BCL11A mRNA Targeting by miR-210: A Possible Network Regulating γ-Globin Gene Expression
Abstract
:1. Introduction
2. Results
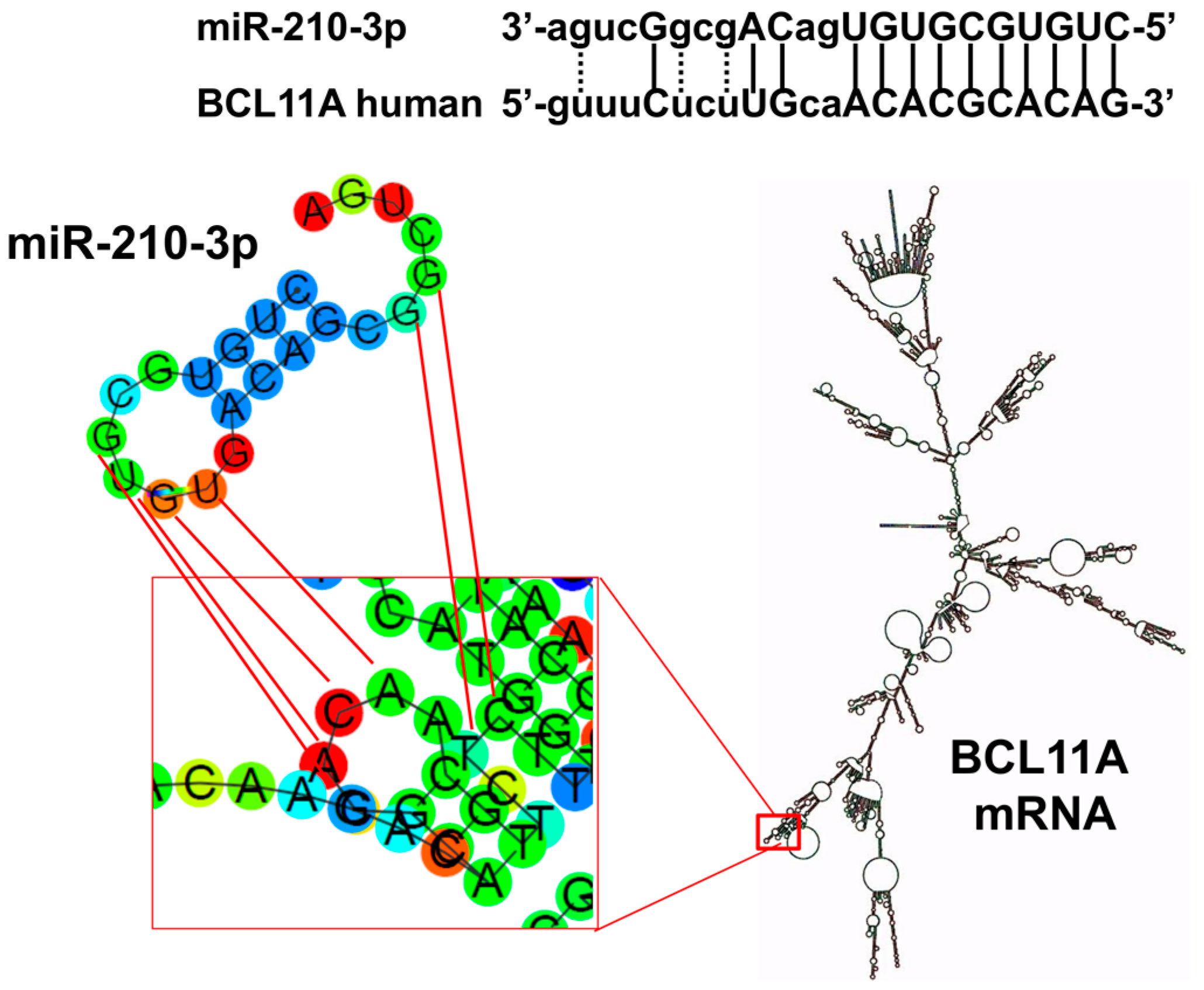
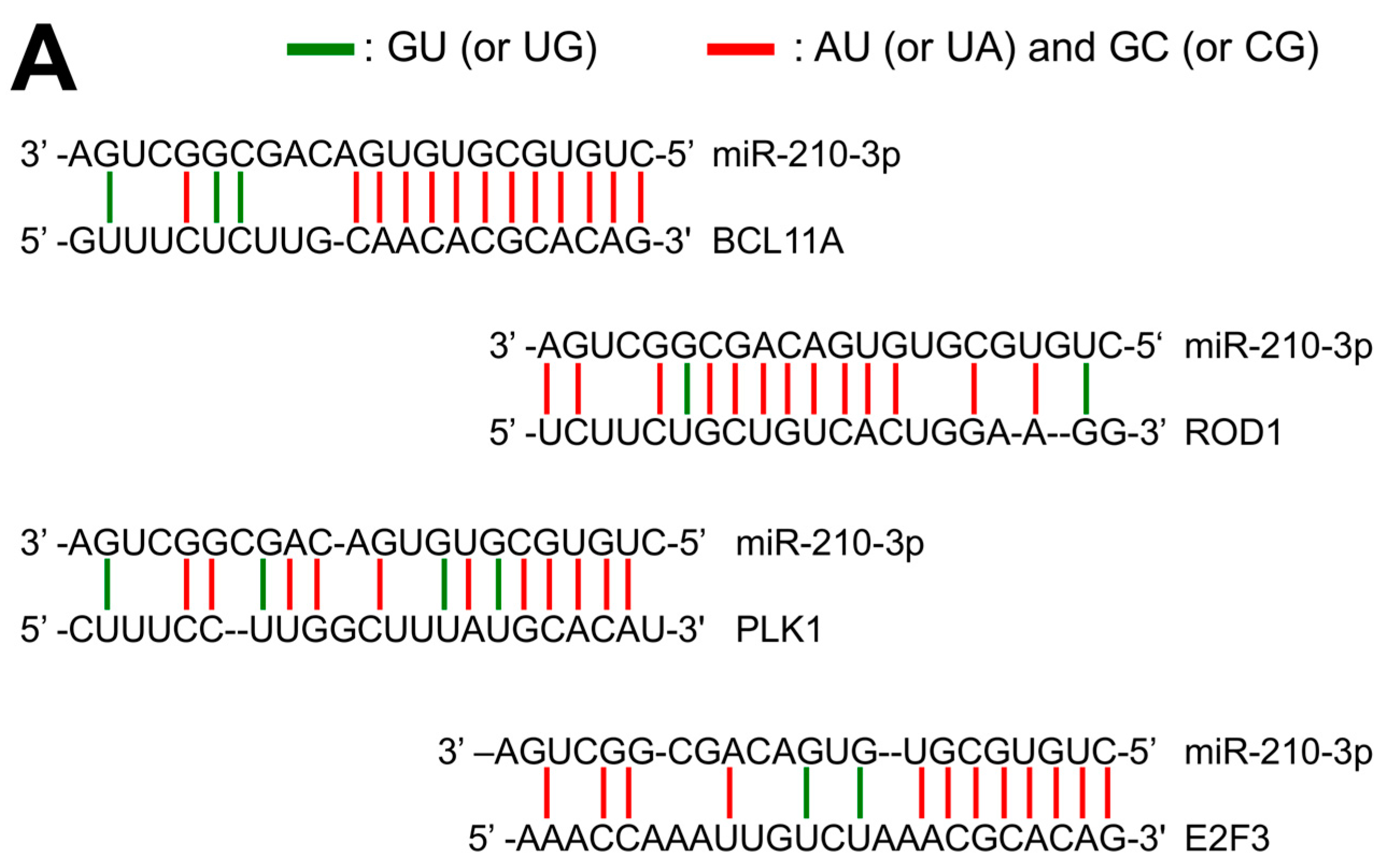
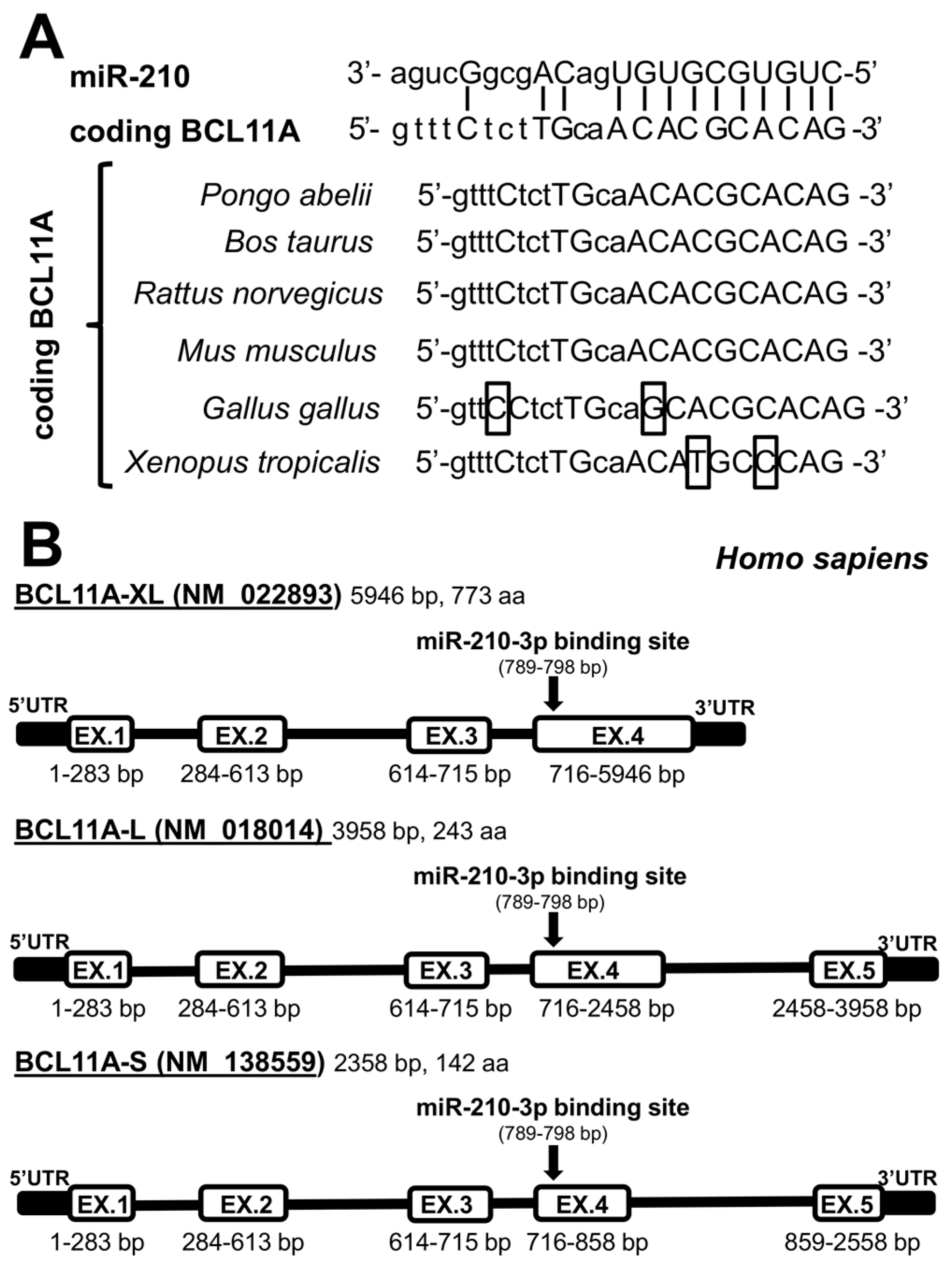
2.1. Presence of miR-210 Binding Sites within mRNA Sequences Coding Repressors of γ-Globin Gene Transcription: The Coding Sequence of BCL11A mRNA Contains a Putative miR-210 Binding Site
2.2. Interaction of miR-210 with BCL11A Sequences Mimicking the miR-210 Binding Site of BCL11A mRNA
2.3. The miR-210 Putative Binding Sites of BCL11A Are Conserved through Molecular Evolution
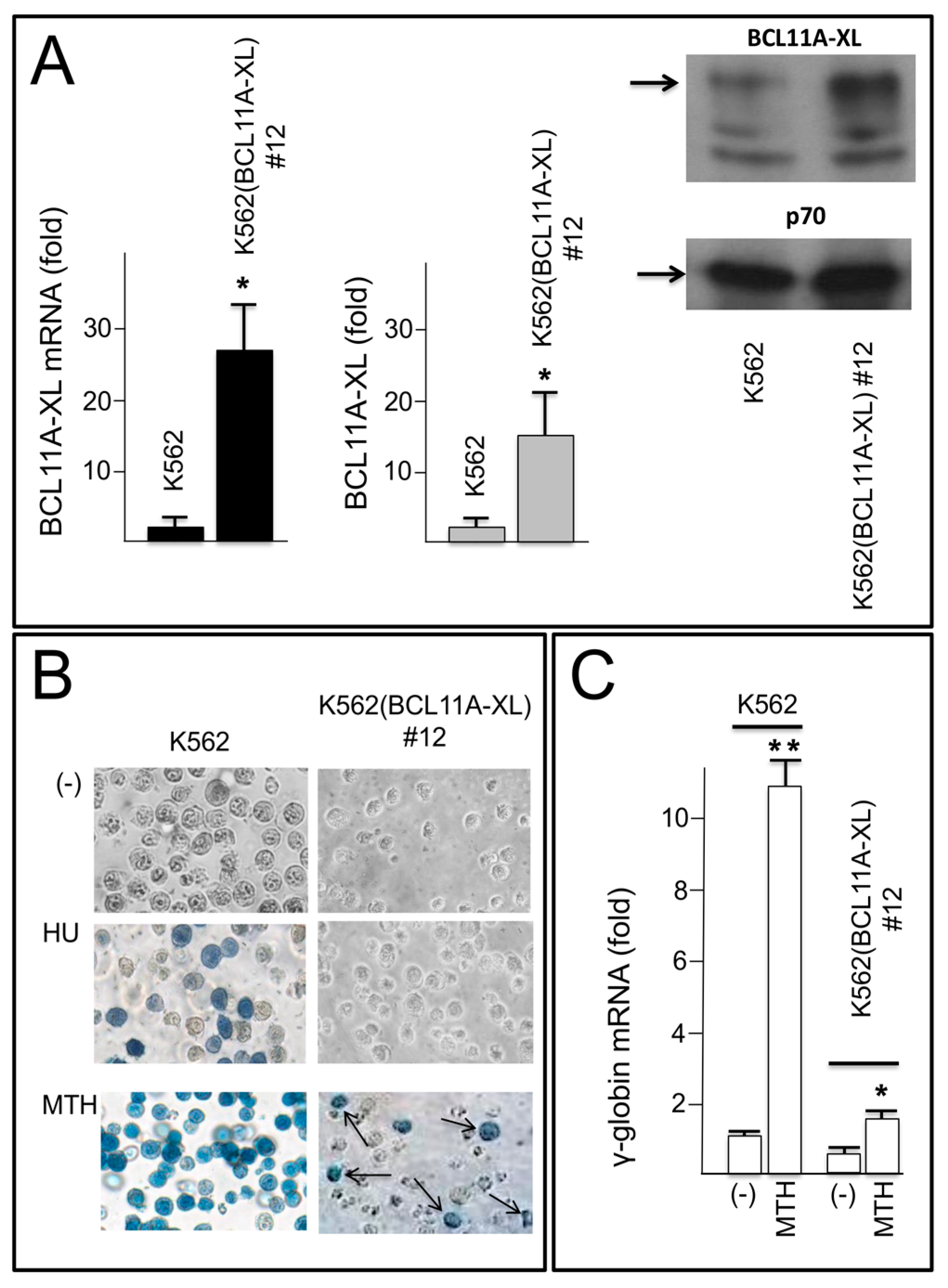
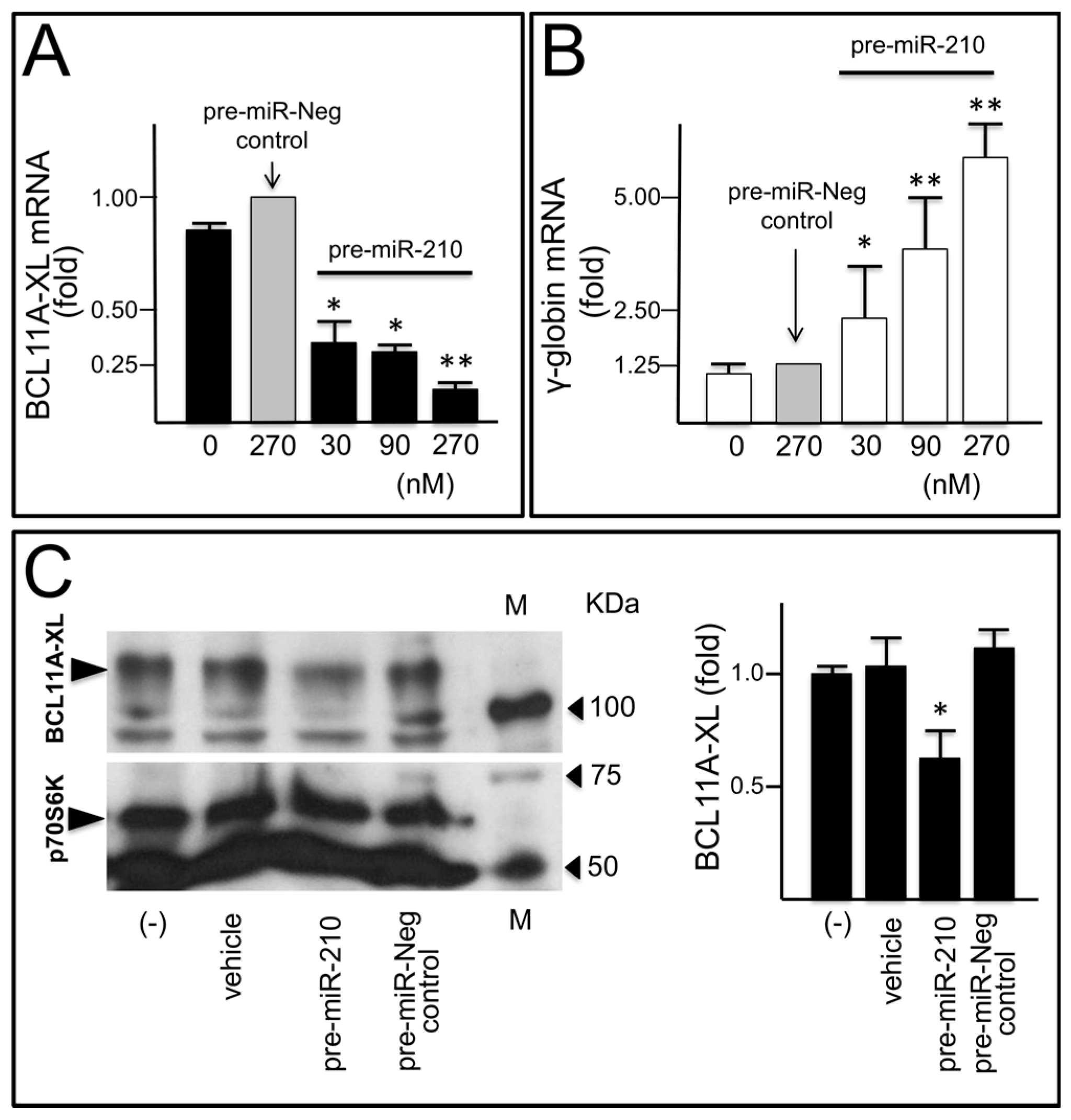
2.4. Decrease of BCL11A-XL and Increase of γ-Globin after Transfection of a K562(BCL11A-XL) Clone with Pre-miR-210
2.5. Transfection of Erythroid Precursor Cells with Pre-miR-210 Leads to a Decrease of BCL11A-XL and an Increase of γ-Globin mRNA
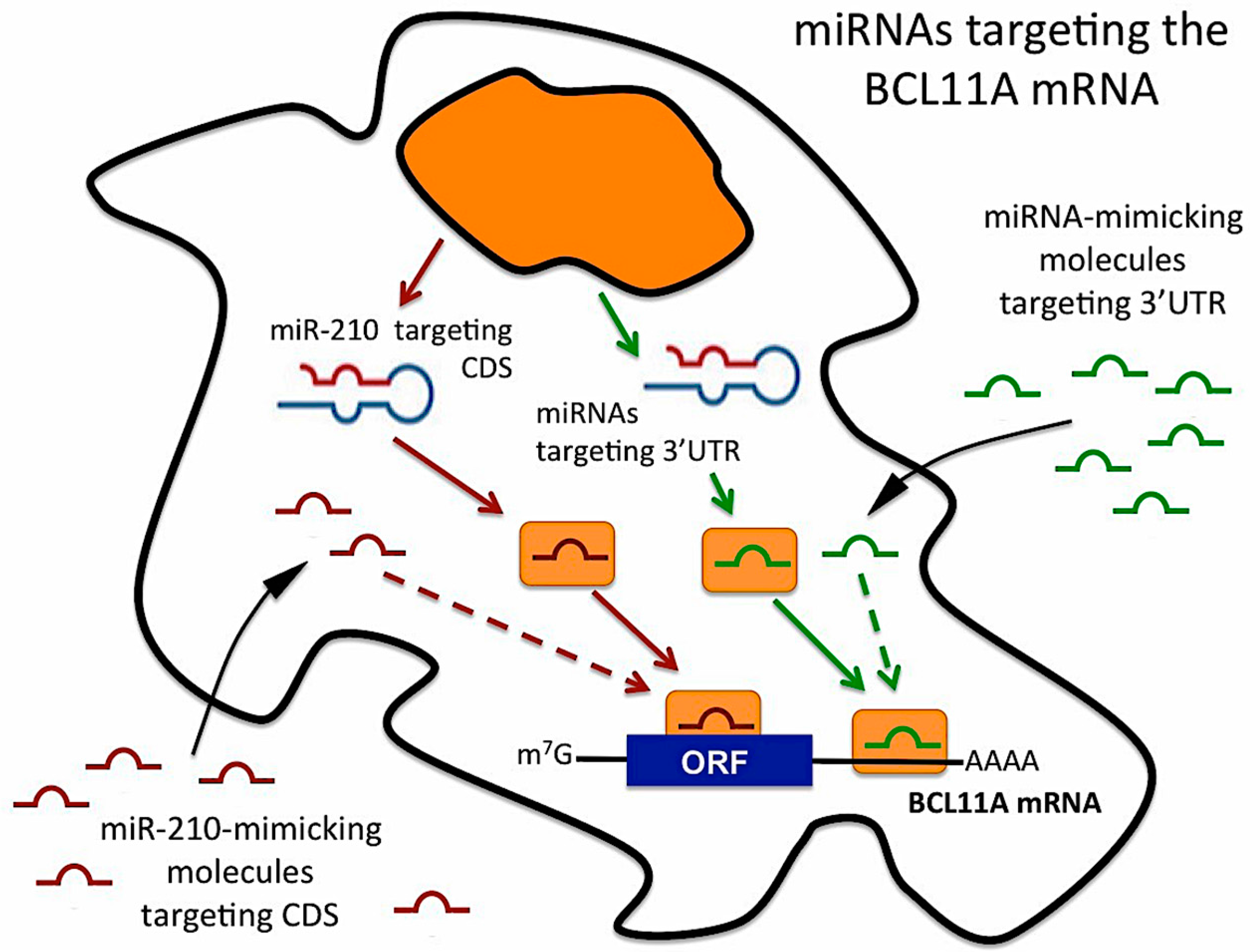
3. Discussion
4. Materials and Methods
4.1. Bioinformatic Analysis
4.2. Biospecific Interaction Analysis (BIA) with Biacore™ X100
4.3. Ethics Statement
4.4. Human Cell Lines and Culture Conditions
4.5. Patients and Erythroid Precursor Cultures
4.6. Transfection Procedure
4.7. RNA Extraction
4.8. Reverse Transcription and Quantitative Real-Time PCR (RT-qPCR)
4.9. Western Blot
4.10. Elisa Assay
4.11. FACS Analysis
4.12. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ErPCs | Erythroid precursor cells |
| HPFH | Hereditary persistence of fetal hemoglobin |
| MTH | Mithramycin |
| RAPA | Rapamycin |
| RSV | Resveratrol |
| BA | Butyric acid |
| HU | Hydroxyurea |
| EPO | Erythropoietin |
| SCF | Stem cell factor |
| PBS | Phosphate buffered saline |
| HbF | Fetal hemoglobin |
| UTR | Untranslated region |
| CDS | Coding DNA sequence |
| UBC | Umbilical cord blood |
| BIA | Biomolecular interaction analysis |
| BCL11A | B-cell lymphoma/leukemia 11A |
| RPL13A | Ribosomal protein L13a |
| GPA | Glycophorin A |
| trfR | Transferrin Receptor |
| p70S6K | Ribosomal protein S6 kinase β-1 |
References
