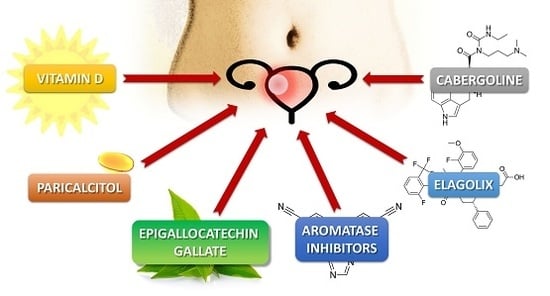
Alternative Oral Agents in Prophylaxis and Therapy of Uterine Fibroids—An Up-to-Date Review
Abstract
:1. Introduction
2. Materials and Methods
3. Discussion
3.1. Vitamin D
3.2. Vitamin D Analogs—Paricalcitol
3.3. Green Tea Extract—Epigallocatechin Gallate
3.4. Elagolix
3.5. Aromatase Inhibitors
3.6. Cabergoline
3.7. Others
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 25(OH)D | 25-hydroxyvitamin D |
| AI | aromatase inhibitor |
| ALK5 | transforming growth factor-β type 1 receptor |
| BCL | B-cell lymphoma |
| CDK | cyclin-dependent kinase |
| COMT | catechol-O-methyltransferase |
| ECG | epicatechin gallate |
| EGC | epigallocatechin |
| EGCG | epigallocatechin gallate |
| ER | estrogen receptor |
| GnRH | gonadotropin-releasing hormone |
| MMP | matrix metalloproteinase |
| mRNA | messenger RNA |
| PCNA | proliferating cell nuclear antigen |
| PPAR | peroxisome proliferator-activated receptor |
| PR | progesterone receptor |
| QoL | quality of life |
| RCT | randomized control trial |
| SPRM | selective progesterone receptor modulator |
| TGF-β | transforming growth factor beta |
| TIMP | tissue inhibitor of metalloproteinase |
| UF | uterine fibroid |
| UPA | ulipristal acetate |
| VDR | vitamin D receptor |
References
- Bulun, S.E. Uterine fibroids. N. Engl. J. Med. 2013, 369, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A.; Laughlin-Tommaso, S.K.; Catherino, W.H.; Lalitkumar, S.; Gupta, D.; Vollenhoven, B. Uterine fibroids. Nat. Rev. Dis. Prim. 2016, 2, 16043. [Google Scholar] [CrossRef] [PubMed]
- Al-Hendy, A.; Myers, E.R.; Stewart, E. Uterine fibroids: Burden and unmet medical need. Semin. Reprod. Med. 2017, 35, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.H. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil. Steril. 2007, 87, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.A.; Palmer, J.R.; Stewart, E.A.; Rosenberg, L. Age-specific incidence rates for self-reported uterine leiomyomata in the black women’s health study. Obstet. Gynecol. 2005, 105, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Dolmans, M.M. Uterine fibroid management: From the present to the future. Hum. Reprod. Update 2016, 22, 665–686. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A. Uterine fibroids. Lancet 2001, 357, 293–298. [Google Scholar] [CrossRef]
- Baird, D.D.; Dunson, D.B.; Hill, M.C.; Cousins, D.; Schectman, J.M. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am. J. Obstet. Gynecol. 2003, 188, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Borahay, M.A.; Asoglu, M.R.; Mas, A.; Adam, S.; Kilic, G.S.; Al-Hendy, A. Estrogen receptors and signaling in fibroids: Role in pathobiology and therapeutic implications. Reprod. Sci. 2017, 24, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Ishi, K.; Serna, V.A.; Kakazu, R.; Bulun, S.E.; Kurita, T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 2010, 151, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Maruo, T.; Ohara, N.; Wang, J.; Matsuo, H. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum. Reprod. Update 2004, 10, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Ciarmela, P.; Islam, M.S.; Reis, F.M.; Gray, P.C.; Bloise, E.; Petraglia, F.; Vale, W.; Castellucci, M. Growth factors and myometrium: Biological effects in uterine fibroid and possible clinical implications. Hum. Reprod. Update 2011, 17, 772–790. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Segars, J.H. The role of angiogenic factors in fibroid pathogenesis: Potential implications for future therapy. Hum. Reprod. Update 2014, 20, 194–216. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Wlodarczyk, M.; Slabuszewska-Jozwiak, A.; Nowicka, G.; Jakiel, G. Influence of vitamin D and transforming growth factor β3 serum concentrations, obesity, and family history on the risk for uterine fibroids. Fertil. Steril. 2016, 106, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Makinen, N.; Mehine, M.; Tolvanen, J.; Kaasinen, E.; Li, Y.; Lehtonen, H.J.; Gentile, M.; Yan, J.; Enge, M.; Taipale, M.; et al. Med12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 2011, 334, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, H.R.; Sarvilinna, N.S.; Sjoberg, J.; Kampjarvi, K.; Pitkanen, E.; Vahteristo, P.; Makinen, N.; Aaltonen, L.A. Med12 mutation frequency in unselected sporadic uterine leiomyomas. Fertil. Steril. 