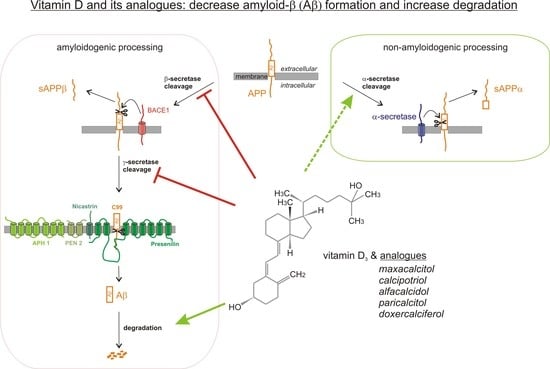
Vitamin D and Its Analogues Decrease Amyloid-β (Aβ) Formation and Increase Aβ-Degradation
Abstract
1. Introduction
2. Results
2.1. Vitamin D Analogues Decrease Total Aβ Level
2.2. Analysis of Non-Amyloidogenic APP Shedding in Presence of Vitamin D Analogues
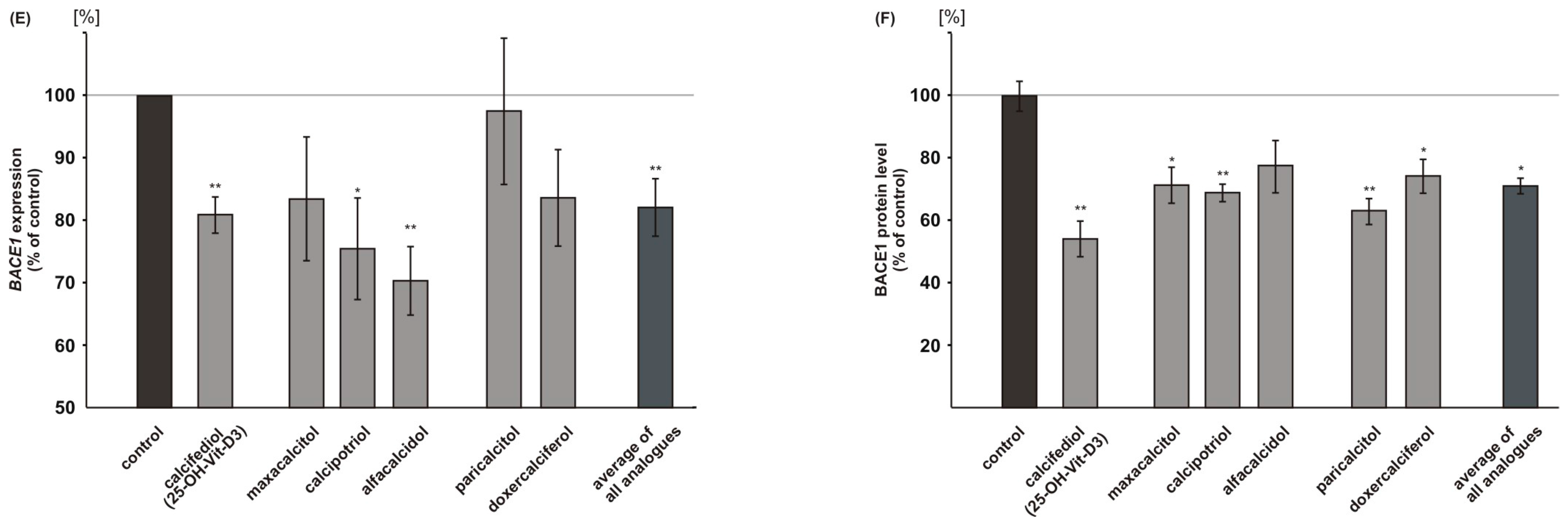
2.3. Vitamin D Analogues Decrease Amyloidogenic β-Secretase Dependent APP Cleavage
2.4. Vitamin D Analogues Decrease γ-Secretase Processing of APP
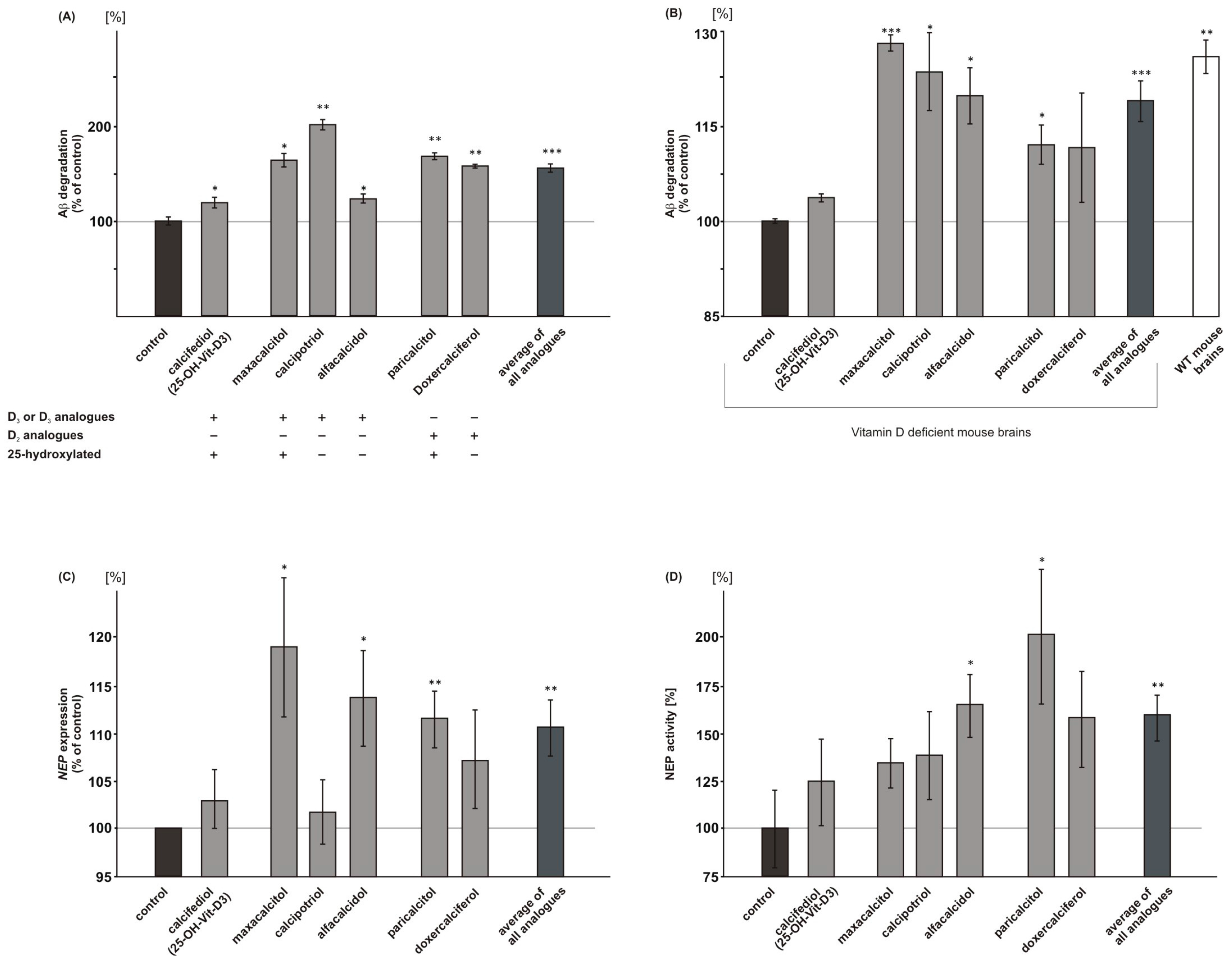
2.5. Vitamin D3 and Vitamin D2 Analogues Increase Aβ-Degradation
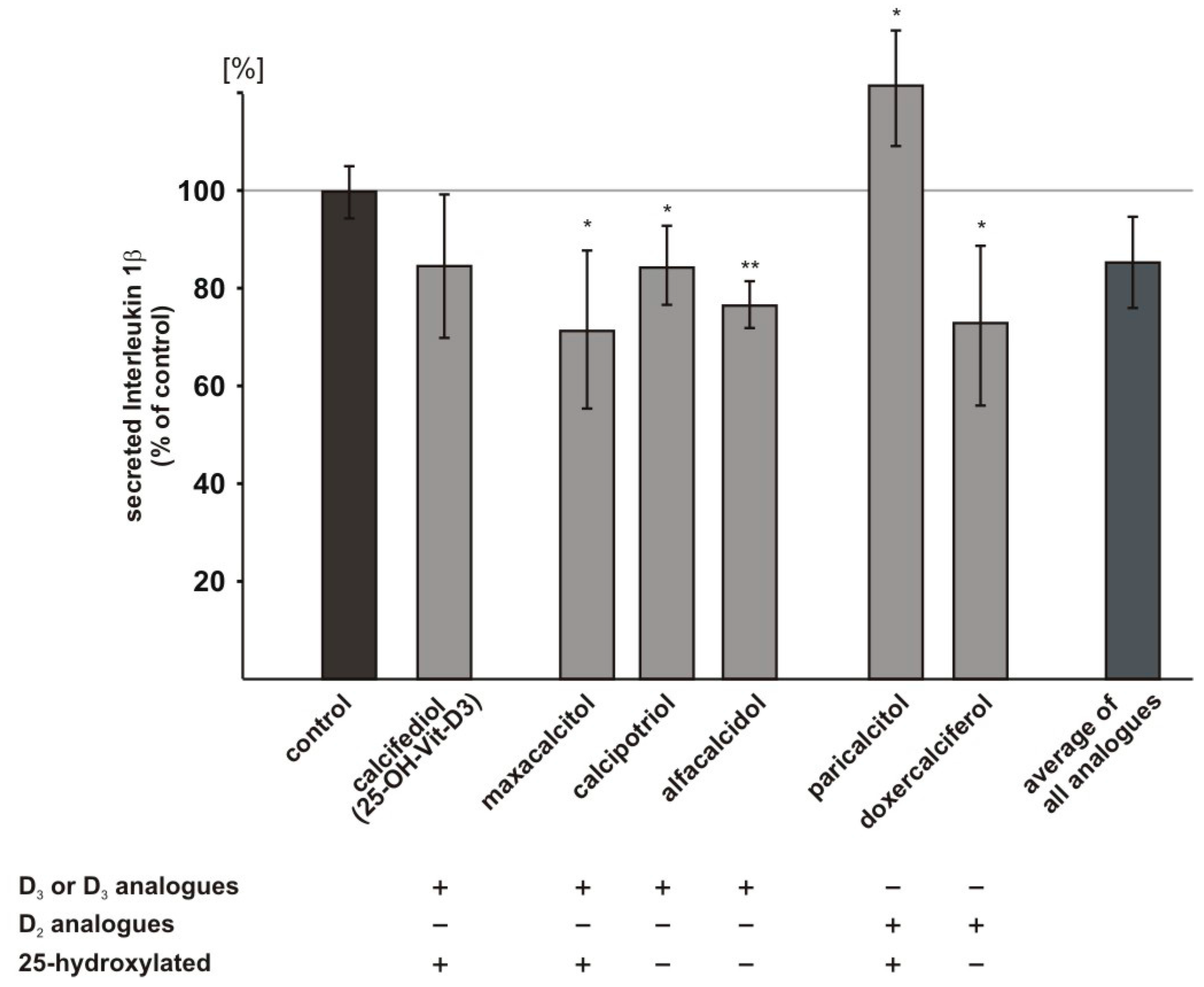
2.6. Influence of Vitamin D3 and Vitamin D2 Analogues on Inflammatory Processes
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and Mice
4.3. Vitamin D Incubations
4.3.1. Cell Culture
4.3.2. Mouse Brains or Purified Membranes
4.4. Determination of Protein Concentration
4.5. Western Blot Experiments
4.6. Determination of Total Aβ-Degradation
4.6.1. Determination of Total Aβ-Degradation in N2a wt Cells.
4.6.2. Determination of Total Aβ-Degradation in Deficient Mouse Brains
4.7. Secretase Activity Assays
4.7.1. Determination of α-, β- and γ-Secretase Activity in Living SH-SY5Y Cells
4.7.2. Determination of β-Secretase Activity in Isolated SH-SY5Y Membranes
4.8. RT-PCR Experiments
4.9. Neprilysin Activity Assay
4.10. Enzyme-Linked Immunosorbent Assay (ELISA)
4.11. Lactate Dehydrogenase (LDH) Activity Assay
4.12. Data Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophy. Res. Commun. 2012, 425, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Masters, C.L.; Simms, G.; Weinman, N.A.; Multhaup, G.; McDonald, B.L.; Beyreuther, K. Amyloid plaque core protein in alzheimer disease and down syndrome. Proc. Natl. Acad. Sci. USA 1985, 82, 4245–4249. [Google Scholar] [CrossRef] [PubMed]
- Grundke-Iqbal, I.; Iqbal, K.; Tung, Y.C.; Quinlan, M.; Wisniewski, H.M.; Binder, L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA 1986, 83, 4913–4917. [Google Scholar] [CrossRef] [PubMed]
- Grundke-Iqbal, I.; Iqbal, K.; Quinlan, M.; Tung, Y.C.; Zaidi, M.S.; Wisniewski, H.M. Microtubule-associated protein tau. A component of alzheimer paired helical filaments. J. Biol. Chem. 1986, 261, 6084–6089. [Google Scholar] [PubMed]
- Sinha, S.; Anderson, J.P.; Barbour, R.; Basi, G.S.; Caccavello, R.; Davis, D.; Doan, M.; Dovey, H.F.; Frigon, N.; Hong, J.; et al. Purification and cloning of amyloid precursor protein β-secretase from human brain. Nature 1999, 402, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R.; Bennett, B.D.; Babu-Khan, S.; Kahn, S.; Mendiaz, E.A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R.; et al. β-secretase cleavage of alzheimer’s amyloid precursor protein by the transmembrane aspartic protease bace. Science 1999, 286, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Kimberly, W.T.; LaVoie, M.J.; Ostaszewski, B.L.; Ye, W.; Wolfe, M.S.; Selkoe, D.J. Gamma-secretase is a membrane protein complex comprised of presenilin, nicastrin, aph-1, and pen-2. Proc. Natl. Acad. Sci. USA 2003, 100, 6382–6387. [Google Scholar] [CrossRef] [PubMed]
