hASC and DFAT, Multipotent Stem Cells for Regenerative Medicine: A Comparison of Their Potential Differentiation In Vitro
Abstract
:1. Introduction
2. Results
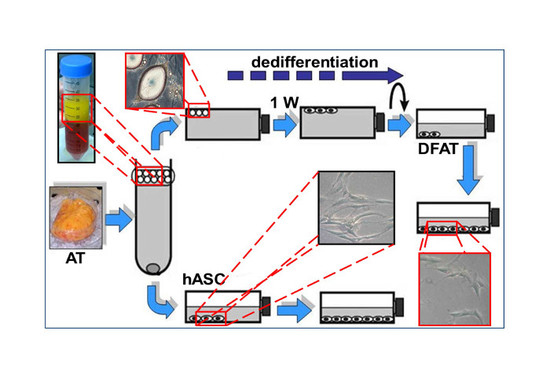
2.1. Dedifferentiated Mature Adipocyte (DFAT) and Human Adipose Stem Cell (hASC) Cultures
2.2. Expression of Cell-Surface Antigens by DFAT and hASC
2.3. Dedifferentiation Analysis by Real-Time RT-PCR
2.4. Generation Time, Viability, and Proliferation Capacity
2.5. Evaluation of Differentiation
2.5.1. Adipogenic Differentiation
2.5.2. Osteogenic Differentiation
2.5.3. Chondrogenic Differentiation
3. Discussion
4. Materials and Methods
4.1. Human Adipose Tissue Specimen Collection
4.2. Isolation of Stem Cells from Adipose Tissue (hASCs)
4.3. Extraction and Dedifferentiation of Mature Adipocytes from Adipose Tissue (DFAT)
4.4. Flow Cytometric Analysis
4.5. Curves of Viability
4.6. Cell Seeding, Culture, and Differentiation
4.7. Cell Differentiation Analysis
4.8. RNA Isolation and Reverse Transcriptase Quantitative Real-Time PCR
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wei, X.; Yang, X.; Han, Z.P.; Qu, F.F.; Shao, L.; Shi, Y.F. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol. Sin. 2013, 34, 747–754. [Google Scholar]
- Gnecchi, M.; Melo, L.G. Bone marrow-derived mesenchymal stem cells: Isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol. Biol. 2009, 482, 281–294. [Google Scholar] [PubMed]
- Tallone, T.; Realini, C.; Böhmel, A.; Kornfeld, C.; Vassalli, G.; Moccetti, T.; Bardelli, S.; Soldati, G. Adult human adipose tissue contains several types of multipotent cells. J. Cardiovasc. Transl. Res. 2011, 4, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Abumaree, M.H.; Al Jumah, M.A.; Kalionis, B.; Jawdat, D.; Al Khaldi, A.; Al Talabani, A.A.; Knawy, B.A. Phenotypic and functional characterization of mesenchymal stem cells from chorionic villi of human term placenta. Stem Cell Rev. 2013, 9, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Soncini, M.; Vertua, E.; Gibelli, L.; Zorzi, F.; Denegri, M.; Albertini, A.; Wengler, G.S.; Parolini, O. Isolation and characterization of mesenchymal cells from human fetal membranes. J. Tissue Eng. Regen. Med. 2007, 1, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Karamzadeh, R.; Eslaminejad, M.B.; Aflatoonia, R. Isolation, characterization and comparative differentiation of human dental pulp stem cells derived from permanent teeth by using two different methods. J. Vis. Exp. 2012, 69, 4372. [Google Scholar] [CrossRef] [PubMed]
- Kossowska-Tomaszczuk, K.; De Geyter, C.; De Geyter, M.; Martin, I.; Holzgreve, W.; Scherberich, A.; Zhang, H. The multipotency of luteinizing granulosa cells collected from mature ovarian follicles. Stem Cells 2009, 27, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Riva, F.; Omes, C.; Bassani, R.; Nappi, R.E.; Mazzini, G.; Icaro Cornaglia, A.; Casasco, A. In vitro culture system for mesenchymal progenitor cells derived from waste human ovarian follicular fluid. Reprod. Biomed. Online 2014, 29, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Maioli, M.; Leonardi, E.; Olivi, E.; Pasquinelli, G.; Valente, S.; Mendez, A.J.; Ricordi, C.; Raffaini, M.; Tremolada, C.; et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant. 2013, 22, 2063–2077. