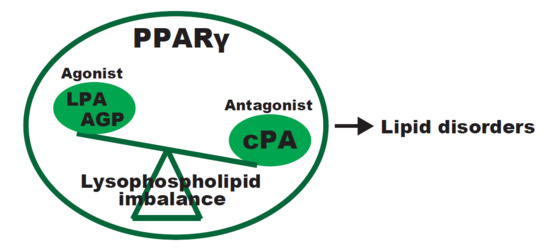
Lysophospholipid-Related Diseases and PPARγ Signaling Pathway
Abstract
:1. PPARγ and Lysophospholipids
2. Lysophospholipid and Vascular Pathologies
3. Lysophospholipids and Vascular Dementia
4. Lysophospholipids and Spinal Cord Injury (SCI)
5. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| PPAR | Peroxisome proliferator-activated receptor |
| LPA | Lysophosphatidic acid |
| AGP | 1-O-octadecenyl-2-hydroxy-sn-glycero-3-phosphate |
| LPC | Lysophosphatidylcholine |
| cPA | Cyclic phosphatidic acid |
| S1P | Sphingosine 1-phosphate |
| SPC | Lysosphingomyelin |
| KLF9 | Kruppel-like factor 9 |
| mox-LDL | Mildly oxidized low-density lipoprotein |
| NF-κB | Nuclear factor-κB |
| AP-1 | Activator protein 1 |
| PLA2 | Phospholipase A2 |
| AD | Alzheimer’s disease |
| SCI | Spinal cord injury |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| NOS | Nitric oxide synthase |
| CNS | Central nervous system |
References
- Schneider, G.; Sellers, Z.P.; Abdel-Latif, A.; Morris, A.J.; Ratajczak, M.Z. Bioactive lipids, LPC and LPA, are novel prometastatic factors and their tissue levels increase in response to radio/chemotherapy. Mol. Cancer Res. 2014, 12, 1560–1573. [Google Scholar] [CrossRef] [PubMed]
- Tigyi, G. Aiming drug discovery at lysophosphatidic acid targets. Br. J. Pharmacol. 2010, 161, 241–270. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Herr, D.R.; Noguchi, K.; Yung, Y.C.; Lee, C.W.; Mutoh, T.; Lin, M.E.; Teo, S.T.; Park, K.E.; Mosley, A.N.; et al. LPA receptors: Subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 157–186. [Google Scholar] [CrossRef] [PubMed]
- Siess, W.; Zangl, K.J.; Essler, M.; Bauer, M.; Brandl, R.; Corrinth, C.; Bittman, R.; Tigyi, G.; Aepfelbacher, M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc. Natl. Acad. Sci. USA 1999, 96, 6931–6936. [Google Scholar] [CrossRef] [PubMed]
- Tigyi, G.; Parrill, A.L. Molecular mechanisms of lysophosphatidic acid action. Prog. Lipid Res. 2003, 42, 498–526. [Google Scholar] [CrossRef]
- Tigyi, G. Selective ligands for lysophosphatidic acid receptor subtypes: Gaining control over the endothelial differentiation gene family. Mol. Pharmacol. 2001, 60, 1161–1164. [Google Scholar] [PubMed]
- Sano, T.; Baker, D.; Virag, T.; Wada, A.; Yatomi, Y.; Kobayashi, T.; Igarashi, Y.; Tigyi, G. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J. Biol. Chem. 2002, 277, 21197–21206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Baker, D.L.; Yasuda, S.; Makarova, N.; Balazs, L.; Johnson, L.R.; Marathe, G.K.; McIntyre, T.M.; Xu, Y.; Prestwich, G.D.; et al. Lysophosphatidic acid induces neointima formation through PPARγ activation. J. Exp. Med. 2004, 199, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Chui, P.C.; Guan, H.P.; Lehrke, M.; Lazar, M.A. PPARγ regulates adipocyte cholesterol metabolism via oxidized LDL receptor 1. J. Clin. Investig. 2005, 115, 2244–2256. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Tsukahara, R.; Yasuda, S.; Makarova, N.; Valentine, W.J.; Allison, P.; Yuan, H.; Baker, D.L.; Li, Z.; Bittman, R.; et al. Different residues mediate recognition of 1-O-oleyllysophosphatidic acid and rosiglitazone in the ligand binding domain of peroxisome proliferator-activated receptor γ. J. Biol. Chem. 2006, 281, 3398–3407. [Google Scholar] [CrossRef] [PubMed]
- Gaits, F.; Fourcade, O.; Le Balle, F.; Gueguen, G.; Gaige, B.; Gassama-Diagne, A.; Fauvel, J.; Salles, J.P.; Mauco, G.; Simon, M.F.; et al. Lysophosphatidic acid as a phospholipid mediator: Pathways of synthesis. FEBS Lett. 1997, 410, 54–58. [Google Scholar] [CrossRef]