- Haass, C. Take five—BACE and the γ-secretase quartet conduct alzheimer’s amyloid β-peptide generation. EMBO J. 2004, 23, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Buxbaum, J.D.; Liu, K.N.; Luo, Y.; Slack, J.L.; Stocking, K.L.; Peschon, J.J.; Johnson, R.S.; Castner, B.J.; Cerretti, D.P.; Black, R.A. Evidence that tumor necrosis factor α converting enzyme is involved in regulated α-secretase cleavage of the alzheimer amyloid protein precursor. J. Biol. Chem. 1998, 273, 27765–27767. [Google Scholar] [CrossRef] [PubMed]
- Lammich, S.; Kojro, E.; Postina, R.; Gilbert, S.; Pfeiffer, R.; Jasionowski, M.; Haass, C.; Fahrenholz, F. Constitutive and regulated α-secretase cleavage of alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc. Natl. Acad. Sci. USA 1999, 96, 3922–3927. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.H.; Wang, H.; Dislich, B.; Colombo, A.; Zeitschel, U.; Ellwart, J.W.; Kremmer, E.; Rossner, S.; Lichtenthaler, S.F. Adam10 is the physiologically relevant, constitutive α-secretase of the amyloid precursor protein in primary neurons. EMBO J. 2010, 29, 3020–3032. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Tomioka, S.; Sorimachi, H.; Saido, T.C.; Maruyama, K.; Okuyama, A.; Fujisawa-Sehara, A.; Ohno, S.; Suzuki, K.; Ishiura, S. Membrane-anchored metalloprotease MDC9 has an α-secretase activity responsible for processing the amyloid precursor protein. Biochem. J. 1999, 343, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Iwata, N.; Tsubuki, S.; Takaki, Y.; Shirotani, K.; Lu, B.; Gerard, N.P.; Gerard, C.; Hama, E.; Lee, H.J.; Saido, T.C. Metabolic regulation of brain Aβ by neprilysin. Science 2001, 292, 1550–1552. [Google Scholar] [CrossRef] [PubMed]
- Farris, W.; Mansourian, S.; Chang, Y.; Lindsley, L.; Eckman, E.A.; Frosch, M.P.; Eckman, C.B.; Tanzi, R.E.; Selkoe, D.J.; Guenette, S. Insulin-degrading enzyme regulates the levels of insulin, amyloid β-protein, and the β-amyloid precursor protein intracellular domain in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 4162–4167. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.; Grimm, H.S.; Patzold, A.J.; Zinser, E.G.; Halonen, R.; Duering, M.; Tschape, J.A.; De Strooper, B.; Muller, U.; Shen, J.; et al. Regulation of cholesterol and sphingomyelin metabolism by amyloid-β and presenilin. Nature Cell Biol. 2005, 7, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.; Kuchenbecker, J.; Grosgen, S.; Burg, V.K.; Hundsdorfer, B.; Rothhaar, T.L.; Friess, P.; de Wilde, M.C.; Broersen, L.M.; Penke, B.; et al. Docosahexaenoic acid reduces amyloid beta production via multiple pleiotropic mechanisms. J. Biol. Chem. 2011, 286, 14028–14039. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.; Zinser, E.G.; Grosgen, S.; Hundsdorfer, B.; Rothhaar, T.L.; Burg, V.K.; Kaestner, L.; Bayer, T.A.; Lipp, P.; Muller, U.; et al. Amyloid precursor protein (APP) mediated regulation of ganglioside homeostasis linking alzheimer’s disease pathology with ganglioside metabolism. PLoS ONE 2012, 7, e34095. [Google Scholar] [CrossRef] [PubMed]