2014, 102, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Soave, I.; Marci, R. Uterine leiomyomata: The snowball effect. Curr. Med. Res. Opin. 2017, 33, 1909–1911. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Margolis, M.K.; Castelli-Haley, J.; Fuldeore, M.J.; Owens, C.D.; Coyne, K.S. Impact of uterine fibroid symptoms on health-related quality of life of US women: Evidence from a cross-sectional survey. Curr. Med. Res. Opin. 2017, 33, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Yang, H.; Du, E.X.; Kelkar, S.S.; Winkel, C. The direct and indirect costs of uterine fibroid tumors: A systematic review of the literature between 2000 and 2013. Am. J. Obstet. Gynecol. 2015, 213, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, E.R.; Clark, A.D.; Banks, N.K.; Henne, M.B.; Stegmann, B.J.; Segars, J.H. The estimated annual cost of uterine leiomyomata in the United States. Am. J. Obstet. Gynecol. 2012, 206, 211.e1–211.e9. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Ozminkowski, R.J.; Carls, G.S.; Wang, S.; Gibson, T.B.; Stewart, E.A. The direct and indirect cost burden of clinically significant and symptomatic uterine fibroids. J. Occup. Environ. Med. 2007, 49, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Faustino, F.; Martinho, M.; Reis, J.; Aguas, F. Update on medical treatment of uterine fibroids. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 216, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Belland, L. Contemporary management of uterine fibroids: Focus on emerging medical treatments. Curr. Med. Res. Opin. 2015, 31, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A. Clinical practice. Uterine fibroids. N. Engl. J. Med. 2015, 372, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.L.; Segars, J.H. Is vitamin D the answer for prevention of uterine fibroids? Fertil. Steril. 2015, 104, 559–560. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Donnez, O.; Dolmans, M.M. Safety of treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. Expert Opin. Drug Saf. 2016, 15, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Donnez, O.; Courtoy, G.E.; Dolmans, M.M. The place of selective progesterone receptor modulators in myoma therapy. Minerva Ginecol. 2016, 68, 313–320. [Google Scholar] [PubMed]
- Biglia, N.; Carinelli, S.; Maiorana, A.; D’Alonzo, M.; Lo Monte, G.; Marci, R. Ulipristal acetate: A novel pharmacological approach for the treatment of uterine fibroids. Drug Des. Dev. Ther. 2014, 8, 285–292. [Google Scholar] [CrossRef]
- Donnez, J.; Arriagada, P.; Donnez, O.; Dolmans, M.M. Current management of myomas: The place of medical therapy with the advent of selective progesterone receptor modulators. Curr. Opin. Obstet. Gynecol. 2015, 27, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Commandeur, A.E.; Styer, A.K.; Teixeira, J.M. Epidemiological and genetic clues for molecular mechanisms involved in uterine leiomyoma development and growth. Hum. Reprod. Update 2015, 21, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Sabry, M.; Al-Hendy, A. Innovative oral treatments of uterine leiomyoma. Obstet. Gynecol. Int. 2012, 2012, 943635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Al-Hendy, M.; Richard-Davis, G.; Montgomery-Rice, V.; Rajaratnam, V.; Al-Hendy, A. Antiproliferative and proapoptotic effects of epigallocatechin gallate on human leiomyoma cells. Fertil. Steril. 2010, 94, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Brakta, S.; Diamond, J.S.; Al-Hendy, A.; Diamond, M.P.; Halder, S.K. Role of vitamin D in uterine fibroid biology. Fertil. Steril. 2015, 104, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Archer, D.F.; Stewart, E.A.; Jain, R.I.; Feldman, R.A.; Lukes, A.S.; North, J.D.; Soliman, A.M.; Gao, J.; Ng, J.W.; Chwalisz, K. Elagolix for the management of heavy menstrual bleeding associated with uterine fibroids: Results from a phase 2a proof-of-concept study. Fertil. Steril. 2017, 108, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Sharan, C.; Al-Hendy, O.; Al-Hendy, A. Paricalcitol, a vitamin D receptor activator, inhibits tumor formation in a murine model of uterine fibroids. Reprod. Sci. 2014, 21, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, T.; Xie, S.; Tu, R.; Cao, Y.; Guo, X.; Zhou, J.; Zhou, X.; Cao, L. Gestrinone inhibits growth of human uterine leiomyoma may relate to activity regulation of ERα, Src and P38 MAPK. Biomed. Pharmacother. 2012, 66, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Al-Hendy, A. Hypovitaminosis D and high serum transforming growth factor β-3: Important biomarkers for uterine fibroids risk. Fertil. Steril. 2016, 106, 1648–1649. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [PubMed]