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.P.; Lau, N.C.; Garrett-Engele, P.; Grimson, A.; Schelter, J.M.; Castle, J.; Bartel, D.P.; Linsley, P.S.; Johnson, J.M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005, 433, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S. The microRNA Registry. Nucleic Acids Res. 2004, 32, D109–D111. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, N.; Zuccato, C.; Finotti, A.; Lampronti, I.; Borgatti, M.; Gambari, R. Involvement of miRNA in erythroid differentiation. Epigenomics 2012, 4, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Gambari, R.; Fabbri, E.; Borgatti, M.; Lampronti, I.; Finotti, A.; Brognara, E.; Bianchi, N.; Manicardi, A.; Marchelli, R.; Corradini, R. Targeting microRNAs involved in human diseases: A novel approach for modification of gene expression and drug development. Biochem. Pharmacol. 2011, 82, 1416–1429. [Google Scholar] [CrossRef] [PubMed]
- Felli, N.; Fontana, L.; Pelosi, E.; Botta, R.; Bonci, D.; Facchiano, F.; Liuzzi, F.; Lulli, V.; Morsilli, O.; Santoro, S.; et al. MicroRNAs 221 and 222 inhibit normal erythropoiesis and erythroleukemic cell growth via kit receptor down-modulation. Proc. Natl. Acad. Sci. USA 2005, 102, 18081–18086. [Google Scholar] [CrossRef] [PubMed]
- Choong, M.L.; Yang, H.H.; McNiece, I. MicroRNA expression profiling during human cord blood-derived CD34 cell erythropoiesis. Exp. Hematol. 2007, 35, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Georgantas, R.W., 3rd; Hildreth, R.; Morisot, S.; Alder, J.; Liu, C.G.; Heimfeld, S.; Calin, G.A.; Croce, C.M.; Civin, C.I. CD34+ hematopoietic stem-progenitor cell microRNA expression and function: A circuit diagram of differentiation control. Proc. Natl. Acad. Sci. USA 2007, 104, 2750–2755. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Z.; Lodish, H.F. MicroRNAs as regulators of mammalian hematopoiesis. Semin. Immunol. 2005, 17, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Kluiver, J.; Kroesen, B.J.; Poppema, S.; van den Berg, A. The role of microRNAs in normal hematopoiesis and hematopoietic malignancies. Leukemia 2006, 20, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Masaki, S.; Ohtsuka, R.; Abe, Y.; Muta, K.; Umemura, T. Expression patterns of microRNAs 155 and 451 during normal human erythropoiesis. Biochem. Biophys. Res. Commun. 2007, 364, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Bruchova, H.; Yoon, D.; Agarwal, A.M.; Mendell, J.; Prchal, J.T. Regulated expression of microRNAs in normal and polycythemia vera erythropoiesis. Exp. Hematol. 