- Osenkowski, P.; Ye, W.; Wang, R.; Wolfe, M.S.; Selkoe, D.J. Direct and potent regulation of gamma-secretase by its lipid microenvironment. J. Biol. Chem. 2008, 283, 22529–22540. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.; Rothhaar, T.L.; Grosgen, S.; Burg, V.K.; Hundsdorfer, B.; Haupenthal, V.J.; Friess, P.; Kins, S.; Grimm, H.S.; Hartmann, T. Trans fatty acids enhance amyloidogenic processing of the alzheimer amyloid precursor protein (APP). J. Nutr. Biochem. 2012, 23, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Burg, V.K.; Grimm, H.S.; Rothhaar, T.L.; Grosgen, S.; Hundsdorfer, B.; Haupenthal, V.J.; Zimmer, V.C.; Mett, J.; Weingartner, O.; Laufs, U.; et al. Plant sterols the better cholesterol in alzheimer’s disease? A mechanistical study. J. Neurosci. 2013, 33, 16072–16087. [Google Scholar] [CrossRef] [PubMed]
- Lemkul, J.A.; Bevan, D.R. Aggregation of alzheimer’s amyloid β-peptide in biological membranes: A molecular dynamics study. Biochemistry 2013, 52, 4971–4980. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.; Haupenthal, V.J.; Mett, J.; Stahlmann, C.P.; Blumel, T.; Mylonas, N.T.; Endres, K.; Grimm, H.S.; Hartmann, T. Oxidized docosahexaenoic acid species and lipid peroxidation products increase amyloidogenic amyloid precursor protein processing. Neurodegener. Dis. 2016, 16, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Rothhaar, T.L.; Grosgen, S.; Haupenthal, V.J.; Burg, V.K.; Hundsdorfer, B.; Mett, J.; Riemenschneider, M.; Grimm, H.S.; Hartmann, T.; Grimm, M.O. Plasmalogens inhibit app processing by directly affecting γ-secretase activity in alzheimer’s disease. Sci. World J. 2012, 2012, 141240. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.; Grimm, H.S.; Tomic, I.; Beyreuther, K.; Hartmann, T.; Bergmann, C. Independent inhibition of alzheimer disease β- and γ-secretase cleavage by lowered cholesterol levels. J. Biol. Chem. 2008, 283, 11302–11311. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.; Mett, J.; Stahlmann, C.P.; Haupenthal, V.J.; Blumel, T.; Stotzel, H.; Grimm, H.S.; Hartmann, T. Eicosapentaenoic acid and docosahexaenoic acid increase the degradation of amyloid-β by affecting insulin-degrading enzyme. Biochem. Cell Biol. 2016, 94, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Pierucci, F.; Garcia-Gil, M.; Frati, A.; Bini, F.; Martinesi, M.; Vannini, E.; Mainardi, M.; Luzzati, F.; Peretto, P.; Caleo, M.; et al. Vitamin D3 protects against Aβ peptide cytotoxicity in differentiated human neuroblastoma SH-SY5Y cells: A role for S1P1/p38MAPK/ATF4 axis. Neuropharmacology 2017, 116, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Raha, S.; Lee, H.J.; Yumnam, S.; Hong, G.E.; Saralamma, V.V.G.; Ha, Y.L.; Kim, J.O.; Kim, Y.S.; Heo, J.D.; Lee, S.J.; et al. Vitamin D2 suppresses amyloid-β 25–35 induced microglial activation in BV2 cells by blocking the NF-κB inflammatory signaling pathway. Life sci. 2016, 161, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; He, L.Y.; Zhang, M.; Wang, F.; Liu, F.; Peng, W.X. 1,25-dihydroxyvitamin D3 regulates expression of LRP1 and rage in vitro and in vivo, enhancing Aβ1–40 brain-to-blood efflux and peripheral uptake transport. Neuroscience 2016, 322, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.; Lehmann, J.; Mett, J.; Zimmer, V.C.; Grosgen, S.; Stahlmann, C.P.; Hundsdorfer, B.; Haupenthal, V.J.; Rothhaar, T.L.; Herr, C.; et al. Impact of vitamin D on amyloid precursor protein processing and amyloid-β peptide degradation in alzheimer’s disease. Neurodegener. Dis. 2014, 13, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.; Mett, J.; Hartmann, T. The impact of vitamin E and other fat-soluble vitamins on alzheimer’s disease. Int. J. Mol. Sci. 2016, 17, 1785. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Shah, J. Role of vitamin D in amyloid clearance via LRP-1 upregulation in alzheimer’s disease: A potential therapeutic target? J. Chem. Neuroanat. 2017, 85, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Jimenez, F.J.; Molina, J.A.; de Bustos, F.; Orti-Pareja, M.; Benito-Leon, J.; Tallon-Barranco, A.; Gasalla, T.; Porta, J.; Arenas, J. Serum levels of β-carotene, α-carotene and vitamin A in patients with alzheimer’s disease. Eur. J. Neurol. 1999, 6, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Mangialasche, F.; Xu, W.; Kivipelto, M.; Costanzi, E.; Ercolani, S.; Pigliautile, M.; Cecchetti, R.; Baglioni, M.; Simmons, A.; Soininen, H.; et al. Tocopherols and tocotrienols plasma levels are associated with cognitive impairment. Neurobiol. aging 2012, 33, 2282–2290. [Google Scholar] [CrossRef] [PubMed]
- Lopes da Silva, S.; Vellas, B.; Elemans, S.; Luchsinger, J.; Kamphuis, P.; Yaffe, K.; Sijben, J.; Groenendijk, M.; Stijnen, T. Plasma nutrient status of patients with alzheimer’s disease: Systematic review and meta-analysis. Alzheimers Dement. 2014, 10, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Presse, N.; Shatenstein, B.; Kergoat, M.J.; Ferland, G. Low vitamin k intakes in community-dwelling elders at an early stage of alzheimer’s disease. J. Am. Diet. Assoc. 2008, 108, 2095–2099. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Honda, Y.; Hayashida, N.; Iwamoto, J.; Kanoko, T.; Satoh, K. Vitamin K deficiency and osteopenia in elderly women with alzheimer’s disease. Arch. Phys. Med. Rehabil. 2005, 86, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Souberbielle, J.C.; Schott, A.M.; de Decker, L.; Berrut, G.; Beauchet, O. Vitamin D in the elderly: 5 points to remember. Geriatrie et Psychologie Neuropsychiatrie du Vieillissement 2011, 9, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Beauchet, O. Vitamin D-mentia: Randomized clinical trials should be the next step. Neuroepidemiology 2011, 37, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Khemka, V.K.; Ganguly, A.; Roy, D.; Ganguly, U.; Chakrabarti, S. Vitamin D and alzheimer’s disease: Neurocognition to therapeutics. Int. J. Alzheimers Dis. 2015, 2015, 192747. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin d receptor and 1 α-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M.; Sakiyama, R.; Coty, W.A. Restricted transport of vitamin D and a derivatives through the rat blood-brain barrier. J. Neurochem. 1985, 44, 1138–1141. [Google Scholar] [CrossRef] [PubMed]