- Sugiura, T.; Nakane, S.; Kishimoto, S.; Waku, K.; Yoshioka, Y.; Tokumura, A.; Hanahan, D.J. Occurrence of lysophosphatidic acid and its alkyl ether-linked analog in rat brain and comparison of their biological activities toward cultured neural cells. Biochim. Biophys. Acta 1999, 1440, 194–204. [Google Scholar] [CrossRef]
- McIntyre, T.M.; Pontsler, A.V.; Silva, A.R.; St Hilaire, A.; Xu, Y.; Hinshaw, J.C.; Zimmerman, G.A.; Hama, K.; Aoki, J.; Arai, H.; et al. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARγ agonist. Proc. Natl. Acad. Sci. USA 2003, 100, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Tsukahara, R.; Fujiwara, Y.; Yue, J.; Cheng, Y.; Guo, H.; Bolen, A.; Zhang, C.; Balazs, L.; Re, F.; et al. Phospholipase D2-dependent inhibition of the nuclear hormone receptor PPARγ by cyclic phosphatidic acid. Mol. Cell 2010, 39, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T. PPARγ Networks in Cell Signaling: Update and Impact of Cyclic Phosphatidic Acid. J. Lipids 2013, 2013, 246597. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y. Cyclic phosphatidic acid—A unique bioactive phospholipid. Biochim. Biophys. Acta 2008, 1781, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D. Atherogenesis in perspective: Hypercholesterolemia and inflammation as partners in crime. Nat. Med. 2002, 8, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Spano, S.; Cericola, C.; Pesce, A.; Massaro, A.; Millo, E.; Luini, A.; Corda, D.; Bolognesi, M. CtBP/BARS: A dual-function protein involved in transcription co-repression and Golgi membrane fission. EMBO J. 2003, 22, 3122–3130. [Google Scholar] [CrossRef] [PubMed]
- Thumser, A.E.; Voysey, J.E.; Wilton, D.C. The binding of lysophospholipids to rat liver fatty acid-binding protein and albumin. Biochem. J. 1994, 301, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; Lee, H.; Azuma, T.; Stossel, T.P.; Turck, C.W.; Karliner, J.S. Gelsolin binding and cellular presentation of lysophosphatidic acid. J. Biol. Chem. 2000, 275, 14573–14578. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, R.; Haniu, H.; Matsuda, Y.; Tsukahara, T. Heart-type fatty-acid-binding protein (FABP3) is a lysophosphatidic acid-binding protein in human coronary artery endothelial cells. FEBS Open Bio 2014, 4, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Tsukahara, R.; Haniu, H.; Matsuda, Y.; Murakami-Murofushi, K. Cyclic phosphatidic acid inhibits the secretion of vascular endothelial growth factor from diabetic human coronary artery endothelial cells through peroxisome proliferator-activated receptor γ. Mol. Cell. Endocrinol. 2015, 412, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.J.; Schwartz, B.; Washington, M.; Kennedy, A.; Webster, K.; Belinson, J.; Xu, Y. Electrospray ionization mass spectrometry analysis of lysophospholipids in human ascitic fluids: Comparison of the lysophospholipid contents in malignant vs nonmalignant ascitic fluids. Anal. Biochem. 2001, 290, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Nakane, S.; Kishimoto, S.; Waku, K.; Yoshioka, Y.; Tokumura, A. Lysophosphatidic acid, a growth factor-like lipid, in the saliva. J. Lipid Res. 2002, 43, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Nakane, S.; Tokumura, A.; Waku, K.; Sugiura, T. Hen egg yolk and white contain high amounts of lysophosphatidic acids, growth factor-like lipids: Distinct molecular species compositions. Lipids 2001, 36, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Subauste, A.R.; Das, A.K.; Li, X.; Elliott, B.G.; Evans, C.; El Azzouny, M.; Treutelaar, M.; Oral, E.; Leff, T.; Burant, C.F. Alterations in lipid signaling underlie lipodystrophy secondary to AGPAT2 mutations. Diabetes 2012, 61, 2922–2931. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.K.; Arioglu, E.; De Almeida, S.; Akkoc, N.; Taylor, S.I.; Bowcock, A.M.; Barnes, R.I.; Garg, A. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat. Genet. 2002, 31, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Markan, K.; Temple, K.A.; Deplewski, D.; Brady, M.J.; Cohen, R.N. The nuclear receptor corepressors NCoR and SMRT decrease peroxisome proliferator-activated receptor γ transcriptional activity and repress 3T3-L1 adipogenesis. J. Biol. Chem. 2005, 280, 13600–13605. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; He, Q.; Peng, B.; Chiao, P.J.; Ye, J. Regulation of nuclear translocation of HDAC3 by IκBα is required for tumor necrosis factor inhibition of peroxisome proliferator-activated receptor γ function. J. Biol. Chem. 2006, 281, 4540–4547. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Longo, R.; Fiorino, E.; Silva, R.; Mitro, N.; Cermenati, G.; Gilardi, F.; Desvergne, B.; Andolfo, A.; Magagnotti, C.; et al. HDAC3 is a molecular brake of the metabolic switch supporting white adipose tissue browning. Nat. Commun. 2017, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Haniu, H.; Matsuda, Y. Cyclic phosphatidic acid inhibits alkyl-glycerophosphate-induced downregulation of histone deacetylase 2 expression and suppresses the inflammatory response in human coronary artery endothelial cells. Int. J. Med. Sci. 2014, 11, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Haniu, H.; Matsuda, Y.; Murakmi-Murofushi, K. Short-term treatment with a 2-carba analog of cyclic phosphatidic acid induces lowering of plasma cholesterol levels in ApoE-deficient mice. Biochem. Biophys. Res. Commun. 2016, 473, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Dietschy, J.M.; Turley, S.D. Thematic review series: Brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 2004, 45, 1375–1397. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.C.; Stoddard, N.C.; Mirendil, H.; Chun, J. Lysophosphatidic Acid signaling in the nervous system. Neuron 2015, 85, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The pathobiology of vascular dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef] [PubMed]
- Venkat, P.; Chopp, M.; Chen, J. Models and mechanisms of vascular dementia. Exp. Neurol. 2015, 272, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.C.; Stoddard, N.C.; Chun, J. LPA receptor signaling: Pharmacology, physiology, and pathophysiology. J. Lipid Res. 2014, 55, 1192–1214. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Yamagishi, S.; Matsuda, Y.; Haniu, H. Lysophosphatidic acid signaling regulates the KLF9-PPARγ axis in human induced pluripotent stem cell-derived neurons. Biochem. Biophys. Res. Commun. 2017, 491, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Kaczynski, J.; Cook, T.; Urrutia, R. Sp1- and Kruppel-like transcription factors. Genome Biol. 2003, 4, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Chan, Y.S.; Loh, Y.H.; Cai, J.; Tong, G.Q.; Lim, C.A.; Robson, P.; Zhong, S.; Ng, H.H. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat. Cell Biol. 2008, 10, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Scobie, K.N.; Hall, B.J.; Wilke, S.A.; Klemenhagen, K.C.; Fujii-Kuriyama, Y.; Ghosh, A.; Hen, R.; Sahay, A. Kruppel-like factor 9 is necessary for late-phase neuronal maturation in the developing dentate gyrus and during adult hippocampal neurogenesis. J. Neurosci. 2009, 29, 9875–9887. [Google Scholar] [CrossRef] [PubMed]