- Parazzini, F.; Di Martino, M.; Candiani, M.; Vigano, P. Dietary components and uterine leiomyomas: A review of published data. Nutr. Cancer 2015, 67, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D and immune function: Understanding common pathways. Curr. Osteoporos. Rep. 2009, 7, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mateo, G.T.; Fernandez-Millara, V.; Bellon, T.; Liappas, G.; Ruiz-Ortega, M.; Lopez-Cabrera, M.; Selgas, R.; Aroeira, L.S. Paricalcitol reduces peritoneal fibrosis in mice through the activation of regulatory T cells and reduction in IL-17 production. PLoS ONE 2014, 9, e108477. [Google Scholar] [CrossRef] [PubMed]
- Protic, O.; Toti, P.; Islam, M.S.; Occhini, R.; Giannubilo, S.R.; Catherino, W.H.; Cinti, S.; Petraglia, F.; Ciavattini, A.; Castellucci, M.; et al. Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell Tissue Res. 2016, 364, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Paffoni, A.; Somigliana, E.; Vigano, P.; Benaglia, L.; Cardellicchio, L.; Pagliardini, L.; Papaleo, E.; Candiani, M.; Fedele, L. Vitamin D status in women with uterine leiomyomas. J. Clin. Endocrinol. Metab. 2013, 98, E1374–E1378. [Google Scholar] [CrossRef] [PubMed]
- Lerchbaum, E.; Rabe, T. Vitamin D and female fertility. Curr. Opin. Obstet. Gynecol. 2014, 26, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Oskovi Kaplan, Z.A.; Tasci, Y.; Topcu, H.O.; Erkaya, S. 25-Hydroxy vitamin D levels in premenopausal turkish women with uterine leiomyoma. Gynecol. Endocrinol. 2017, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Ford, E.S.; Tsai, J.; Li, C.; Croft, J.B. Factors associated with vitamin D deficiency and inadequacy among women of childbearing age in the United States. ISRN Obstet. Gynecol. 2012, 2012, 691486. [Google Scholar] [CrossRef] [PubMed]
- Sabry, M.; Halder, S.K.; Allah, A.S.; Roshdy, E.; Rajaratnam, V.; Al-Hendy, A. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: A cross-sectional observational study. Int. J. Womens Health 2013, 5, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.D.; Hill, M.C.; Schectman, J.M.; Hollis, B.W. Vitamin D and the risk of uterine fibroids. Epidemiology 2013, 24, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Blauer, M.; Rovio, P.H.; Ylikomi, T.; Heinonen, P.K. Vitamin D inhibits myometrial and leiomyoma cell proliferation in vitro. Fertil. Steril. 2009, 91, 1919–1925. [Google Scholar] [CrossRef] [PubMed]
- Sharan, C.; Halder, S.K.; Thota, C.; Jaleel, T.; Nair, S.; Al-Hendy, A. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil. Steril. 2011, 95, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Goodwin, J.S.; Al-Hendy, A. 1,25-Dihydroxyvitamin D3 reduces TGF-β3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J. Clin. Endocrinol. Metab. 2011, 96, E754–E762. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Sharan, C.; Al-Hendy, A. 1,25-Dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the eker rat model. Biol. Reprod. 2012, 86, 116. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Osteen, K.G.; Al-Hendy, A. 1,25-Dihydroxyvitamin D3 reduces extracellular matrix-associated protein expression in human uterine fibroid cells. Biol. Reprod. 2013, 89, 150. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality—A review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Karczmarewicz, E.; Bayer, M.; Carter, G.; Chlebna-Sokol, D.; Czech-Kowalska, J.; Debski, R.; Decsi, T.; Dobrzanska, A.; Franek, E.; et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in central europe-recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol. Pol. 2013, 64, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Al-Baaj, F.; Yadav, P.; Al-Rifai, A. Paricalcitol in secondary hyperparathyroidism and the survival benefit in patients with chronic kidney disease. J. Ren. Care 2011, 37, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Verstuyf, A.; Verlinden, L.; Allewaert, K.; Branisteanu, D.; Mathieu, C.; van Baelen, H. Non-hypercalcemic pharmacological aspects of vitamin D analogs. Biochem. Pharmacol. 1995, 50, 577–583. [Google Scholar] [CrossRef]
- EMC. Zemplar Soft Capsules 1 mcg. Available online: https://www.Medicines.Org.Uk/emc/medicine/20464 (accessed on 16 November 2017).
