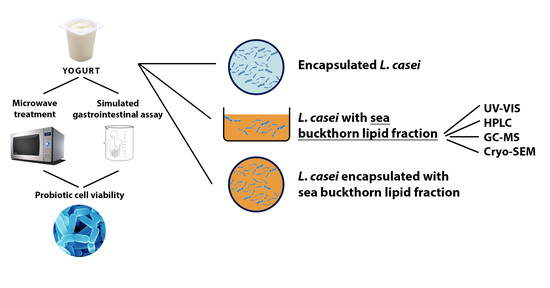
Characterization of a Sea Buckthorn Extract and Its Effect on Free and Encapsulated Lactobacillus casei
Abstract
:1. Introduction
2. Results
2.1. Sea Buckthorn Lipid Fraction: Characterization
2.1.1. HPLC-PDA and UV-VIS Spectroscopy Analysis
2.1.2. GS-MS Analysis of FAMES
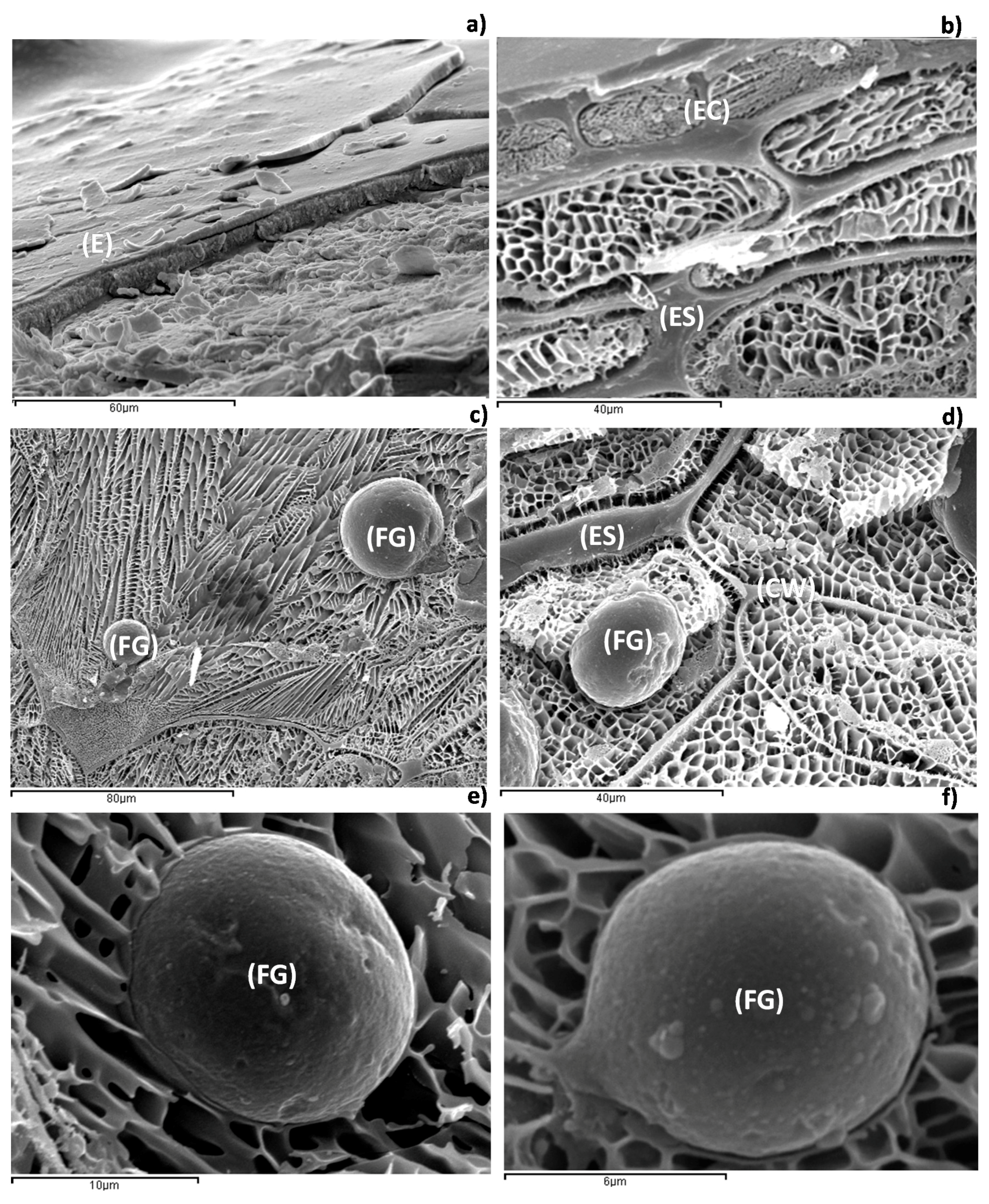
2.1.3. Low-Temperature Scanning Electron Microscopy (Cryo-SEM)
2.2. Probiotic Cell Encapsulation
2.3. Capsules Characterization. Entrapment Efficiency
2.4. Influence of Heat Treatment on the Probiotic Cells Viability
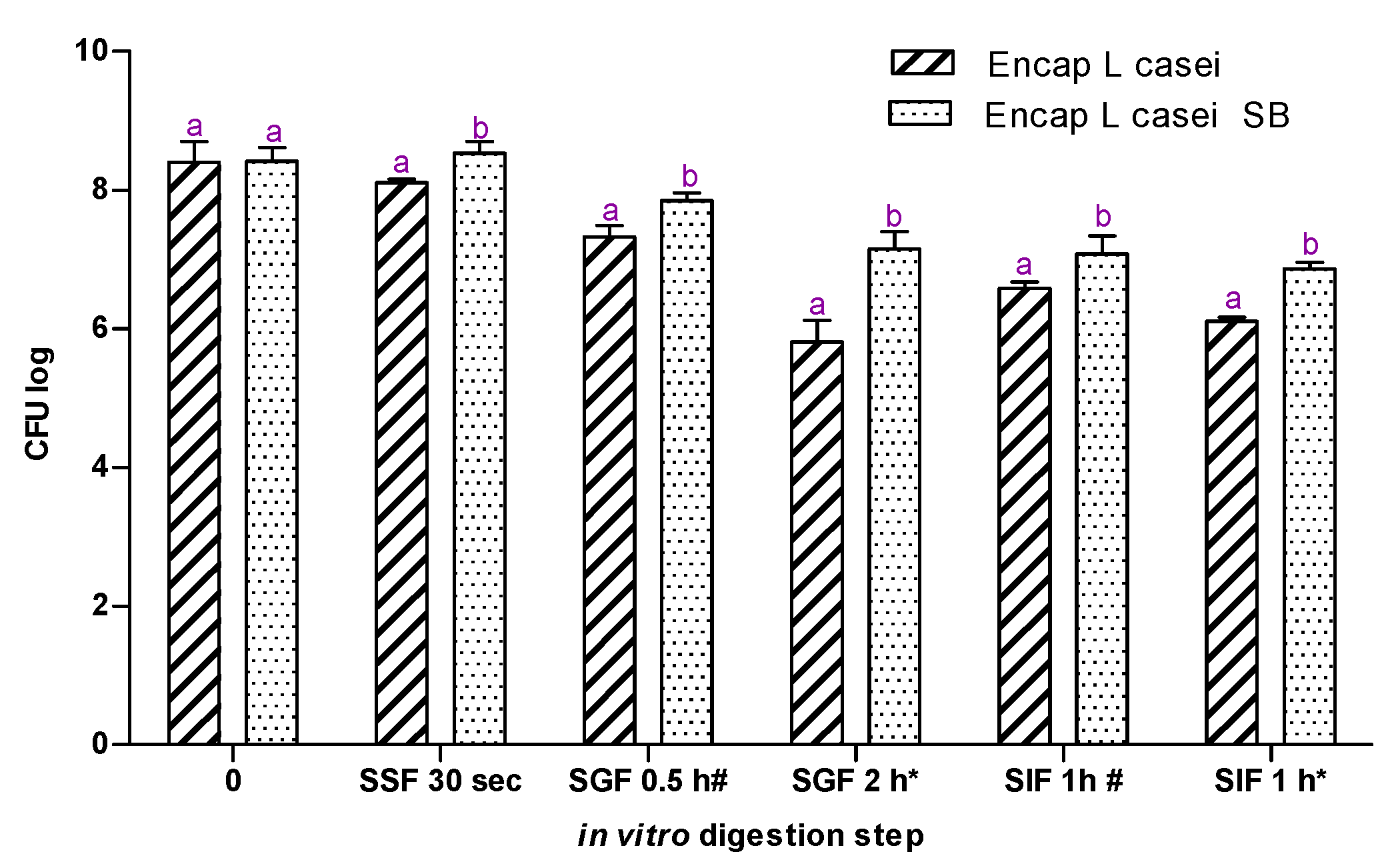
2.5. In Vitro Gastrointestinal Model Assay
3. Discussion
4. Materials and Methods
4.1. Sea Buckthorn (Hippophae rhamnoides) Lipid Fraction: Obtaining and Characterization
4.1.1. HPLC-PDA and UV-VIS Analysis
4.1.2. GS-MS Analysis of FAMEs
4.1.3. Low-Temperature Scanning Electron Microscopy (Cryo-SEM)
4.2. Bacterial Strains and Culture Conditions
4.3. Probiotic Cells Encapsulation
4.4. Capsules Characterization, Entrapment Efficiency and Sphericity
4.5. Heat Treatment
4.6. In Vitro Gastrointestinal Model
4.7. Probiotic Cells Viability Test
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coghetto, C.C.; Flores, S.H.; Brinques, G.B.; Záchia Ayub, M.A. Viability and alternative uses of a dried powder, microencapsulated lactobacillus plantarum without the use of cold chain or dairy products. LWT Food Sci. Technol. 2016, 71, 54–59. [Google Scholar] [CrossRef]
- Sardes, M.E. Probiotics: Definition, sources, selection, and uses. Clin. Infect. Dis. 2008, 1, S58–S61. [Google Scholar] [CrossRef]
- Butel, M.J. Probiotics, gut microbiota and health. Médecine et Maladies Infectieuses 2014, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.; Bik, E.M.; Bernstein, C.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.; Nelson, K.; Relman, D. Diversity of the human intestinal microbial flora. Science 2005, 5728, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Marelli, G.; Papaleo, E.; FerrariI, A. Lactobacilli for prevention of urogenital infections: A review. Eur. Rev. Med. Farmacol. 2004, 8, 87–95. [Google Scholar] [CrossRef]
- Aguirre, M.; Venema, K. The art of targeting gut microbiota for tackling human obesity. Genes Nutr. 2015, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ivey, K.L.; Hodgson, J.M.; Kerr, D.A.; Thompson, P.L.; Stojceski, B.; Prince, R.L. The effect of yoghurt and its probiotics on blood pressure and serum lipid profile; a randomised controlled trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Tapia, N.C.; González-Rodríguez, L.; Jeong, M.; López-Ramírez, Y.; Barbero-Becerra, V.; Juárez-Hernández, E.; Romero-Flores, J.L.; Arrese, M.; Méndez-Sánchez, N.; Uribe, M. Current evidence on the use of probiotics in liver diseases. J. Funct. Food 2015, 17, 137–151. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Lee, B.H. New perspectives on probiotics in health and disease. Food Sci. Hum. Wellness 2015, 4, 56–65. [Google Scholar] [CrossRef]
- Vajro, P.; Poeta, M.; Pierri, L.; Pizza, C.; D’Aniello, R.; Sangermano, M.; Massa, G.; Paolella, G. Chapter 33—Probiotics to treat visceral obesity and related liver disease. In Nutrition in the Prevention and Treatment of Abdominal Obesity; Watson, R.R., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 363–380. [Google Scholar]
- Amara, A.A.; Shibl, A. Role of probiotics in health improvement, infection control and disease treatment and management. Saudi Pharm. J. 2015, 23, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.S.; Liu, C.C.; Xiang, H.; Zheng, X.F.; Peng, G.J.; Zhang, X.; Du, G.H.; Qin, X.M. Investigation on the antidepressant effect of sea buckthorn seed oil through the GC-MS-based metabolomics approach coupled with multivariate analysis. Food Funct. 2015, 6, 3585–3592. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Ullah, S. Sea buckthorn seed oil protects against the oxidative stress produced by thermally oxidized lipids. Food Chem. 2015, 186, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, J.; Dong, X.; Han, Y.; Ma, Q.; Ding, Y.; Zhao, F.; Zhang, J.; Chen, H.; Xu, Q.; et al. Carotenoid accumulation affects redox status, starch metabolism, and flavonoid/anthocyanin accumulation in citrus. BMC Plant Biol. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Monego, D.L.; da Rosa, M.B.; do Nascimento, P.C. Applications of computational chemistry to the study of the antiradical activity of carotenoids: A review. Food Chem. 2017, 217, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Gunenc, A.; Khoury, C.; Legault, C.; Mirrashed, H.; Rijke, J.; Hosseinian, F. Seabuckthorn as a novel prebiotic source improves probiotic viability in yogurt. LWT Food Sci. Technol. 2016, 66, 490–495. [Google Scholar] [CrossRef]
- Kumari, A.; Angmo, K.; Monika Bhalla, T.C. Probiotic attributes of indigenous Lactobacillus spp. Isolated from traditional fermented foods and beverages of north-western Himalayas using in vitro screening and principal component analysis. J. Food Sci. Technol. 2016, 53, 2463–2475. [Google Scholar] [CrossRef] [PubMed]