- Bialkowska, A.B.; Yang, V.W.; Mallipattu, S.K. Kruppel-like factors in mammalian stem cells and development. Development 2017, 144, 737–754. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Ceru, M.P. In search for novel strategies towards neuroprotection and neuroregeneration: Is PPARalpha a promising therapeutic target? Neural. Regen. Res. 2015, 10, 1409–1412. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, A.; Minghetti, L. Regulation of Glial Cell Functions by PPARγ Natural and Synthetic Agonists. PPAR Res. 2008, 2008, 864140. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, L.; Bernardo, A.; Ceru, M.P. Peroxisome proliferator-activated receptors (PPARs) and peroxisomes in rat cortical and cerebellar astrocytes. J. Neurocytol. 2001, 30, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, A.; Bianchi, D.; Magnaghi, V.; Minghetti, L. Peroxisome proliferator-activated receptor γ agonists promote differentiation and antioxidant defenses of oligodendrocyte progenitor cells. J. Neuropathol. Exp. Neurol. 2009, 68, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Cimini, A.; Cristiano, L.; Benedetti, E.; D’Angelo, B.; Ceru, M.P. PPARs Expression in Adult Mouse Neural Stem Cells: Modulation of PPARs during Astroglial Differentiaton of NSC. PPAR Res. 2007, 2007, 48242. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.A.; Polk, D.B.; Krishna, U.; Israel, D.A.; Yan, F.; DuBois, R.N.; Peek, R.M., Jr. Activation of peroxisome proliferator-activated receptor γ suppresses nuclear factor κ B-mediated apoptosis induced by Helicobacter pylori in gastric epithelial cells. J. Biol. Chem. 2001, 276, 31059–31066. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhang, J.; Lin, Y.; Zhu, X.; Zhao, L.; Ahmad, M.; Ehrengruber, M.U.; Chen, Y.E. Early stimulation and late inhibition of peroxisome proliferator-activated receptor γ (PPARγ) gene expression by transforming growth factor β in human aortic smooth muscle cells: Role of early growth-response factor-1 (EGR-1), activator protein 1 (AP1) and Smads. Biochem. J. 2003, 370, 1019–1025. [Google Scholar] [PubMed]
- Chen, Y.C.; Wu, J.S.; Tsai, H.D.; Huang, C.Y.; Chen, J.J.; Sun, G.Y.; Lin, T.N. Peroxisome proliferator-activated receptor γ (PPARγ) and neurodegenerative disorders. Mol. Neurobiol. 2012, 46, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, A.; Minghetti, L. PPARγ agonists as regulators of microglial activation and brain inflammation. Curr. Pharm. Des. 2006, 12, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L. Lipidomics of Alzheimer’s disease: Current status. Alzheimers Res. Ther. 2012, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.M.; Wang, Y.; Duan, X.; Wenk, M.R.; Kalaria, R.N.; Chen, C.P.; Lai, M.K.; Shui, G. Brain lipidomes of subcortical ischemic vascular dementia and mixed dementia. Neurobiol. Aging 2014, 35, 2369–2381. [Google Scholar] [CrossRef] [PubMed]
- Liebisch, G.; Drobnik, W.; Lieser, B.; Schmitz, G. High-throughput quantification of lysophosphatidylcholine by electrospray ionization tandem mass spectrometry. Clin. Chem. 2002, 48, 2217–2224. [Google Scholar] [PubMed]
- Kume, N.; Cybulsky, M.I.; Gimbrone, M.A., Jr. Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J. Clin. Investig. 1992, 90, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Li, N.J.; Liu, W.T.; Li, W.; Li, S.Q.; Chen, X.H.; Bi, K.S.; He, P. Plasma metabolic profiling of Alzheimer’s disease by liquid chromatography/mass spectrometry. Clin. Biochem. 2010, 43, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xiao, Y.J.; Zhu, K.; Baudhuin, L.M.; Lu, J.; Hong, G.; Kim, K.S.; Cristina, K.L.; Song, L.; Williams, F.S.; et al. Unfolding the pathophysiological role of bioactive lysophospholipids. Curr. Drug Targets Immune Endocr. Metabol. Disord. 2003, 3, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Spinal Cord Injury (SCI) 2016 Facts and Figures at a Glance. J. Spinal Cord Med. 2016, 39, 493–494.