- Zhang, Y.; Kong, J.; Deb, D.K.; Chang, A.; Li, Y.C. Vitamin D receptor attenuates renal fibrosis by suppressing the renin-angiotensin system. J. Am. Soc. Nephrol. 2010, 21, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Stavenuiter, A.W.; Farhat, K.; Vila Cuenca, M.; Schilte, M.N.; Keuning, E.D.; Paauw, N.J.; ter Wee, P.M.; Beelen, R.H.; Vervloet, M.G. Protective effects of paricalcitol on peritoneal remodeling during peritoneal dialysis. Biomed. Res. Int. 2015, 2015, 468574. [Google Scholar] [CrossRef] [PubMed]
- Oblak, M.; Mlinsek, G.; Kandus, A.; Buturovic-Ponikvar, J.; Arnol, M. Effects of paricalcitol on biomarkers of inflammation and fibrosis in kidney transplant recipients: Results of a randomized controlled trial. Clin. Nephrol. 2017, 88, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Freundlich, M.; Quiroz, Y.; Zhang, Z.; Zhang, Y.; Bravo, Y.; Weisinger, J.R.; Li, Y.C.; Rodriguez-Iturbe, B. Suppression of renin-angiotensin gene expression in the kidney by paricalcitol. Kidney Int. 2008, 74, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Arici, A.; Sozen, I. Transforming growth factor-β3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil. Steril. 2000, 73, 1006–1011. [Google Scholar] [CrossRef]
- Lee, B.S.; Nowak, R.A. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-β3 (TGFβ3) and altered responses to the antiproliferative effects of TGFβ. J. Clin. Endocrinol. Metab. 2001, 86, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Leppert, P.C.; Baginski, T.; Prupas, C.; Catherino, W.H.; Pletcher, S.; Segars, J.H. Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertil. Steril. 2004, 82, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Sozen, I.; Arici, A. Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertil. Steril. 2002, 78, 1–12. [Google Scholar] [CrossRef]
- Dou, Q.; Tarnuzzer, R.W.; Williams, R.S.; Schultz, G.S.; Chegini, N. Differential expression of matrix metalloproteinases and their tissue inhibitors in leiomyomata: A mechanism for gonadotrophin releasing hormone agonist-induced tumour regression. Mol. Hum. Reprod. 1997, 3, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Dinakarpandian, D.; Nagase, H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta 2000, 1477, 267–283. [Google Scholar] [CrossRef]
- Beresniak, A.; Duru, G.; Berger, G.; Bremond-Gignac, D. Relationships between black tea consumption and key health indicators in the world: An ecological study. BMJ Open 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea and health: Studies in humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Lambert, J.D. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol. Nutr. Food Res. 2011, 55, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, S.; Shimazu, T.; Ohmori, K.; Kikuchi, N.; Nakaya, N.; Nishino, Y.; Tsubono, Y.; Tsuji, I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: The ohsaki study. JAMA 2006, 296, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ahmad, N.; Nieminen, A.L.; Mukhtar, H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (−)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol. Appl. Pharmacol. 2000, 164, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhao, D.Y.; Elliott, S.; Zhao, W.; Curiel, T.J.; Beckman, B.S.; Burow, M.E. Epigallocatechin-3 gallate induces growth inhibition and apoptosis in human breast cancer cells through survivin suppression. Int. J. Oncol. 2007, 31, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.S.; Latief, N.; Ijaz, B.; Ashfaq, U.A. Epigallocatechin gallate as an anti-obesity therapeutic compound: An in silico approach for structure-based drug designing. Nat. Prod. Res. 2017, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Ho, C.T.; Liong, E.C.; Nanji, A.A.; Leung, T.M.; Lau, T.Y.; Fung, M.L.; Tipoe, G.L. Epigallocatechin gallate attenuates fibrosis, oxidative stress, and inflammation in non-alcoholic fatty liver disease rat model through TGF/SMAD, PI3 K/Akt/FoxO1, and NF-κB pathways. Eur. J. Nutr. 2014, 53, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Sriram, N.; Kalayarasan, S.; Manikandan, R.; Arumugam, M.; Sudhandiran, G. Epigallocatechin gallate attenuates fibroblast proliferation and excessive collagen production by effectively intervening TGF-β1 signalling. Clin. Exp. Pharmacol. Physiol. 