2007, 35, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Spinello, I.; Quaranta, M.T.; Pasquini, L.; Pelosi, E.; Petrucci, E.; Pagliuca, A.; Castelli, G.; Mariani, G.; Diverio, D.; Foà, R.; et al. PLZF-mediated control on c-kit expression in CD34+ cells and early erythropoiesis. Oncogene 2009, 28, 2276–2288. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, C.H. MicroRNAs and haematology: Small molecules, big function. Br. J. Haematol. 2007, 137, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.H.; Wang, F.; Yu, J.; Wang, X.S.; Yuan, J.Y.; Zhang, J.W. MicroRNAs are involved in erythroid differentiation control. J. Cell. Biochem. 2009, 107, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Felli, N.; Pedini, F.; Romania, P.; Biffoni, M.; Morsilli, O.; Castelli, G.; Santoro, S.; Chicarella, S.; Sorrentino, A.; Peschle, C.; et al. MicroRNA 223-dependent expression of LMO2 regulates normal erythropoiesis. Haematologica 2009, 94, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Dore, L.C.; Amigo, J.D.; dos Santos, C.O.; Zhang, Z.; Gai, X.; Tobias, J.W.; Yu, D.; Klein, A.M.; Dorman, C.; Wu, W.; et al. A GATA-1-regulated microRNA locus essential for erythropoiesis. Proc. Natl. Acad. Sci. USA 2008, 105, 3333–3338. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Sugiura, K.; Yamamoto, Y.; Yoshioka, Y.; Miyazaki, H.; Komatsu, N.; Ochiya, T.; Kato, T. Identification of erythropoietin-induced microRNAs in haematopoietic cells during erythroid differentiation. Br. J. Haematol. 2008, 142, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, N.; Zuccato, C.; Lampronti, I.; Borgatti, M.; Gambari, R. Expression of miR-210 during erythroid differentiation and induction of γ-globin gene expression. BMB Rep. 2009, 42, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, N.; Finotti, A.; Ferracin, M.; Lampronti, I.; Zuccato, C.; Breveglieri, G.; Brognara, E.; Fabbri, E.; Borgatti, M.; Negrini, M.; et al. Increase of microRNA-210, decrease of raptor gene expression and alteration of mammalian target of rapamycin regulated proteins following mithramycin treatment of human erythroid cells. PLoS ONE 2015, 10, e0121567. [Google Scholar] [CrossRef] [PubMed]