- De Prisco, A.; Maresca, D.; Ongeng, D.; Mauriello, G. Microencapsulation by vibrating technology of the probiotic strain lactobacillus reuteri dsm 17938 to enhance its survival in foods and in gastrointestinal environment. LWT Food Sci. Technol. 2015, 61, 452–462. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.C.; Baines, S.K. In vitro analysis of gastrointestinal tolerance and intestinal cell adhesion of probiotics in goat’s milk ice cream and yogurt. Food. Res. Int. 2012, 49, 619–625. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Bušić, A.; Barišić, L.; Vrsaljko, D.; Karlović, S.; Špoljarić, I.; Vojvodić, A.; Mršić, G.; Komes, D. Emulsion templated microencapsulation of dandelion (Taraxacum officinale L.) polyphenols and β-carotene by ionotropic gelation of alginate and pectin. Food Hydrocoll. 2016, 57, 139–152. [Google Scholar] [CrossRef]
- Coghetto, C.C.; Brinques, G.B.; Siqueira, N.M.; Pletsch, J.; Soares, R.M.D.; Ayub, M.A.Z. Electrospraying microencapsulation of lactobacillus plantarum enhances cell viability under refrigeration storage and simulated gastric and intestinal fluids. J. Funct. Food 2016, 24, 316–326. [Google Scholar] [CrossRef]
- Farnworth, E.R.; Champagne, C.P. Production of Probiotic Cultures and Their Incorporation into Foods. In Probiotics, Prebiotics, and Synbiotics: Bioactive Foods in Health Promotion; Watson, R.R., Preedy, V., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 303–318. [Google Scholar]
- Dulf, F.; Andrei, S.; Bunea, A.; Socaciu, C. Fatty acid and phytosterol contents of some romanian wild and cultivated berry pomaces. Chem. Pap. 2012, 66, 925–934. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Ho, L.S.; Nair, A.; Yusof, H.M.; Kulaveerasingam, H.; Jangi, M.S. Morphometry of lipid bodies in embryo, kernel and mesocarp of oil palm: Its relationship to yield. Am. J. Plant Sci. 2014, 5, 1163–1173. [Google Scholar] [CrossRef]
- Chavarri, M.; Maranon, I.; Ares, R.; Ibanez, F.C.; Marzo, F.; Villaran, M.D.C. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Pop, O.; Brandau, T.; Schwinn, J.; Vodnar, D.; Socaciu, C. The influence of different polymers on viability of bifidobacterium lactis 300b during encapsulation, freeze-drying and storage. J. Food Sci. Technol. 2015, 52, 4146–4155. [Google Scholar] [CrossRef] [PubMed]
- Mapelli-Brahm, P.; Corte-Real, J.; Meléndez-Martínez, A.J.; Bohn, T. Bioaccessibility of phytoene and phytofluene is superior to other carotenoids from selected fruit and vegetable juices. Food Chem. 2017, 229, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Ferreira, I.C. Wastes and by-products: Upcoming sources of carotenoids for biotechnological purposes and health-related applications. Trends Food Sci. Technol. 2017, 62, 33–48. [Google Scholar] [CrossRef]