2015, 42, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Darcha, C. Antifibrotic properties of epigallocatechin-3-gallate in endometriosis. Hum. Reprod. 2014, 29, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Lambert, J.D.; Hou, Z.; Ju, J.; Lu, G.; Hao, X. Molecular targets for the cancer preventive activity of tea polyphenols. Mol. Carcinog. 2006, 45, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.; Taskeen, M.; Mohammad, I.; Huo, C.; Chan, T.H.; Dou, Q.P. Recent advances on tea polyphenols. Front. Biosci. 2012, 4, 111–131. [Google Scholar] [CrossRef]
- Chen, D.; Wan, S.B.; Yang, H.; Yuan, J.; Chan, T.H.; Dou, Q.P. Egcg, green tea polyphenols and their synthetic analogs and prodrugs for human cancer prevention and treatment. Adv. Clin. Chem. 2011, 53, 155–177. [Google Scholar] [PubMed]
- Ahmed, R.S.; Liu, G.; Renzetti, A.; Farshi, P.; Yang, H.; Soave, C.; Saed, G.; El-Ghoneimy, A.A.; El-Banna, H.A.; Foldes, R.; et al. Biological and mechanistic characterization of novel prodrugs of green tea polyphenol epigallocatechin gallate analogs in human leiomyoma cell lines. J. Cell. Biochem. 2016, 117, 2357–2369. [Google Scholar] [CrossRef] [PubMed]
- Roshdy, E.; Rajaratnam, V.; Maitra, S.; Sabry, M.; Allah, A.S.; Al-Hendy, A. Treatment of symptomatic uterine fibroids with green tea extract: A pilot randomized controlled clinical study. Int. J. Womens Health 2013, 5, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Al-Hendy, M.; Richard-Davis, G.; Montgomery-Rice, V.; Sharan, C.; Rajaratnam, V.; Khurana, A.; Al-Hendy, A. Green tea extract inhibits proliferation of uterine leiomyoma cells in vitro and in nude mice. Am. J. Obstet. Gynecol. 2010, 202, 289.e1–289.e9. [Google Scholar] [CrossRef] [PubMed]
- Bartels, C.B.; Cayton, K.C.; Chuong, F.S.; Holthouser, K.; Arian, S.E.; Abraham, T.; Segars, J.H. An evidence-based approach to the medical management of fibroids: A systematic review. Clin. Obstet. Gynecol. 2016, 59, 30–52. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, B.; Jayavelu, S.; Murhekar, K.; Rajkumar, T. Repeated dose studies with pure epigallocatechin-3-gallate demonstrated dose and route dependant hepatotoxicity with associated dyslipidemia. Toxicol. Rep. 2016, 3, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Maggi, R.; Cariboni, A.M.; Marelli, M.M.; Moretti, R.M.; Andre, V.; Marzagalli, M.; Limonta, P. GnRH and GnRH receptors in the pathophysiology of the human female reproductive system. Hum. Reprod. Update 2016, 22. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.; Chwalisz, K.; Carter, D.C.; Klein, C.E. Dose-dependent suppression of gonadotropins and ovarian hormones by elagolix in healthy premenopausal women. J. Clin. Endocrinol. Metab. 2017, 102, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, D.; Guo, Z.; Xie, Q.; Reinhart, G.J.; Madan, A.; Wen, J.; Chen, T.; Huang, C.Q.; Chen, M.; et al. Discovery of sodium R-(+)-4-{2-[5-(2-fluoro-3-methoxyphenyl)-3-(2-fluoro-6-[trifluoromethyl]benzyl)-4-methyl-2,6-dioxo-3,6-dihydro-2H-pyrimidin-1-yl]-1-phenylethylamino}butyrate (elagolix), a potent and orally available nonpeptide antagonist of the human gonadotropin-releasing hormone receptor. J. Med. Chem. 2008, 51, 7478–7485. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, M.; Carr, B.R. Elagolix, a novel, orally bioavailable GnRH antagonist under investigation for the treatment of endometriosis-related pain. Womens Health 2015, 11, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Nakata, D.; Masaki, T.; Tanaka, A.; Yoshimatsu, M.; Akinaga, Y.; Asada, M.; Sasada, R.; Takeyama, M.; Miwa, K.; Watanabe, T.; et al. Suppression of the hypothalamic-pituitary-gonadal axis by TAK-385 (relugolix), a novel, investigational, orally active, small molecule gonadotropin-releasing hormone (GnRH) antagonist: Studies in human gnrh receptor knock-in mice. Eur. J. Pharmacol. 2014, 723, 167–174. [Google Scholar] [CrossRef] [PubMed]
- ObsEva. OBE2109—Uterine Fibroids. Available online: http://www.Obseva.Com/pipeline/obe2109 (accessed on 16 November 2017).