- Gezen-Ak, D.; Atasoy, I.L.; Candas, E.; Alaylioglu, M.; Yilmazer, S.; Dursun, E. Vitamin D receptor regulates amyloid beta 1–42 production with protein disulfide isomerase A3. ACS Chem. Neurosci. 2017, 8, 2335–2346. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J. Therapeutic uses of vitamin D analogues. Am. J. Kidney Dis. 2001, 38, S3–S19. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, S.; Goldsmith, D.; Larsson, T.E.; Massy, Z.A.; Cozzolino, M. Vitamin D metabolites and/or analogs: Which D for which patient? Curren. Vasc. Pharmacol. 2014, 12, 339–349. [Google Scholar] [CrossRef]
- Rohan de Silva, H.A.; Jen, A.; Wickenden, C.; Jen, L.S.; Wilkinson, S.L.; Patel, A.J. Cell-specific expression of β-amyloid precursor protein isoform mrnas and proteins in neurons and astrocytes. Mol. Brain Res. 1997, 47, 147–156. [Google Scholar] [CrossRef]
- Wootton, A.M. Improving the measurement of 25-hydroxy vitamin D. Clin. Biochemist. Rev. 2005, 26, 33–36. [Google Scholar]
- Vieth, R. Why the minimum desirable serum 25-hydroxy vitamin D level should be 75 nmol/L (30 ng/mL). Best Pract. Res. Clin. Endocrinol. 2011, 25, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Kennel, K.A.; Drake, M.T.; Hurley, D.L. Vitamin D deficiency in adults: When to test and how to treat. Mayo Clin. Proc. 2010, 85, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Wu-Wong, J.R.; Nakane, M.; Gagne, G.D.; Brooks, K.A.; Noonan, W.T. Comparison of the pharmacological effects of paricalcitol and doxercalciferol on the factors involved in mineral homeostasis. Int. J. Endocrinol. 2010, 2010, 621687. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Wierzbicka, J.; Nadkarni, S.; Brown, G.; Kutner, A.; Zmijewski, M.A. Antiproliferative activity of double point modified analogs of 1,25-dihydroxyvitamin D(2) against human malignant melanoma cell lines. Int. J. Mol. Sci. 2016, 17, 76. [Google Scholar] [CrossRef] [PubMed]
- Brandi, M.L. Indications on the use of vitamin D and vitamin D metabolites in clinical phenotypes. Clin. Cases Miner. Bone Metab. 2010, 7, 243–250. [Google Scholar] [PubMed]
- Duplancic, D.; Cesarik, M.; Poljak, N.K.; Radman, M.; Kovacic, V.; Radic, J.; Rogosic, V. The influence of selective vitamin D receptor activator paricalcitol on cardiovascular system and cardiorenal protection. Clin. Interv. Aging 2013, 8, 149–156. [Google Scholar] [PubMed]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R.; Alvarez, X.A.; Fernandez-Novoa, L.; Franco, A.; Mangues, R.; Pellicer, A.; Nishimura, T. Brain interleukin-1 β in alzheimer’s disease and vascular dementia. Methods Find. Exp. Clin. Pharmacol. 1994, 16, 141–151. [Google Scholar] [PubMed]
- Perez-Lopez, F.R.; Chedraui, P.; Fernandez-Alonso, A.M. Vitamin D and aging: Beyond calcium and bone metabolism. Maturitas 2011, 69, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.; Qayyum, R. Relation between serum 25-hydroxyvitamin D and c-reactive protein in asymptomatic adults (from the continuous national health and nutrition examination survey 2001 to 2006). Am. J. Cardiol. 2012, 109, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Cannell, J.J.; Hollis, B.W.; Zasloff, M.; Heaney, R.P. Diagnosis and treatment of vitamin D deficiency. Expert Opin. Pharmacother. 2008, 9, 107–118. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J. Hypothesis: Is low prenatal vitamin D a risk-modifying factor for schizophrenia? Schizophr. Res. 1999, 40, 173–177. [Google Scholar] [CrossRef]