- Pintea, A.; Varga, A.; Stepnowski, P.; Socaciu, C.; Culea, M.; Diehl, H.A. Chromatographic analysis of carotenol fatty acid esters in physalis alkekengi and hippophae rhamnoides. Phytochem. Anal. 2005, 16, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wu, J.; Wei, L.; Wu, F. Temperature dependence of aggregated structure of β-carotene by absorption spectral experiment and simulation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 169, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Lenucci, M.S.; Laddomada, B.; Mita, G.; Caretto, S. Effects of sodium alginate bead encapsulation on the storage stability of durum wheat (Triticum durum Desf.) bran oil extracted by supercritical CO2. J. Agric. Food Chem. 2012, 60, 10689–10695. [Google Scholar] [CrossRef] [PubMed]
- Eratte, D.; Wang, B.; Dowling, K.; Barrow, C.J.; Adhikari, B. Survival and fermentation activity of probiotic bacteria and oxidative stability of omega-3 oil in co-microcapsules during storage. J. Funct. Food 2016, 23, 485–496. [Google Scholar] [CrossRef]
- Dulf, F.V. Fatty acids in berry lipids of six sea buckthorn (Hippophae rhamnoides L., subspecies Carpatica) cultivars grown in romania. Chem. Cent. J. 2012, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Liu, L.P.; Jiao, L.X.; Fan, M.T. Volatile profile of sea buckthorn wines, raw juices and must in Qinghai (China). Int. J. Food Prop. 2011, 14, 776–785. [Google Scholar] [CrossRef]
- Socaci, S.A.; Socaciu, C.; Tofană, M.; Raţi, I.V.; Pintea, A. In-tube extraction and gc-ms analysis of volatile components from wild and cultivated sea buckthorn (Hippophae rhamnoides L. ssp. Carpatica) berry varieties and juice. Phytochem. Anal. 2013, 24, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Peredo, A.G.; Beristain, C.I.; Pascual, L.A.; Azuara, E.; Jimenez, M. The effect of prebiotics on the viability of encapsulated probiotic bacteria. LWT Food Sci. Technol. 2016, 73, 191–196. [Google Scholar] [CrossRef]
- Pop, O.L.; Vodnar, D.C.; Suharoschi, R.; Mudura, E.; Socaciu, C.L. Plantarumatcc 8014 entrapment with prebiotics and lucerne green juice and their behavior in simulated gastrointestinal conditions. J. Food Process Eng. 2015. [Google Scholar] [CrossRef]
- Laos, K.; Lõugas, T.; Mändmets, A.; Vokk, R. Encapsulation of β-carotene from sea buckthorn (Hippophaë rhamnoides L.) juice in furcellaran beads. Innov. Food Sci. Emerg. 2007, 8, 395–398. [Google Scholar] [CrossRef]