- Struthers, R.S.; Nicholls, A.J.; Grundy, J.; Chen, T.; Jimenez, R.; Yen, S.S.; Bozigian, H.P. Suppression of gonadotropins and estradiol in premenopausal women by oral administration of the nonpeptide gonadotropin-releasing hormone antagonist elagolix. J. Clin. Endocrinol. Metab. 2009, 94, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Giudice, L.C.; Lessey, B.A.; Abrao, M.S.; Kotarski, J.; Archer, D.F.; Diamond, M.P.; Surrey, E.; Johnson, N.P.; Watts, N.B.; et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N. Engl. J. Med. 2017, 377, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Perricos, A.; Wenzl, R. Efficacy of elagolix in the treatment of endometriosis. Expert Opin. Pharmacother. 2017, 18, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Abbvie. AbbVie Presents Pivotal Phase 3 Data on Investigational Treatment Elagolix at the World Congress on Endometriosis. Available online: https://news.Abbvie.Com/news/abbvie-presents-pivotal-phase-3-data-on-investigational-treatment-elagolix-at-world-congress-on-endometriosis.Htm (accessed on 16 November 2017).
- Shozu, M.; Murakami, K.; Inoue, M. Aromatase and leiomyoma of the uterus. Semin. Reprod. Med. 2004, 22, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Czajka-Oraniec, I.; Simpson, E.R. Aromatase research and its clinical significance. Endokrynol. Pol. 2010, 61, 126–134. [Google Scholar] [PubMed]
- American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice. Committee opinion No. 663: Aromatase inhibitors in gynecologic practice. Obstet. Gynecol. 2016, 127, e170–e174. [Google Scholar] [CrossRef]
- Attar, E.; Bulun, S.E. Aromatase inhibitors: The next generation of therapeutics for endometriosis? Fertil. Steril. 2006, 85, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Slopien, R.; Meczekalski, B. Aromatase inhibitors in the treatment of endometriosis. Prz. Menopauzalny 2016, 15, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.P.; Legro, R.S.; Coutifaris, C.; Alvero, R.; Robinson, R.D.; Casson, P.; Christman, G.M.; Ager, J.; Huang, H.; Hansen, K.R.; et al. Letrozole, gonadotropin, or clomiphene for unexplained infertility. N. Engl. J. Med. 2015, 373, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Protic, O.; Giannubilo, S.R.; Toti, P.; Tranquilli, A.L.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Uterine leiomyoma: Available medical treatments and new possible therapeutic options. J. Clin. Endocrinol. Metab. 2013, 98, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Parsanezhad, M.E.; Azmoon, M.; Alborzi, S.; Rajaeefard, A.; Zarei, A.; Kazerooni, T.; Frank, V.; Schmidt, E.H. A randomized, controlled clinical trial comparing the effects of aromatase inhibitor (letrozole) and gonadotropin-releasing hormone agonist (triptorelin) on uterine leiomyoma volume and hormonal status. Fertil. Steril. 2010, 93, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Giannubilo, S.R.; Ciavattini, A.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Management of fibroids in perimenopausal women. Curr. Opin. Obstet. Gynecol. 2015, 27, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.R.; Lambrinoudaki, I.; Lumsden, M.; Mishra, G.D.; Pal, L.; Rees, M.; Santoro, N.; Simoncini, T. Menopause. Nat. Rev. Dis. Prim. 2015, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- Sumitani, H.; Shozu, M.; Segawa, T.; Murakami, K.; Yang, H.J.; Shimada, K.; Inoue, M. In situ estrogen synthesized by aromatase P450 in uterine leiomyoma cells promotes cell growth probably via an autocrine/intracrine mechanism. Endocrinology 2000, 141, 3852–3861. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Reierstad, S.; Demura, M.; Rademaker, A.W.; Kasai, T.; Inoue, M.; Usui, H.; Shozu, M.; Bulun, S.E. High aromatase expression in uterine leiomyoma tissues of african-american women. J. Clin. Endocrinol. Metab. 2009, 94, 1752–1756. [Google Scholar] [CrossRef] [PubMed]
- Kashani, B.N.; Centini, G.; Morelli, S.S.; Weiss, G.; Petraglia, F. Role of medical management for uterine leiomyomas. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 34, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Kaunitz, A.M. Aromatase inhibitor therapy for uterine bleeding in a postmenopausal woman with leiomyomata. Menopause 2007, 14, 941–943. [Google Scholar] [CrossRef] [PubMed]
