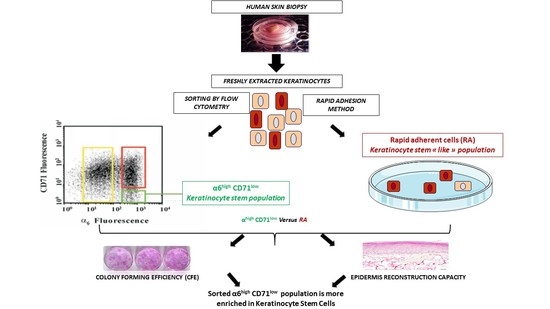
α6 Integrin (α6high)/Transferrin Receptor (CD71)low Keratinocyte Stem Cells Are More Potent for Generating Reconstructed Skin Epidermis Than Rapid Adherent Cells
Abstract
:1. Introduction
2. Results
2.1. Optimization of the Rapid Adhesion Method
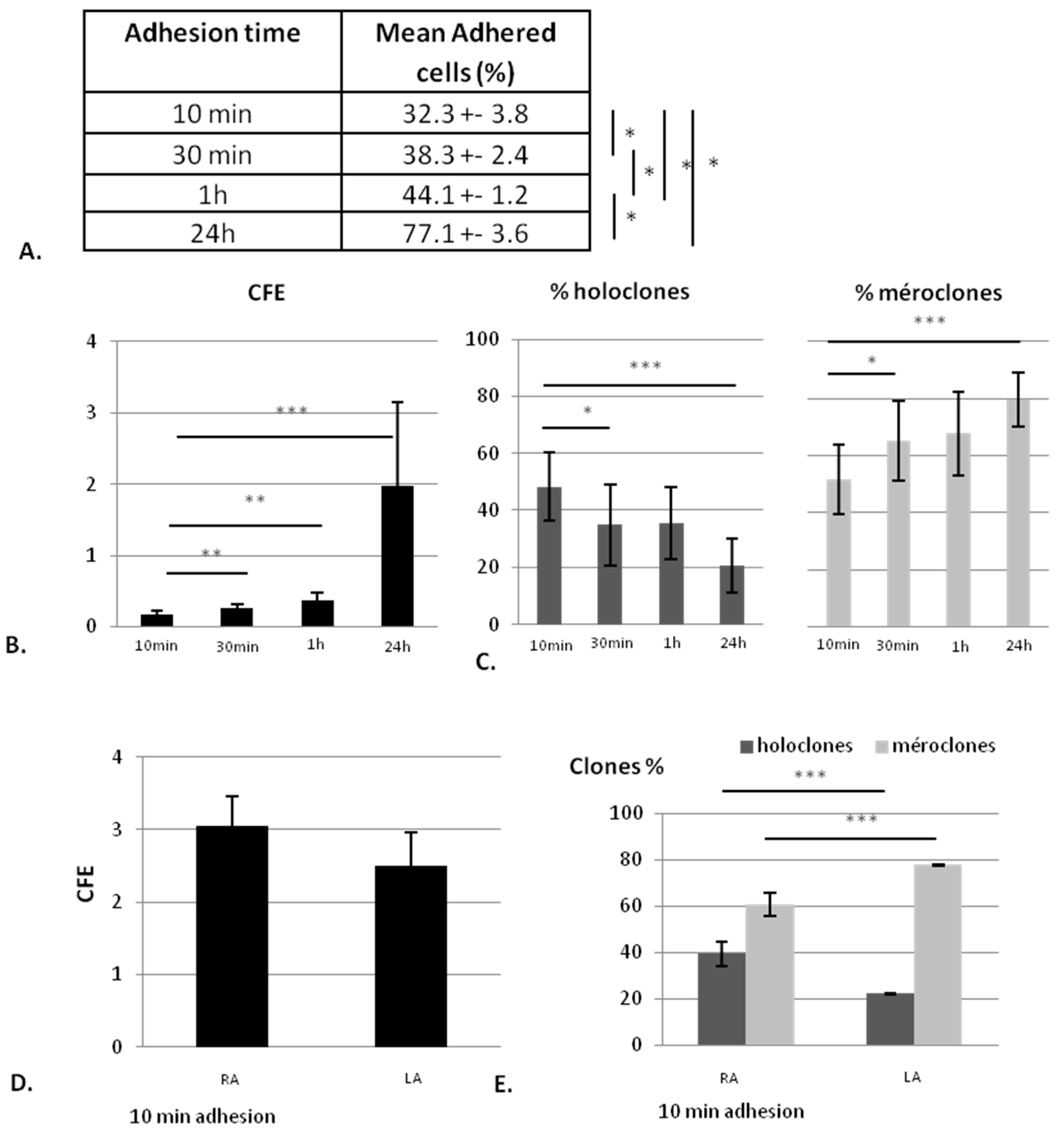
2.1.1. The KSC Enrichment is Inversely