- Khosravi Zanjani, M.A.; Ghiassi Tarzi, B.; Sharifan, A.; Mohammadi, N. Microencapsulation of probiotics by calcium alginate-gelatinized starch with chitosan coating and evaluation of survival in simulated human gastro-intestinal condition. Iran. J. Pharm. Res. 2014, 13, 843–852. [Google Scholar] [PubMed]
- Silva, D.; Pinto, L.F.V.; Bozukova, D.; Santos, L.F.; Serro, A.P.; Saramago, B. Chitosan/alginate based multilayers to control drug release from ophthalmic lens. Colloid Surf. B Biointerface 2016, 147, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Woo, I.S.; Rhee, I.K.; Park, H.D. Differential damage in bacterial cells by microwave radiation on the basis of cell wall structure. Appl. Environ. Microbiol. 2000, 66, 2243–2247. [Google Scholar] [CrossRef] [PubMed]
- Haddaji, N.; Mahdhi, A.K.; Krifi, B.; Ismail, M.B.; Bakhrouf, A. Change in cell surface properties of lactobacillus casei under heat shock treatment. FEMS Microbiol. Lett. 2015, 362. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.C.; Lan, C.C.E.; Huang, T.Y.; Chen, K.W.; Chai, C.Y.; Chen, W.T.; Fang, A.H.; Chen, Y.H.; Wu, C.S. Heat-killed and live: Lactobacillus reuteri gmnl-263 exhibit similar effects on improving metabolic functions in high-fat diet-induced obese rats. Food Funct. 2016, 7, 2374–2388. [Google Scholar] [CrossRef] [PubMed]
- Liévin-Le Moal, V. A gastrointestinal anti-infectious biotherapeutic agent: The heat-treated Lactobacillus LB. Therap. Adv. Gastroenterol. 2016, 9, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Sidira, M.; Karapetsas, A.; Galanis, A.; Kanellaki, M.; Kourkoutas, Y. Effective survival of immobilized lactobacillus casei during ripening and heat treatment of probiotic dry-fermented sausages and investigation of the microbial dynamics. Meat Sci. 2014, 96, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Hartvig, D.; Hausner, H.; Wendin, K.; Bredie, W.L.P. Quinine sensitivity influences the acceptance of sea-buckthorn and grapefruit juices in 9- to 11-year-old children. Appetite 2014, 74, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Darjani, P.; Hosseini Nezhad, M.; Kadkhodaee, R.; Milani, E. Influence of prebiotic and coating materials on morphology and survival of a probiotic strain of lactobacillus casei exposed to simulated gastrointestinal conditions. LWT Food Sci. Technol. 2016, 73, 162–167. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Socaciu, C. Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chem. 2016, 209, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.-S.; Wong, S.-L.; Lee, P.-P.; Lee, J.-S.; Ti, T.B.; Zhang, Z.; Poncelet, D.; Ravindra, P.; Phan, S.-H.; Yim, Z.-H. Effects of starch filler on the physical properties of lyophilized calcium-alginate beads and the viability of encapsulated cells. Carbohydr. Polym. 2011, 83, 225–232. [Google Scholar] [CrossRef]