- Silva, N.A.; Sousa, N.; Reis, R.L.; Salgado, A.J. From basics to clinical: A comprehensive review on spinal cord injury. Prog. Neurobiol. 2014, 114, 25–57. [Google Scholar] [CrossRef] [PubMed]
- Bareyre, F.M. Neuronal repair and replacement in spinal cord injury. J. Neurol. Sci. 2008, 265, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Ha, K.Y.; Kim, S.I. Spinal Cord Injury and Related Clinical Trials. Clin. Orthop. Surg. 2017, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Goldshmit, Y.; Matteo, R.; Sztal, T.; Ellett, F.; Frisca, F.; Moreno, K.; Crombie, D.; Lieschke, G.J.; Currie, P.D.; Sabbadini, R.A.; et al. Blockage of lysophosphatidic acid signaling improves spinal cord injury outcomes. Am. J. Pathol. 2012, 181, 978–992. [Google Scholar] [CrossRef] [PubMed]
- Santos-Nogueira, E.; Lopez-Serrano, C.; Hernandez, J.; Lago, N.; Astudillo, A.M.; Balsinde, J.; Estivill-Torrus, G.; de Fonseca, F.R.; Chun, J.; Lopez-Vales, R. Activation of Lysophosphatidic Acid Receptor Type 1 Contributes to Pathophysiology of Spinal Cord Injury. J. Neurosci. 2015, 35, 10224–10235. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V. FTY720 (fingolimod) in Multiple Sclerosis: Therapeutic effects in the immune and the central nervous system. Br. J. Pharmacol. 2009, 158, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Sadahira, Y.; Ruan, F.; Hakomori, S.; Igarashi, Y. Sphingosine 1-phosphate, a specific endogenous signaling molecule controlling cell motility and tumor cell invasiveness. Proc. Natl. Acad. Sci. USA 1992, 89, 9686–9690. [Google Scholar] [CrossRef] [PubMed]
- McTigue, D.M. Potential Therapeutic Targets for PPARgamma after Spinal Cord Injury. PPAR Res. 2008, 2008, 517162. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsukahara, T.; Matsuda, Y.; Haniu, H. Lysophospholipid-Related Diseases and PPARγ Signaling Pathway. Int. J. Mol. Sci. 2017, 18, 2730. https://doi.org/10.3390/ijms18122730
Tsukahara T, Matsuda Y, Haniu H. Lysophospholipid-Related Diseases and PPARγ Signaling Pathway. International Journal of Molecular Sciences. 2017; 18(12):2730. https://doi.org/10.3390/ijms18122730
Chicago/Turabian StyleTsukahara, Tamotsu, Yoshikazu Matsuda, and Hisao Haniu. 2017. "Lysophospholipid-Related Diseases and PPARγ Signaling Pathway" International Journal of Molecular Sciences 18, no. 12: 2730. https://doi.org/10.3390/ijms18122730