Proportional to the Adhesion Time
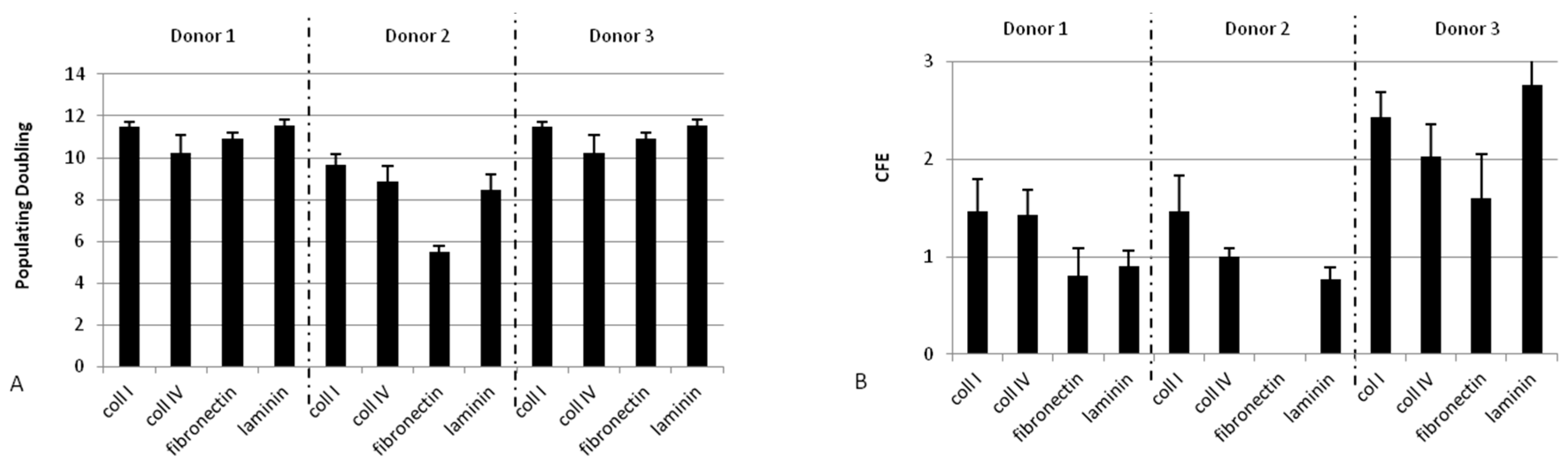
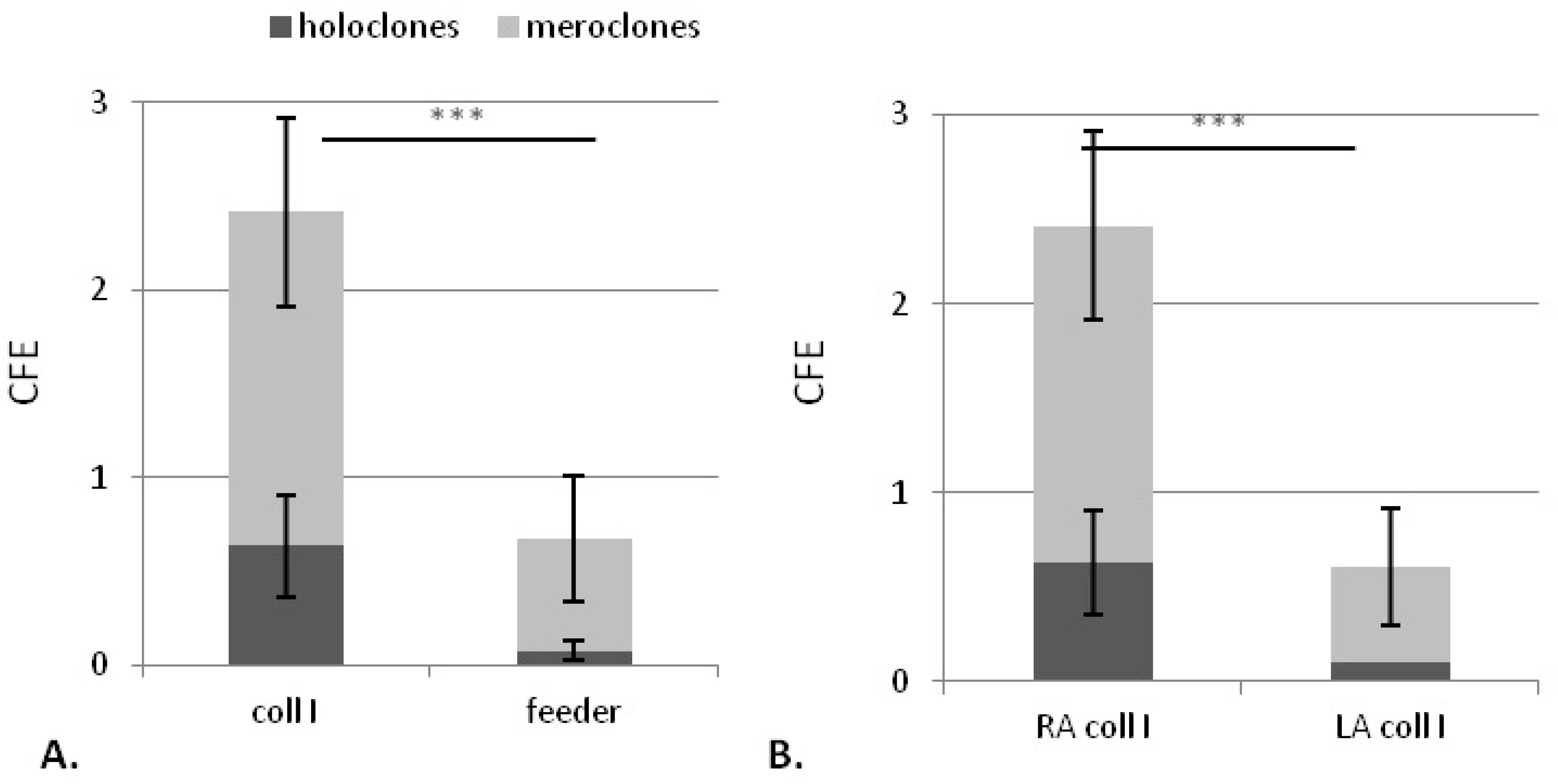
2.1.2. Collagen Type I Leads to Maintenance of Clonogenic Capacity of Isolated Cells