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, G.; Brenta, F.; Jaber, O.; Laberinti, E.; Faga, A. Lipofilling for functional reconstruction of the sole of the foot. Foot 2014, 24, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Benazzo, F.; Botta, L.; Scaffino, M.F.; Caliogna, L.; Marullo, M.; Fusi, S.; Gastaldi, G. Trabecular Titanium can induce in vitro osteogenic differentiation of human adipose derived stem cells without osteogenic factors. J. Biomed. Mater. Res. 2014, 102, 2061–2071. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.S.; Park, J.B.; Kim, H.S.; Kim, D.S.; Park, D.J.; Kang, S.J. Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Med. J. 2011, 52, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Oswald, J.; Boxberger, S.; Jorgensen, B.; Feldmann, S.; Ehninger, G.; Bornhauser, M.; Werner, C. Mesenchymal stem cells can be differentiated Into endothelial cells in vitro. Stem Cells 2004, 22, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Rytlewski, J.A.; Alejandra Aldon, M.; Lewis, E.W.; Suggs, L.J. Mechanisms of tubulogenesis and endothelial phenotype expression by MSCs. Microvasc. Res. 2015, 99, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Poloni, A.; Maurizi, G.; Leoni, P.; Serrani, F.; Mancini, S.; Frontini, A.; Zingaretti, M.C.; Siquini, W.; Sarazani, R.; Cinti, S. Human dedifferentiated adipocytes show smilar properties to bone marrow-derived mesenchymal stem cells. Stem Cells 2012, 30, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. The adipose organ at a glance. Dis. Models Mech. 2012, 5, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Frontini, A.; Cinti, S. Convertible visceral fat as a therapeutic target to curb obesity. Nat. Rev. Drug Discov. 2016, 15, 405–424. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, R.; Zingaretti, M.C.; Murano, I.; Vitali, A.; Frontini, A.; Giannulis, I.; Barbatelli, G.; Marcucci, F.; Bordicchia, M.; Sarzani, R.; et al. In vivo physiological transdifferentiation of adult adipose cells. Stem Cells 2009, 27, 2761–2768. [Google Scholar] [CrossRef] [PubMed]
- Lessard, J.; Pelletier, M.; Biertho, L.; Biron, S.; Marceau, S.; Hould, F.S.; Lebel, S.; Moustarah, F.; Lescelleur, O.; Marceau, P.; et al. Characterization of dedifferentiating human mature adipocytes from the visceral and subcutaneous fat compartments: Fibroblast-activation protein alpha and dipeptidyl peptidase 4 as major components of matrix remodeling. PLoS ONE 2015, 10, e0122065. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.S.; Bueno, R.; Silva, W.; Campos, C.F.; Gionbelli, M.P.; Guimaraes, S.E.F.; Silva, F.F.; Lopes, P.S.; Hausman, G.J.; Dodson, M.V. Triennial Growth and Development Symposium: Dedifferentiated fat cells: Potential and perspectives for their use in clinical and animal science purpose. J. Anim. Sci. 2017, 95, 2255–2260. [Google Scholar] [CrossRef] [PubMed]
- Murata, D.; Yamasaki, A.; Matusuzaki, S.; Sunaga, T.; Fujiki, M.; Tokunaga, S.; Misumi, K. Characteristics and multipotency of equine dedifferentiated fat cells. J. Equine Sci. 2016, 27, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, H.; Yonemitsu, N.; Miyabara, S.; Yun, K. Primary cultures of unilocular fat cells: Characteristics of growth in vitro and changes in differentiation properties. Differentiation 1986, 31, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, N.; Momota, Y.; Hashimoto, Y.; Tatsumi, S.; Ando, K.; Omasa, T.; Kotani, J. The osteoblastic differentiation of human dedifferentiated fat cells is higher than that of adipose stem cells from the buccal fat pad. Clin. Oral Investig. 