- Annweiler, C. Vitamin D-mentia: Is vitamin D optional or essential for preventing late-life cognitive decline? J. Am. Geriatr. Soc. 2017, 65, 2155–2157. [Google Scholar] [CrossRef] [PubMed]
- Lemire, P.; Brangier, A.; Beaudenon, M.; Duval, G.T.; Annweiler, C. Cognitive changes under memantine according to vitamin D status in alzheimer patients: An exposed/unexposed cohort pilot study. J. Steroid Biochem. Mol. Biol. 2016, 175, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Etgen, T.; Sander, D.; Bickel, H.; Sander, K.; Forstl, H. Vitamin D deficiency, cognitive impairment and dementia: A systematic review and meta-analysis. Dement. Geriatr. Cogn. 2012, 33, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Van der Schaft, J.; Koek, H.L.; Dijkstra, E.; Verhaar, H.J.; van der Schouw, Y.T.; Emmelot-Vonk, M.H. The association between vitamin D and cognition: A systematic review. Ageing Res. Rev. 2013, 12, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Dickens, A.P.; Lang, I.A.; Langa, K.M.; Kos, K.; Llewellyn, D.J. Vitamin D, cognitive dysfunction and dementia in older adults. CNS Drugs 2011, 25, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Allali, G.; Allain, P.; Bridenbaugh, S.; Schott, A.M.; Kressig, R.W.; Beauchet, O. Vitamin D and cognitive performance in adults: A systematic review. Eur. J. Neurol. 2009, 16, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, M.K.; Wong, L.; Somerville, M.J.; Yoong, L.K.; Bergeron, C.; Parmentier, M.; McLachlan, D.R. Reduction of calbindin-28k mrna levels in alzheimer as compared to huntington hippocampus. Mol. Brain Res. 1993, 18, 32–42. [Google Scholar] [CrossRef]
- Balion, C.; Griffith, L.E.; Strifler, L.; Henderson, M.; Patterson, C.; Heckman, G.; Llewellyn, D.J.; Raina, P. Vitamin D, cognition, and dementia: A systematic review and meta-analysis. Neurology 2012, 79, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Bojesen, S.E.; Nordestgaard, B.G. Reduced 25-hydroxyvitamin d and risk of alzheimer’s disease and vascular dementia. Alzheimers Dement. 2014, 10, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Littlejohns, T.J.; Henley, W.E.; Lang, I.A.; Annweiler, C.; Beauchet, O.; Chaves, P.H.; Fried, L.; Kestenbaum, B.R.; Kuller, L.H.; Langa, K.M.; et al. Vitamin D and the risk of dementia and alzheimer disease. Neurology 2014, 83, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Mokry, L.E.; Ross, S.; Morris, J.A.; Manousaki, D.; Forgetta, V.; Richards, J.B. Genetically decreased vitamin D and risk of alzheimer disease. Neurology 2016, 87, 2567–2574. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Gattoni-Celli, M.; Zhu, H.; Bhat, N.R.; Sambamurti, K.; Gattoni-Celli, S.; Kindy, M.S. Vitamin D3-enriched diet correlates with a decrease of amyloid plaques in the brain of AβPP transgenic mice. J. Alzheimer’s Dis. 2011, 25, 295–307. [Google Scholar]
- Koh, Y.H.; von Arnim, C.A.; Hyman, B.T.; Tanzi, R.E.; Tesco, G. Bace is degraded via the lysosomal pathway. J. Biol. Chem. 2005, 280, 32499–32504. [Google Scholar] [CrossRef] [PubMed]