- Hilario, S.G.; Bozzini, N.; Borsari, R.; Baracat, E.C. Action of aromatase inhibitor for treatment of uterine leiomyoma in perimenopausal patients. Fertil. Steril. 2009, 91, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Varelas, F.K.; Papanicolaou, A.N.; Vavatsi-Christaki, N.; Makedos, G.A.; Vlassis, G.D. The effect of anastrazole on symptomatic uterine leiomyomata. Obstet. Gynecol. 2007, 110, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Shozu, M.; Murakami, K.; Segawa, T.; Kasai, T.; Inoue, M. Successful treatment of a symptomatic uterine leiomyoma in a perimenopausal woman with a nonsteroidal aromatase inhibitor. Fertil. Steril. 2003, 79, 628–631. [Google Scholar] [CrossRef]
- Doherty, L.; Mutlu, L.; Sinclair, D.; Taylor, H. Uterine fibroids: Clinical manifestations and contemporary management. Reprod. Sci. 2014, 21, 1067–1092. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lu, D.; Navaratnam, K.; Shi, G. Aromatase inhibitors for uterine fibroids. Cochrane Database Syst. Rev. 2013, CD009505. [Google Scholar] [CrossRef] [PubMed]
- Duhan, N.; Madaan, S.; Sen, J. Role of the aromatase inhibitor letrozole in the management of uterine leiomyomas in premenopausal women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 171, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Sayyah-Melli, M.; Mobasseri, M.; Gharabaghi, P.M.; Ouladsahebmadarek, E.; Rahmani, V. Comparing the effect of aromatase inhibitor (letrozole) + cabergoline (dostinex) and letrozole alone on uterine myoma regression, a randomized clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 210, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Leone Roberti Maggiore, U.; Scala, C.; Venturini, P.L.; Ferrero, S. Preoperative treatment with letrozole in patients undergoing laparoscopic myomectomy of large uterine myomas: A prospective non-randomized study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, N.; Ghirardi, V.; Remorgida, V.; Venturini, P.L.; Ferrero, S. Three-month treatment with triptorelin, letrozole and ulipristal acetate before hysteroscopic resection of uterine myomas: Prospective comparative pilot study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 192, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Rains, C.P.; Bryson, H.M.; Fitton, A. Cabergoline. A review of its pharmacological properties and therapeutic potential in the treatment of hyperprolactinaemia and inhibition of lactation. Drugs 1995, 49, 255–279. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, S.; Manyonda, I.T. Medical management of fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 655–676. [Google Scholar] [CrossRef] [PubMed]
- Melli, M.S.; Farzadi, L.; Madarek, E.O. Comparison of the effect of gonadotropin-releasing hormone analog (diphereline) and cabergoline (dostinex) treatment on uterine myoma regression. Saudi Med. J. 2007, 3, 445–450. [Google Scholar]
- Vahdat, M.; Kashanian, M.; Ghaziani, N.; Sheikhansari, N. Evaluation of the effects of cabergoline (dostinex) on women with symptomatic myomatous uterus: A randomized trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 206, 74–78. [Google Scholar] [CrossRef] [PubMed]
- ESHRE Capri Workshop Group. Noncontraceptive health benefits of combined oral contraception. Hum. Reprod. Update 2005, 11, 513–525. [Google Scholar] [CrossRef]
- Munro, M.G.; Critchley, H.O.; Fraser, I.S.; FIGO Menstrual Disorders Working Group. The figo classification of causes of abnormal uterine bleeding in the reproductive years. Fertil. Steril. 2011, 95, 2204–2208. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Yang, T.; Kong, F.; Zhou, Q. Oral contraceptive use and uterine leiomyoma risk: A meta-analysis based on cohort and case-control studies. Arch. Gynecol. Obstet. 2013, 288, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Fraser, I.S.; Romer, T.; Parke, S.; Zeun, S.; Mellinger, U.; Machlitt, A.; Jensen, J.T. Effective treatment of heavy and/or prolonged menstrual bleeding with an oral contraceptive containing estradiol valerate and dienogest: A randomized, double-blind phase III trial. Hum. Reprod. 2011, 26, 2698–2708. [Google Scholar] [CrossRef] [PubMed]
- Bromham, D.R.; Booker, M.W.; Rose, G.L.; Wardle, P.G.; Newton, J.R. A multicentre comparative study of gestrinone and danazol in the treatment of endometriosis. J. Obstet. Gynaecol. 1995, 15, 188–194. [Google Scholar] [CrossRef]
- Selak, V.; Farquhar, C.; Prentice, A.; Singla, A. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst. Rev. 2007, CD000068. [Google Scholar] [CrossRef]
