-) | ROS and NO formation | [138] |
| AD postmortem brains | p67phox, p47phox, and p40phox | Increasing of redox pathways | [126] | |
| MCI postmortem brains | NOXs activity, gp91phox, p47phox | Microglia activation | [127] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, D.-H.; Lee, J. A Mini-Review of the NADPH Oxidases in Vascular Dementia: Correlation with NOXs and Risk Factors for VaD. Int. J. Mol. Sci. 2017, 18, 2500. https://doi.org/10.3390/ijms18112500
Choi D-H, Lee J. A Mini-Review of the NADPH Oxidases in Vascular Dementia: Correlation with NOXs and Risk Factors for VaD. International Journal of Molecular Sciences. 2017; 18(11):2500. https://doi.org/10.3390/ijms18112500
Chicago/Turabian StyleChoi, Dong-Hee, and Jongmin Lee. 2017. "A Mini-Review of the NADPH Oxidases in Vascular Dementia: Correlation with NOXs and Risk Factors for VaD" International Journal of Molecular Sciences 18, no. 11: 2500. https://doi.org/10.3390/ijms18112500