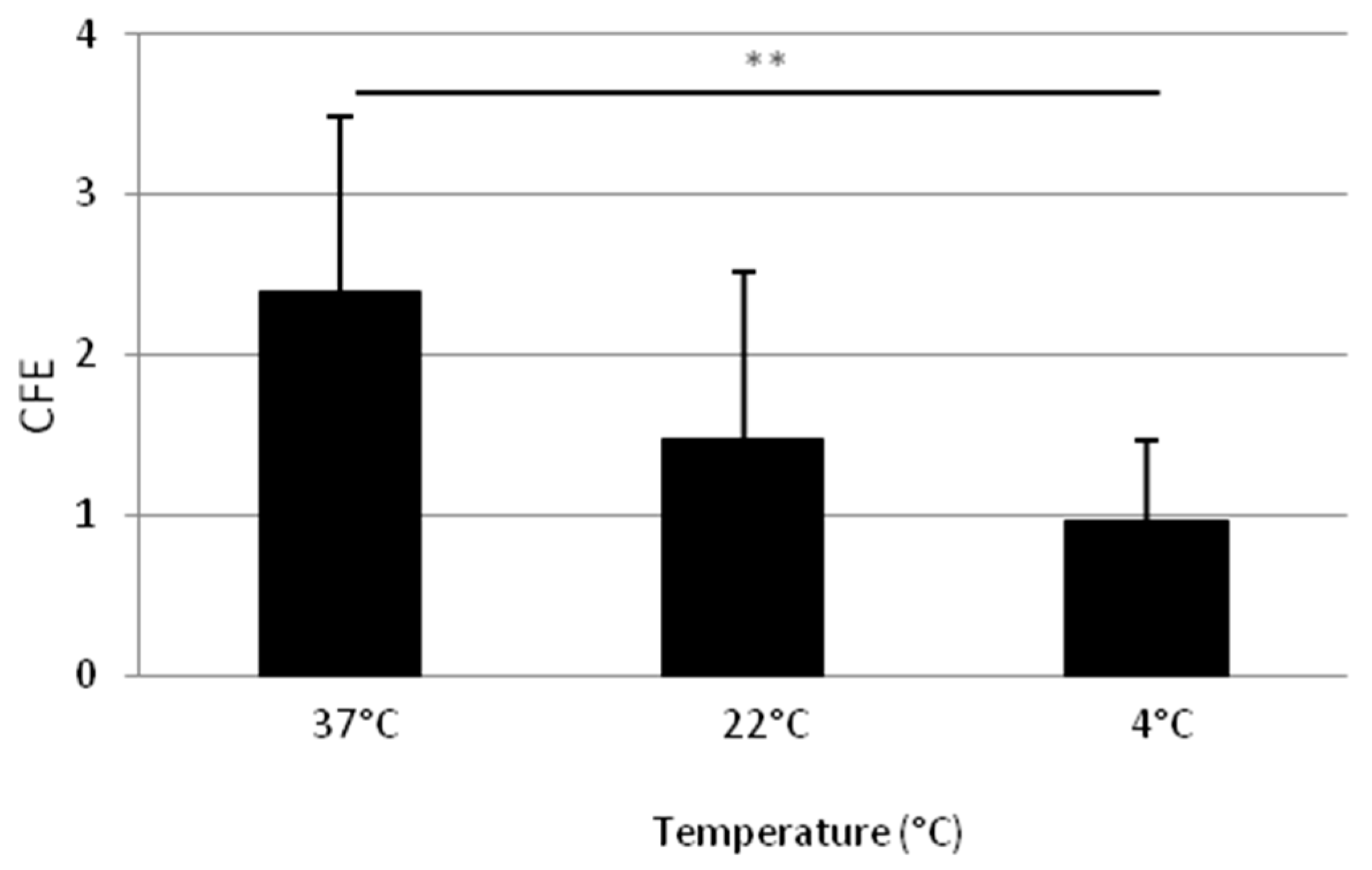
2.1.3. Adhesion at 37 °C Leads to a Higher Clonogenic Potential of Isolated Cells
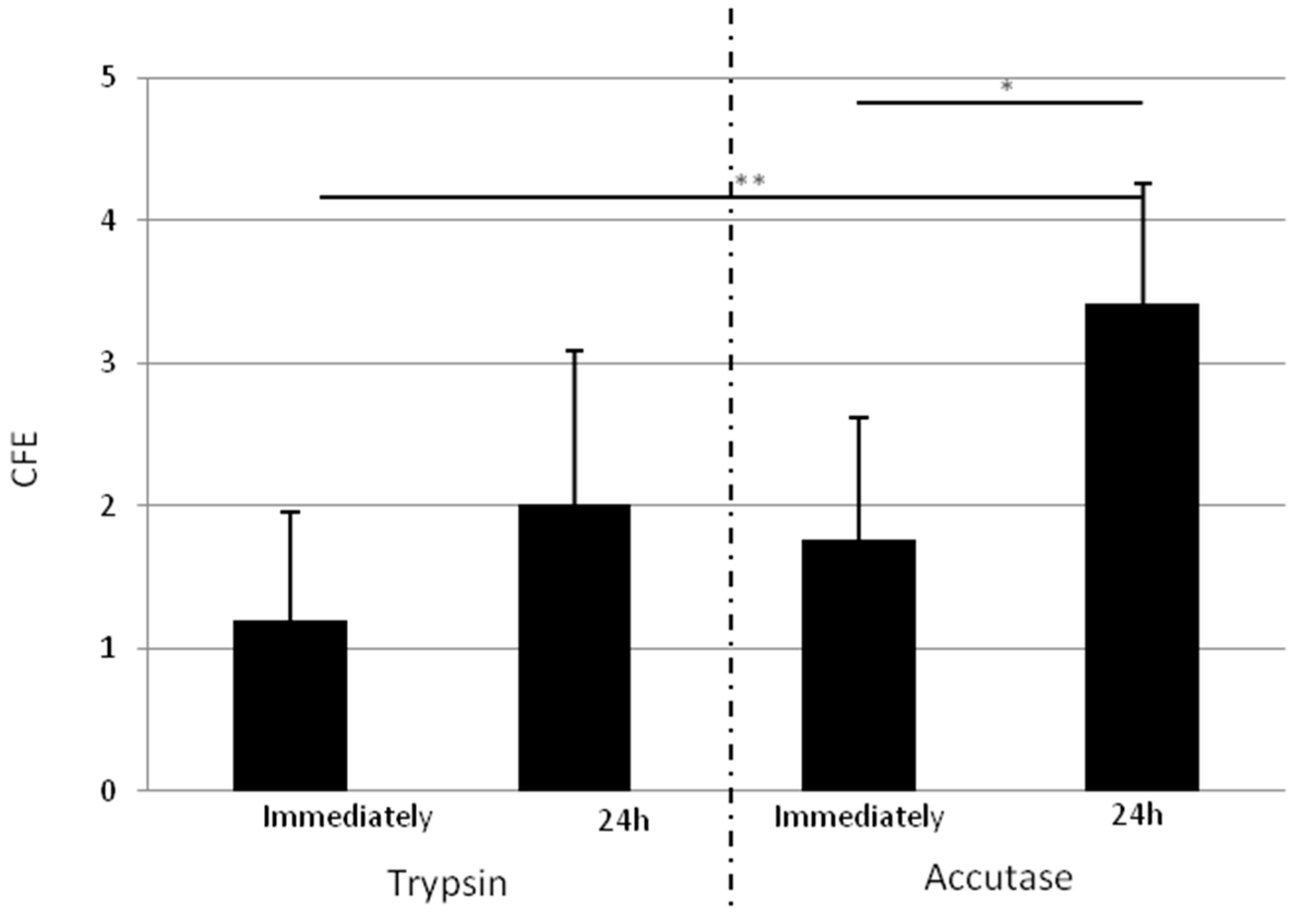