- Coutinho, E.M. Gestrinone in the treatment of myomas. Acta Obstet. Gynecol. Scand. Suppl. 1989, 150, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, E.M. Treatment of large fibroids with high doses of gestrinone. Gynecol. Obstet. Investig. 1990, 30, 44–47. [Google Scholar] [CrossRef]
- Coutinho, E.M.; Goncalves, M.T. Long-term treatment of leiomyomas with gestrinone. Fertil. Steril. 1989, 51, 939–946. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar] [PubMed]
- Feng, T.; Wei, Y.; Lee, R.J.; Zhao, L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017, 12, 6027–6044. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.; Amin, H.M.; Lai, R.; Aggarwal, B.B. Curcumin (diferuloylmethane) inhibits constitutive NF-κB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem. Pharmacol. 2005, 70, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Mendoza, M.; Payson, M.; Catherino, W.H. Curcumin, a nutritional supplement with antineoplastic activity, enhances leiomyoma cell apoptosis and decreases fibronectin expression. Fertil. Steril. 2009, 91, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Tsuiji, K.; Takeda, T.; Li, B.; Wakabayashi, A.; Kondo, A.; Kimura, T.; Yaegashi, N. Inhibitory effect of curcumin on uterine leiomyoma cell proliferation. Gynecol. Endocrinol. 2011, 27, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Grygielko, E.T.; Martin, W.M.; Tweed, C.; Thornton, P.; Harling, J.; Brooks, D.P.; Laping, N.J. Inhibition of gene markers of fibrosis with a novel inhibitor of transforming growth factor-β type I receptor kinase in puromycin-induced nephritis. J. Pharmacol. Exp. Ther. 2005, 313, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Laping, N.J.; Everitt, J.I.; Frazier, K.S.; Burgert, M.; Portis, M.J.; Cadacio, C.; Gold, L.I.; Walker, C.L. Tumor-specific efficacy of transforming growth factor-βRI inhibition in Eker rats. Clin. Cancer Res. 2007, 13, 3087–3099. [Google Scholar] [CrossRef] [PubMed]
- Chwalisz, K.; Taylor, H. Current and emerging medical treatments for uterine fibroids. Semin. Reprod. Med. 2017, 35, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Guo, K.M.; Wei, Y.M.; Ma, M.M.; Cai, J.R.; Xia, T.T.; Ye, Q.J. Aspirin inhibits the proliferation of human uterine leiomyoma cells by downregulation of k-Ras-p110α interaction. Oncol. Rep. 2017, 38, 2507–2517. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Margolin, S.B.; Nowak, R.A. Pirfenidone: A novel pharmacological agent that inhibits leiomyoma cell proliferation and collagen production. J. Clin. Endocrinol. Metab. 1998, 83, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Hayton, C.; Chaudhuri, N. Managing idiopathic pulmonary fibrosis: Which drug for which patient? Drugs Aging 2017. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.H.; Fouad, H.; Bahashwan, S.; Al-Hendy, A. Towards non-surgical therapy for uterine fibroids: Catechol-O-methyl transferase inhibitor shrinks uterine fibroid lesions in the Eker rat model. Hum. Reprod. 2011, 26, 3008–3018. [Google Scholar] [CrossRef] [PubMed]
- Young, M.V.; Schwartz, G.G.; Wang, L.; Jamieson, D.P.; Whitlatch, L.W.; Flanagan, J.N.; Lokeshwar, B.L.; Holick, M.F.; Chen, T.C. The prostate 25-hydroxyvitamin d-1 α-hydroxylase is not influenced by parathyroid hormone and calcium: Implications for prostate cancer chemoprevention by vitamin D. Carcinogenesis 2004, 25, 967–971. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciebiera, M.; Łukaszuk, K.; Męczekalski, B.; Ciebiera, M.; Wojtyła, C.; Słabuszewska-Jóźwiak, A.; Jakiel, G. Alternative Oral Agents in Prophylaxis and Therapy of Uterine Fibroids—An Up-to-Date Review. Int. J. Mol. Sci. 2017, 18, 2586. https://doi.org/10.3390/ijms18122586
Ciebiera M, Łukaszuk K, Męczekalski B, Ciebiera M, Wojtyła C, Słabuszewska-Jóźwiak A, Jakiel G. Alternative Oral Agents in Prophylaxis and Therapy of Uterine Fibroids—An Up-to-Date Review. International Journal of Molecular Sciences. 2017; 18(12):2586. https://doi.org/10.3390/ijms18122586
Chicago/Turabian StyleCiebiera, Michał, Krzysztof Łukaszuk, Błażej Męczekalski, Magdalena Ciebiera, Cezary Wojtyła, Aneta Słabuszewska-Jóźwiak, and Grzegorz Jakiel. 2017. "Alternative Oral Agents in Prophylaxis and Therapy of Uterine Fibroids—An Up-to-Date Review" International Journal of Molecular Sciences 18, no. 12: 2586. https://doi.org/10.3390/ijms18122586