- Sarakul, O.; Vattanaviboon, P.; Tanaka, Y.; Fucharoen, S.; Abe, Y.; Svasti, S.; Umemura, T. Enhanced erythroid cell differentiation in hypoxic condition is in part contributed by miR-210. Blood Cells Mol. Dis. 2013, 51, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Bavelloni, A.; Poli, A.; Fiume, R.; Blalock, W.; Matteucci, A.; Ramazzotti, G.; McCubrey, J.A.; Cocco, L.; Faenza, I. PLC-β 1 regulates the expression of miR-210 during mithramycin-mediated erythroid differentiation in K562 cells. Oncotarget 2014, 5, 4222–4231. [Google Scholar] [CrossRef] [PubMed]
- Sawant, M.; Colah, R.; Ghosh, K.; Nadkarni, A. Does HbF induction by hydroxycarbamide work through MIR210 in sickle cell anaemia patients? Br. J. Haematol. 2016, 173, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Finotti, A.; Gambari, R. Recent trends for novel options in experimental biological therapy of β-thalassemia. Expert Opin. Biol. Ther. 2014, 14, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Finotti, A.; Breda, L.; Lederer, C.W.; Bianchi, N.; Zuccato, C.; Kleanthous, M.; Rivella, S.; Gambari, R. Recent trends in the gene therapy of β-thalassemia. J. Blood Med. 2015, 6, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.S.; Hong, X.; Wang, G. Induction of endogenous gamma-globin gene expression with decoy oligonucleotide targeting Oct-1 transcription factor consensus sequence. J. Hematol. Oncol. 2009, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Best, S.; Menzel, S.; Silver, N.; Lai, M.I.; Surdulescu, G.L.; Spector, T.D.; Thein, S.L. cMYB is involved in the regulation of fetal hemoglobin production in adults. Blood 2006, 108, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Liu, K.; Sun, C.W.; Pawlik, K.M.; Townes, T.M. KLF1 regulates BCL11A expression and gamma- to β-globin gene switching. Nat. Genet. 2010, 42, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, V.G.; Menne, T.F.; Xu, J.; Akie, T.E.; Lettre, G.; Van Handel, B.; Mikkola, H.K.; Hirschhorn, J.N.; Cantor, A.B.; Orkin, S.H. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 2008, 322, 1839–1842. [Google Scholar] [CrossRef] [PubMed]