- Qing, H.; Zhou, W.; Christensen, M.A.; Sun, X.; Tong, Y.; Song, W. Degradation of bace by the ubiquitin-proteasome pathway. FASEB J. 2004, 18, 1571–1573. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Diaz, S.; Larriba, M.J.; Lopez-Otin, C.; Munoz, A. Vitamin D: Proteases, protease inhibitors and cancer. Cell Cycle 2010, 9, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Deng, Y.; Luo, Y.; Zhang, S.; Zou, H.; Cai, F.; Wada, K.; Song, W. Control of BACE1 degradation and APP processing by ubiquitin carboxyl-terminal hydrolase L1. J. Neurochem. 2012, 120, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Max, D.; Brandsch, C.; Schumann, S.; Kuhne, H.; Frommhagen, M.; Schutkowski, A.; Hirche, F.; Staege, M.S.; Stangl, G.I. Maternal vitamin D deficiency causes smaller muscle fibers and altered transcript levels of genes involved in protein degradation, myogenesis, and cytoskeleton organization in the newborn rat. Mol. Nutr. Food Res. 2014, 58, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Briones, T.L.; Darwish, H. Vitamin D mitigates age-related cognitive decline through the modulation of pro-inflammatory state and decrease in amyloid burden. J. Neuroinflamm. 2012, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Takasugi, N.; Tomita, T.; Hayashi, I.; Tsuruoka, M.; Niimura, M.; Takahashi, Y.; Thinakaran, G.; Iwatsubo, T. The role of presenilin cofactors in the γ-secretase complex. Nature 2003, 422, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Dries, D.R.; Yu, G. Assembly, maturation, and trafficking of the γ-secretase complex in alzheimer’s disease. Curr. Alzheimer Res. 2008, 5, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Allinson, T.M.; Parkin, E.T.; Condon, T.P.; Schwager, S.L.; Sturrock, E.D.; Turner, A.J.; Hooper, N.M. The role of ADAM10 and ADAM17 in the ectodomain shedding of angiotensin converting enzyme and the amyloid precursor protein. Eur. J. Biochem. 2004, 271, 2539–2547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, M.; Dong, Y.; Zhang, X.; Liu, X.; Chen, Z.; Zhu, Y.; Wang, H.; Liu, X.; Zhu, J.; et al. 1α, 25-dihydroxyvitamin D3 up-regulates Il-34 expression in SH-SY5Y neural cells. Innate Immun. 2017, 23, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Grimm, M.O.; Stahlmann, C.P.; Mett, J.; Haupenthal, V.J.; Zimmer, V.C.; Lehmann, J.; Hundsdorfer, B.; Endres, K.; Grimm, H.S.; Hartmann, T. Vitamin E: Curse or benefit in alzheimer’s disease? A systematic investigation of the impact of α-, γ- and δ-tocopherol on ass generation and degradation in neuroblastoma cells. J. Nutr. Health Aging 2015, 19, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.; Haupenthal, V.J.; Rothhaar, T.L.; Zimmer, V.C.; Grosgen, S.; Hundsdorfer, B.; Lehmann, J.; Grimm, H.S.; Hartmann, T. Effect of different phospholipids on α-secretase activity in the non-amyloidogenic pathway of alzheimer’s disease. Int. J. Mol. Sci. 2013, 14, 5879–5898. [Google Scholar] [CrossRef] [PubMed]
- Miners, J.S.; Verbeek, M.M.; Rikkert, M.O.; Kehoe, P.G.; Love, S. Immunocapture-based fluorometric assay for the measurement of neprilysin-specific enzyme activity in brain tissue homogenates and cerebrospinal fluid. J. Neurosci. Methods 2008, 167, 229–236. [Google Scholar] [CrossRef] [PubMed]