- Sandoval-Castilla, O.; Lobato-Calleros, C.; Garcia-Galindo, H.S.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Textural properties of alginate-pectin beads and survivability of entrapped lb. Casei in simulated gastrointestinal conditions and in yoghurt. Food Res. Int. 2010, 43, 111–117. [Google Scholar] [CrossRef]
- Pop, O.L.; Brandau, T.; Vodnar, D.C.; Socaciu, C. Study of bifidobacterium lactic 300b survival during encapsulation, coating and freeze drying process and the release in alkaline media. Bull. Univ. Agric. Sci. Vet. 2012, 69, 372–379. [Google Scholar] [CrossRef]
- Aste, T.; Weaire, D. The Pursuit of Perfect Packing, 2nd ed.; Taylor and Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
| Fatty Acids | SE Lipid Fraction | SE from Capsules | ||
|---|---|---|---|---|
| % | SD | % | SD | |
| Caprylic | 0.04 | 0.01 | 0.01 | 0.01 |
| Capric | 0.03 | 0.01 | 0.02 | 0.01 |
| Lauric | 0.04 | 0.01 | 0.05 | 0.01 |
| Myristic | 0.18 | 0.02 | 0.20 | 0.02 |
| Z-11-Tetradecenoic acid | 0.05 | 0.01 | 0.02 | 0.01 |
| Pentadecanoic | 0.07 | 0.02 | 0.06 | 0.01 |
| Palmitic | 26.59 | 1.33 | 27.94 | 1.45 |
| 7-Hexadecenoic acid | 0.03 | 0.01 | 0.03 | 0.01 |
| Palmitoleic | 26.2 | 1.31 | 25.15 | 1.30 |
| Margaric acid | 0.02 | 0.01 | 0.04 | 0.01 |
| Stearic | 1.11 | 0.06 | 1.13 | 0.06 |
| Oleic | 27.73 | 1.40 | 27.83 | 1.35 |
| Vaccenic | 10.73 | 0.55 | 10.05 | 0.45 |
| Linoleic | 5.42 | 0.25 | 5.73 | 0.29 |
| Alfa-linolenic | 1.45 | 0.07 | 1.36 | 0.07 |
| Arachidic | 0.14 | 0.02 | 0.14 | 0.01 |
| Gondoic | 0.11 | 0.02 | 0.16 | 0.02 |
| Behenic | 0.02 | 0.01 | 0.03 | 0.01 |
| Erucic | 0.03 | 0.01 | 0.08 | 0.01 |
| Fatty Acids | SE lipid Fraction | SE from Capsules |
|---|---|---|
| ΣSFAs | 28.25 ± 1.41 ba | 29.62 ± 1.48 ba |
| ΣMUFAs | 64.88 ± 3.24 aa | 63.29 ± 3.16 aa |
| ΣPUFAs | 6.87 ± 0.34 ca | 7.09 ± 0.35 ca |
| n-3 PUFA | 1.45 ± 0.07 a | 1.36 ± 0.07 a |
| n-6 PUFA | 5.42 ± 0.27 a | 5.73 ± 0.29 a |
| n-6/n-3 | 3.74 a | 4.21 a |
| PUFAs/SFAs | 0.24 a | 0.24 a |
| Microparticles | Microparticles Size (µm) (n = 10) | Encapsulation Yield (%) (n = 10) |
|---|---|---|
| Alginate 1.5% | 1255.5 ± 12.7 | 96.13 ± 0.28 a |
| Alginate 1.5% and 10% SE | 1285.5 ± 1.3 | 98.46 ± 1.08 b |