- Basak, A.; Sankaran, V.G. Regulation of the fetal hemoglobin silencing factor BCL11A. 2016, 1368, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Wang, Y.; Liu, R.; Zhang, Y.; Xu, Z.; Wang, Y.; Wu, Y.; Liu, M.; Cerruti, L.; Zou, F.; et al. Human fetal globin gene expression is regulated by LYAR. Nucleic Acids Res. 2014, 42, 9740–9752. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, N.; Cosenza, L.C.; Lampronti, I.; Finotti, A.; Breveglieri, G.; Zuccato, C.; Fabbri, E.; Marzaro, G.; Chilin, A.; De Angelis, G.; et al. Structural and Functional Insights on an Uncharacterized Aγ-Globin-Gene Polymorphism Present in Four β0-Thalassemia Families with High Fetal Hemoglobin Levels. Mol. Diagn. Therapy 2016, 20, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, V.G.; Menne, T.F.; Šćepanović, D.; Vergilio, J.A.; Ji, P.; Kim, J.; Thiru, P.; Orkin, S.H.; Lander, E.S.; Lodish, H.F. MicroRNA-15a and -16-1 act via MYB to elevate fetal hemoglobin expression in human trisomy 13. Proc. Natl. Acad. Sci. USA 2011, 108, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Lulli, V.; Romania, P.; Morsilli, O.; Cianciulli, P.; Gabbianelli, M.; Testa, U.; Giuliani, A.; Marziali, G. MicroRNA-486-3p regulates γ-globin expression in human erythroid cells by directly modulating BCL11A. PLoS ONE 2013, 8, e60436. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, B.; Jiang, F.; Wang, D.; Liu, H.; Yan, Y.; Dong, H.; Wang, F.; Gong, B.; Zhu, Y.; et al. A feedback loop consisting of microRNA 23a/27a and the β-like globin suppressors KLF3 and SP1 regulates globin gene expression. Mol. Cell. Biol. 2013, 33, 3994–4007. [Google Scholar] [CrossRef] [PubMed]
- Finotti, A.; Borgatti, M.; Bianchi, N.; Zuccato, C.; Lampronti, I.; Gambari, R. Orphan Drugs and Potential Novel Approaches for Therapies of β-Thalassemia: Current Status and Future Expectations. Expert Opin. Orphan Drugs 2016, 4, 299–315. [Google Scholar] [CrossRef]
- Fasanaro, P.; Greco, S.; Lorenzi, M.; Pescatori, M.; Brioschi, M.; Kulshreshtha, R.; Banfi, C.; Stubbs, A.; Calin, G.A.; Ivan, M.; et al. An integrated approach for experimental target identification of hypoxia-induced miR-210. J. Biol. Chem. 2009, 284, 35134–35143. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wu, J.; Xu, N.; Xie, W.; Li, M.; Li, J.; Jiang, Y.; Yang, B.B.; Zhang, Y. MiR-210 disturbs mitotic progression through regulating a group of mitosis-related genes. Nucleic Acids Res. 2013, 41, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Fasanaro, P.; Romani, S.; Voellenkle, C.; Maimone, B.; Capogrossi, M.C.; Martelli, F. ROD1 is a seedless target gene of hypoxia-induced miR-210. PLoS ONE 2012, 7, e44651. [Google Scholar] [CrossRef] [PubMed]