2014, 18, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Erickson, G.R.; Gimble, J.M.; Franklin, D.M.; Rice, H.E.; Awad, H.; Guilak, F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 2002, 290, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K.; Kondo, D.; Okazaki, Y.; Kano, K. A novel preadipocyte cell line established from mouse adult mature adipocytes. Biochem. Biophys. Res. Commun. 2004, 321, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Sugawara, A.; Yamashita, J.; Ogura, H.; Sato, S. Dedifferentiated fat cells: An alternative source of adult multipotent cells from the adipose tissues. Int. J. Oral Sci. 2011, 3, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kano, K.; Kondo, D.; Fukuda, N.; Iribe, Y.; Tanaka, N.; Matsubara, Y.; Sakuma, T.; Satomi, A.; Otaki, M.; et al. Mature Adipocyte-Derived Dedifferentiated Fat Cells Exhibit Multilineage Potential. J. Cell. Physiol. 2008, 215, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Oedayrajsingh Varma, M.J.; Van Ham, S.M.; Knippenberg, M.; Helder, M.N.; Klein-Nulend, J.; Schoten, T.E.; Ritt, M.J.; Van Milligen, F.J. Adipose Tissue Derived Mesenchymal Stem Cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy 2006, 8, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; Mc Intosh, K.; Zvonica, S.; Garrett, S.; Flyod, Z.E.; Kloster, A.; Halvoren, Y.; Storms, R.W.; Goh, B.; Kilroy, G.; et al. Immunophenotype of Human Adipose-Derived Cells: Temporal changes in stromal-associated and stem-associated markers. Stem Cells 2006, 24, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Yanxia, Z.; Tianqing, L.; Kedong, S.; Xiubo, F.; Xuehu, M.; Zhanfeng, C. Adipose derived stem cell: A better stem cell than BMSC. Cell Biochem. Funct. 2008, 26, 664–675. [Google Scholar]
- Shah, F.S.; Li, J.; Zanata, F.; Curley, J.L.; Martin, E.C.; Wu, X.; Dietrich, M.; Devireddy, R.V.; Wade, J.W.; Gimble, J.M. The relative functionality of freshly isolated and cryopreserved Human Adipose-Derived Stromal/Stem Cells. Cells Tissue Organs 2016, 201, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Tocci, A.; Forte, L. Mesenchymal stem cell: Use and perspectives. Hematol. J. 2003, 4, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Lu, X.W.; Wu, M.K.; Wang, H.; Zhan, Y.J.; Sato, S.; Shen, J.F. The phenotype and tissue-specific nature of multipotent cells derived from human mature adipocytes. Biochem. Biophys. Res. Commun. 2014, 444, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Lafont, J.E. Lack of oxygen in articular cartilage: Consequences for chondrocyte biology. Int. J. Exp. Pathol. 2010, 91, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Aitken, K.J.; Tolg, C.; Panchal, T.; Leslie, B.; Yu, J.; Elkelini, M.; Sabha, N.; Tse, D.J.; Lorenzo, A.J.; Hassouna, M.; et al. Mammalian target of rapamycin (mTOR) induces proliferation and de-differentiation responses to three coordinate pathophysiologic stimuli (mechanical strain, hypoxia, and extracellular matrix remodeling) in rat bladder smooth muscle. Am. J. Pathol. 2010, 176, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Jumabay, M.; Boström, K. Dedifferentiated fat cells: A cell source for regenerative medicine. World J. Stem Cells 2015, 7, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Nayoung, K.; Seok-Goo, C. Clinical applications of mesenchymal stem cells. Korean J. Intern. Med. 2013, 28, 387–402. [Google Scholar]