| Treatment Time (s) | 0 | 10 | 30 | 60 | 90 | 120 | ||
|---|---|---|---|---|---|---|---|---|
| Microwave Power/Sample | ||||||||
| 100 W | YLS | CFU log/g | 7.58 ± 0.07 | 6.69 ± 0.21 | 7.28 ± 0.14 | 7.26 ± 0.05 | 7.75 ± 0.32 | 8.33 ± 0.41 |
| YLE | 7.89 ± 0.5 | 8.36 ± 0.12 | 7.32 ± 0.22 | 7.31 ± 0.5 | 7.45 ± 0.02 | 8.03 ± 0.14 | ||
| YLES | 8.17 ± 0.21 | 8.53 ± 0.4 | 8.42 ± 0.13 | 8.63 ± 0.18 | 8.31 ± 0.5 | 9.05 ± 0.22 | ||
| Temp °C | 4 ± 0.2 | 5.5 ± 0.2 | 7.14 ± 0.2 | 13.2 ± 0.2 | 19.5 ± 0.2 | 23.2 ± 0.2 | ||
| 450 W | YLS | CFU log/g | 7.47 ± 0.12 | 7.74 ± 0.23 | 7.44 ± 0.2 | 8.12 ± 0.4 | 8.66 ± 0.24 | 8.87 ± 0.09 |
| YLE | 7.89 ± 0.51 | 7.65 ± 0.2 | 7.39 ± 0.12 | 7.36 ± 0.15 | 8.11 ± 0.8 | 8.98 ± 0.18 | ||
| YLES | 7.78 ± 0.29 | 7.80 ± 0.02 | 7.62 ± 0.05 | 7.56 ± 0.5 | 7.79 ± 0.17 | 9.21 ± 0.13 | ||
| Temp °C | 4 ± 0.2 | 6.3 ± 0.2 | 12 ± 0.2 | 18.7 ± 0.2 | 39 ± 0.2 | 41 ± 0.2 | ||
| 850 W | YLS | CFU log/g | 7.65 ± 0.03 | 7.65 ± 0.45 | 7.52 ± 0.32 | 7.77 ± 0.15 | 6.16 ± 0.16 | 3.81 ± 0.4 |
| YLE | 7.89 ± 0.22 | 7.84 ± 0.41 | 7.68 ± 0.35 | 8.38 ± 0.05 | 6.83 ± 0.09 | 4.12 ± 0.12 | ||
| YLES | 8.06 ± 0.19 | 7.89 ± 0.16 | 7.84 ± 0.24 | 8.86 ± 0.41 | 7.69 ± 0.32 | 5.56 ± 0.24 | ||
| Temp °C | 4 ± 0.2 | 11.4 ± 0.2 | 14.9 ± 0.2 | 30 ± 0.2 | 55 ± 0.2 | 64.5 ± 0.2 | ||
| Yogurt trials | SE* | Sample Coding |
|---|---|---|
| Yogurt with L. casei and 10% SE | 10% | YLS |
| Yogurt with L. casei encapsulated | - | YLE |
| Yogurt with L. casei and 10% SE encapsulated | 10% | YLSE |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, O.L.; Dulf, F.V.; Cuibus, L.; Castro-Giráldez, M.; Fito, P.J.; Vodnar, D.C.; Coman, C.; Socaciu, C.; Suharoschi, R. Characterization of a Sea Buckthorn Extract and Its Effect on Free and Encapsulated Lactobacillus casei. Int. J. Mol. Sci. 2017, 18, 2513. https://doi.org/10.3390/ijms18122513
Pop OL, Dulf FV, Cuibus L, Castro-Giráldez M, Fito PJ, Vodnar DC, Coman C, Socaciu C, Suharoschi R. Characterization of a Sea Buckthorn Extract and Its Effect on Free and Encapsulated Lactobacillus casei. International Journal of Molecular Sciences. 2017; 18(12):2513. https://doi.org/10.3390/ijms18122513
Chicago/Turabian StylePop, Oana Lelia, Francisc Vasile Dulf, Lucian Cuibus, Marta Castro-Giráldez, Pedro J. Fito, Dan Cristian Vodnar, Cristina Coman, Carmen Socaciu, and Ramona Suharoschi. 2017. "Characterization of a Sea Buckthorn Extract and Its Effect on Free and Encapsulated Lactobacillus casei" International Journal of Molecular Sciences 18, no. 12: 2513. https://doi.org/10.3390/ijms18122513