- Nakada, C.; Tsukamoto, Y.; Matsuura, K.; Nguyen, T.L.; Hijiya, N.; Uchida, T.; Sato, F.; Mimata, H.; Seto, M.; Moriyama, M. Overexpression of miR-210, a downstream target of HIF1α, causes centrosome amplification in renal carcinoma cells. J. Pathol. 2011, 224, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Finotti, A.; Gasparello, J.; Breveglieri, G.; Cosenza, L.C.; Montagner, G.; Bresciani, A.; Altamura, S.; Bianchi, N.; Martini, E.; Gallerani, E.; et al. Development and characterization of K562 cell clones expressing BCL11A-XL: Decreased hemoglobin production with fetal hemoglobin inducers and its rescue with mithramycin. Exp. Hematol. 2015, 43, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Fibach, E.; Bianchi, N.; Borgatti, M.; Prus, E.; Gambari, R. Mithramycin induces fetal hemoglobin production in normal and thalassemic human erythroid precursor cells. Blood 2003, 102, 1276–1281. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, E.; Montagner, G.; Bianchi, N.; Finotti, A.; Borgatti, M.; Lampronti, I.; Cabrini, G.; Gambari, R. MicroRNA miR-93-5p regulates expression of IL-8 and VEGF in neuroblastoma SK-N-AS cells. Oncol. Rep. 2016, 35, 2866–2872. [Google Scholar] [CrossRef] [PubMed]
- Schnall-Levin, M.; Rissland, O.S.; Johnston, W.K.; Perrimon, N.; Bartel, D.P.; Berger, B. Unusually effective microRNA targeting within repeat-rich coding regions of mammalian mRNAs. Genome Res. 2011, 21, 1395–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Z.; Rajewsky, N. The impact of miRNA target sites in coding sequences and in 3′UTRs. PLoS ONE 2011, 6, e18067. [Google Scholar] [CrossRef] [PubMed]
- Grimson, A.; Farh, K.K.; Johnston, W.K.; Garrett-Engele, P.; Lim, L.P.; Bartel, D.P. MicroRNA targeting specificity in mammals: Determinants beyond seed pairing. Mol. Cell 2007, 27, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Brognara, E.; Fabbri, E.; Montagner, G.; Gasparello, J.; Manicardi, A.; Corradini, R.; Bianchi, N.; Finotti, A.; Breveglieri, G.; Borgatti, M.; et al. High levels of apoptosis are induced in human glioma cell lines by co-administration of peptide nucleic acids targeting miR-221 and miR-222. Int. J. Oncol. 