| Analogues | Statistical Test | WT+ | WT+ | WT+ | WT- | WT- | Deficient+ |
|---|---|---|---|---|---|---|---|
| WT− | Deficient+ | Deficient− | Deficient+ | Deficient− | Deficient− | ||
| calcifediol | t test 1 | 0.028 | 0.000 | 0.000 | 0.005 | 0.000 | 0.026 |
| Bonferroni 2 | 0.039 | 0.000 | 0.000 | 0.099 | 0.001 | 0.450 | |
| alfacalcidol | t test | 0.050 | 0.000 | 0.000 | 0.003 | 0.000 | 0.144 |
| Bonferroni | 0.239 | 0.000 | 0.000 | 0.010 | 0.000 | 0.878 | |
| calcipotriol | t test | 0.858 | 0.002 | 0.002 | 0.000 | 0.000 | 0.964 |
| Bonferroni | 1.000 | 0.001 | 0.001 | 0.002 | 0.002 | 1.000 | |
| doxercalciferol | t test | 0.573 | 0.499 | 0.014 | 0.145 | 0.000 | 0.040 |
| Bonferroni | 1.000 | 1.000 | 0.038 | 0.873 | 0.005 | 0.226 | |
| maxacalcitol | t test | 0.015 | 0.000 | 0.000 | 0.001 | 0.000 | 0.606 |
| Bonferroni | 0.167 | 0.000 | 0.000 | 0.001 | 0.000 | 1.000 | |
| paricalcitol | t test | 0.179 | 0.000 | 0.000 | 0.000 | 0.000 | 0.223 |
| Bonferroni | 0.945 | 0.000 | 0.000 | 0.000 | 0.000 | 1.000 |
| Analogues | WT | Deficient | |
|---|---|---|---|
| calcifediol | alfacalcidol | 1.000 | 1.000 |
| calcipotriol | 0.466 | 0.967 | |
| doxercalciferol | 0.066 | 1.000 | |
| maxacalcitol | 1.000 | 1.000 | |
| paricalcitol | 1.000 | 1.000 | |
| alfacalcidol | calcipotriol | 1.000 | 1.000 |
| doxercalciferol | 0.515 | 1.000 | |
| maxacalcitol | 1.000 | 1.000 | |
| paricalcitol | 1.000 | 1.000 | |
| calcipotriol | doxercalciferol | 1.000 | 0.158 |
| maxacalcitol | 1.000 | 1.000 | |
| paricalcitol | 1.000 | 1.000 | |
| doxercalciferol | maxacalcitol | 0.316 | 0.686 |
| paricalcitol | 1.000 | 1.000 | |
| maxacalcitol | paricalcitol | 1.000 | 1.000 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grimm, M.O.W.; Thiel, A.; Lauer, A.A.; Winkler, J.; Lehmann, J.; Regner, L.; Nelke, C.; Janitschke, D.; Benoist, C.; Streidenberger, O.; et al. Vitamin D and Its Analogues Decrease Amyloid-β (Aβ) Formation and Increase Aβ-Degradation. Int. J. Mol. Sci. 2017, 18, 2764. https://doi.org/10.3390/ijms18122764
Grimm MOW, Thiel A, Lauer AA, Winkler J, Lehmann J, Regner L, Nelke C, Janitschke D, Benoist C, Streidenberger O, et al. Vitamin D and Its Analogues Decrease Amyloid-β (Aβ) Formation and Increase Aβ-Degradation. International Journal of Molecular Sciences. 2017; 18(12):2764. https://doi.org/10.3390/ijms18122764
Chicago/Turabian StyleGrimm, Marcus O. W., Andrea Thiel, Anna A. Lauer, Jakob Winkler, Johannes Lehmann, Liesa Regner, Christopher Nelke, Daniel Janitschke, Céline Benoist, Olga Streidenberger, and et al. 2017. "Vitamin D and Its Analogues Decrease Amyloid-β (Aβ) Formation and Increase Aβ-Degradation" International Journal of Molecular Sciences 18, no. 12: 2764. https://doi.org/10.3390/ijms18122764
APA StyleGrimm, M. O. W., Thiel, A., Lauer, A. A., Winkler, J., Lehmann, J., Regner, L., Nelke, C., Janitschke, D., Benoist, C., Streidenberger, O., Stötzel, H., Endres, K., Herr, C., Beisswenger, C., Grimm, H. S., Bals, R., Lammert, F., & Hartmann, T. (2017). Vitamin D and Its Analogues Decrease Amyloid-β (Aβ) Formation and Increase Aβ-Degradation. International Journal of Molecular Sciences, 18(12), 2764. https://doi.org/10.3390/ijms18122764