- Cao, Y.; Sun, Z.; Liao, L.; Meng, Y.; Han, Q.; Chunhua Zhao, R. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem. Biophys. Res. Commun. 2005, 332, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E.; Tumbar, T.; Guasch, G. Socializing with the neighbors: Stem cells and their niche. Cell 2004, 116, 769–778. [Google Scholar] [CrossRef]
- Kragl, M.; Knapp, D.; Nacu, E.; Khattak, S.; Maden, M.; Henning Epperlein, H.; Tanaka, E.M. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature 2009. [Google Scholar] [CrossRef] [PubMed]
- Ab-Sharaniza, R.; Lakshmi, S.; Hanumantha, R.B.R.G.; Tunku, K. Chondrocyte-alginate constructs with or without TGF-b1 produces superior extracellular matrix expression than monolayer cultures. Mol. Cell. Biochem. 2013, 376, 11–20. [Google Scholar]
- Murer, H.; Ammann, E.; Biber, J.; Hopfer, U. The surface membrane of the small intestinal epithelial cell. I. Localization of adenyl cyclase. Biochim. Biophys. Acta 1976, 433, 509–519. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Laforenza, U.; Miceli, E.; Gastaldi, G.; Scaffino, M.; Ventura, U. Solute transporters and aquaporins are impaired in celiac disease. Biol. Cell 2010, 102, 457–467. [Google Scholar] [CrossRef] [PubMed]





| Antigen | hASC | DFAT |
|---|---|---|
| CD14 | 0% | 0% |
| CD34 | 0% | 0.5% |
| CD45 | 0% | 0.2% |
| CD13 | 99.5% | 99.2% |
| CD73 | 97.9% | 95.0% |
| CD90 | 99.5% | 99.0% |
| CD105 | 99.3% | 99.1% |
| Quantification of Lipidic Droplets (Arbitrary Units ± S.E.) | |
|---|---|
| hASC | 2,500,000 * ± 711,000 |
| DFAT | 32,900,000 ± 3,800,000 |
| Specific Primers | ||
|---|---|---|
| Gene | Sense Primers | Antisense Primers |
| ALP | 5′-AGCCCTTCACTGCCATCCTGT-3′ | 5′-ATTCTCTCGTTCACCGCCCAC-3′ |
| RUNX2 | 5′-GGACGAGGCAAGAGTTTCAC-3′ | 5′-TGCCTGCCTGGGGTCTGTAA-3′ |
| LPL | 5′-CTGGACGGTAACAGGAATGT-3′ | 5′-TCCTCCTCCATCCAGTTGAT-3′ |
| GLUT4 | 5′-ATAGGCTCCGAAGATGGGGAA-3′ | 5′-AAACTGCAGGGAGCCAAGCA-3′ |
| Adiponectin | 5′-GGAGATCCAGGTCTTATTGG-3′ | 5′-ACTGAATGCTGAGCGGTATA-3′ |
| Cycling Conditions | ||||
|---|---|---|---|---|
| Lineage | Initial Denaturation and Time | Cycles of Denaturation, Temperature and Time | Annealing Temperature and Time | Elongation Time |
| Osteogenic differentiation | 95 °C—5 min | 40 cycles—95 °C—30 s | 58 °C—30 s | 72 °C—40 s |
| Adipogenic differentiation | 95 °C—5 min | 40 cycles—95 °C—15 s | 61 °C—30 s | 72 °C—45 s |
| Chondrogenic differentiation | 95 °C—5 min | 40 cycles—95 °C—30 s | 61 °C—30 s | 72 °C—45 s |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saler, M.; Caliogna, L.; Botta, L.; Benazzo, F.; Riva, F.; Gastaldi, G. hASC and DFAT, Multipotent Stem Cells for Regenerative Medicine: A Comparison of Their Potential Differentiation In Vitro. Int. J. Mol. Sci. 2017, 18, 2699. https://doi.org/10.3390/ijms18122699
Saler M, Caliogna L, Botta L, Benazzo F, Riva F, Gastaldi G. hASC and DFAT, Multipotent Stem Cells for Regenerative Medicine: A Comparison of Their Potential Differentiation In Vitro. International Journal of Molecular Sciences. 2017; 18(12):2699. https://doi.org/10.3390/ijms18122699
Chicago/Turabian StyleSaler, Marco, Laura Caliogna, Laura Botta, Francesco Benazzo, Federica Riva, and Giulia Gastaldi. 2017. "hASC and DFAT, Multipotent Stem Cells for Regenerative Medicine: A Comparison of Their Potential Differentiation In Vitro" International Journal of Molecular Sciences 18, no. 12: 2699. https://doi.org/10.3390/ijms18122699