2016, 48, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Brognara, E.; Fabbri, E.; Bazzoli, E.; Montagner, G.; Ghimenton, C.; Eccher, A.; Cantù, C.; Manicardi, A.; Bianchi, N.; Finotti, A.; et al. Uptake by human glioma cell lines and biological effects of a peptide-nucleic acids targeting miR-221. J. Neuro-Oncol. 2014, 118, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Lozzio, C.B.; Lozzio, B.B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood 1975, 45, 321–334. [Google Scholar] [PubMed]
- Lampronti, I.; Bianchi, N.; Zuccato, C.; Dall’acqua, F.; Vedaldi, D.; Viola, G.; Potenza, R.; Chiavilli, F.; Breveglieri, G.; Borgatti, M.; et al. Increase in gamma-globin mRNA content in human erythroid cells treated with angelicin analogs. Int. J. Hematol. 2009, 90, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Finotti, A.; Bianchi, N.; Fabbri, E.; Borgatti, M.; Breveglieri, G.; Gasparello, J.; Gambari, R. Erythroid induction of K562 cells treated with mithramycin is associated with inhibition of raptor gene transcription and mammalian target of rapamycin complex 1 (mTORC1) functions. Pharmacol. Res. 2015, 91, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, N.; Osti, F.; Rutigliano, C.; Corradini, F.G.; Borsetti, E.; Tomassetti, M.; Mischiati, C.; Feriotto, G.; Gambari, R. The DNA-binding drugs mithramycin and chromomycin are powerful inducers of erythroid differentiation of human K562 cells. Br. J. Haematol. 1999, 104, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Fibach, E.; Bianchi, N.; Borgatti, M.; Zuccato, C.; Finotti, A.; Lampronti, I.; Prus, E.; Mischiati, C.; Gambari, R. Effects of rapamycin on accumulation of α-, β- and γ-globin mRNAs in erythroid precursor cells from beta-thalassaemia patients. Eur. J. Haematol. 2006, 77, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Fibach, E.; Prus, E.; Bianchi, N.; Zuccato, C.; Breveglieri, G.; Salvatori, F.; Finotti, A.; Lipucci di Paola, M.; Brognara, E.; Lampronti, I.; et al. Resveratrol: Antioxidant activity and induction of fetal hemoglobin in erythroid cells from normal donors and β-thalassemia patients. Int. J. Mol. Med. 2012, 29, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Pule, G.D.; Mowla, S.; Novitzky, N.; Wonkam, A. Hydroxyurea down-regulates BCL11A, KLF-1 and MYB through miRNA-mediated actions to induce γ-globin expression: Implications for new therapeutic approaches of sickle cell disease. Clin. Transl. Med. 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]


| Name | Sequence 5′–3′ | Accession No. | Location |
|---|---|---|---|
| BCL11A | biot-GTT TCT CTT GCA ACA CGC ACA G 1 | NM_022893.3 2 | 789–798 |
| DNA aBCL11A | CTG TGC GTG TTG CAA GAG AAA C | NM_022893.3 2 | 789–798 |
| RNA aBCL11A | CUG UGC GUG UUG CAA GAG AAA C | NM_022893.3 2 | 789–798 |
| miR-210 | CUG UGC GUG UGA CAG CGG CUG A | MIMAT0000267 3 | - |
| miR-221 | AGC UAC AUU GUC UGC UGG GUU UC | MIMAT0000278 3 | - |
| miR-222 | AGC UAC AUC UGG CUA CUG GGU | MIMAT0000279 3 | - |
| Gene Name | Gene Accession Number | Assay Location | Assay ID |
|---|---|---|---|
| BCL11A 1 | NM_022893.3 | Exons 1–2 | Hs00256254_m1 |
| BCL11A 2 | NM_022893.3 | Exons 4–4 | Hs00250581_s1 |
| 18S Ribosomal RNA | X03205.1 | NA | 4310893E |
| RPL13A 3 | NM_012423.3 | Exons 5–6 | Hs03043885_g1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasparello, J.; Fabbri, E.; Bianchi, N.; Breveglieri, G.; Zuccato, C.; Borgatti, M.; Gambari, R.; Finotti, A. BCL11A mRNA Targeting by miR-210: A Possible Network Regulating γ-Globin Gene Expression. Int. J. Mol. Sci. 2017, 18, 2530. https://doi.org/10.3390/ijms18122530
Gasparello J, Fabbri E, Bianchi N, Breveglieri G, Zuccato C, Borgatti M, Gambari R, Finotti A. BCL11A mRNA Targeting by miR-210: A Possible Network Regulating γ-Globin Gene Expression. International Journal of Molecular Sciences. 2017; 18(12):2530. https://doi.org/10.3390/ijms18122530
Chicago/Turabian StyleGasparello, Jessica, Enrica Fabbri, Nicoletta Bianchi, Giulia Breveglieri, Cristina Zuccato, Monica Borgatti, Roberto Gambari, and Alessia Finotti. 2017. "BCL11A mRNA Targeting by miR-210: A Possible Network Regulating γ-Globin Gene Expression" International Journal of Molecular Sciences 18, no. 12: 2530. https://doi.org/10.3390/ijms18122530
APA StyleGasparello, J., Fabbri, E., Bianchi, N., Breveglieri, G., Zuccato, C., Borgatti, M., Gambari, R., & Finotti, A. (2017). BCL11A mRNA Targeting by miR-210: A Possible Network Regulating γ-Globin Gene Expression. International Journal of Molecular Sciences, 18(12), 2530. https://doi.org/10.3390/ijms18122530