2.1.4. Isolated Cells Detached One Day after Adhesion with Accutase Display a Higher Clonogenic Potential
2.2. Comparison of KSC Sorted by Flow Cytometry Following the α6high/CD71low Phenotype to KSC Isolated by Rapid Adhesion Method (RA Cells)
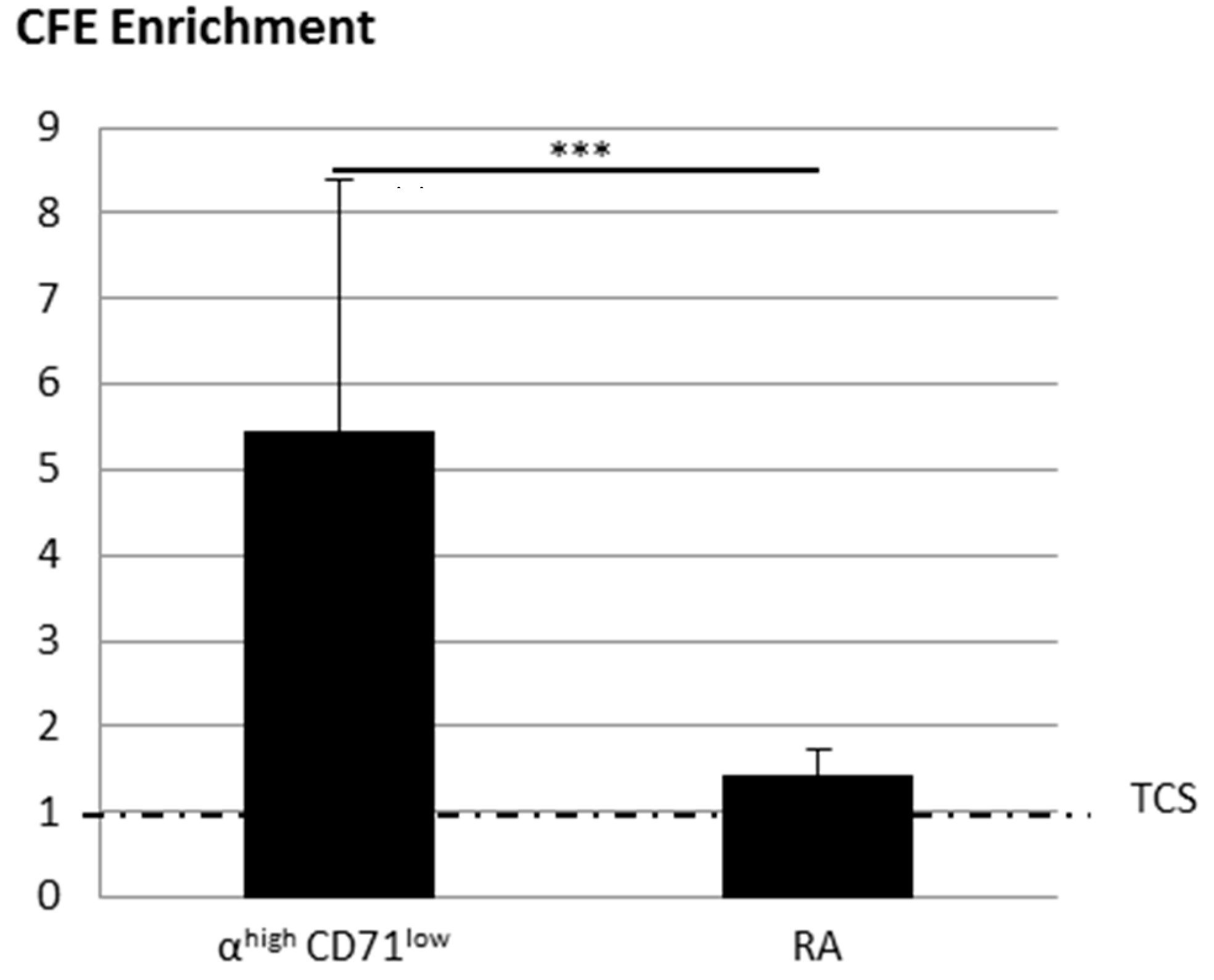
2.2.1. KSC Sorted by Flow Cytometry (α6high/CD71low) Display a Better CFE Enrichment
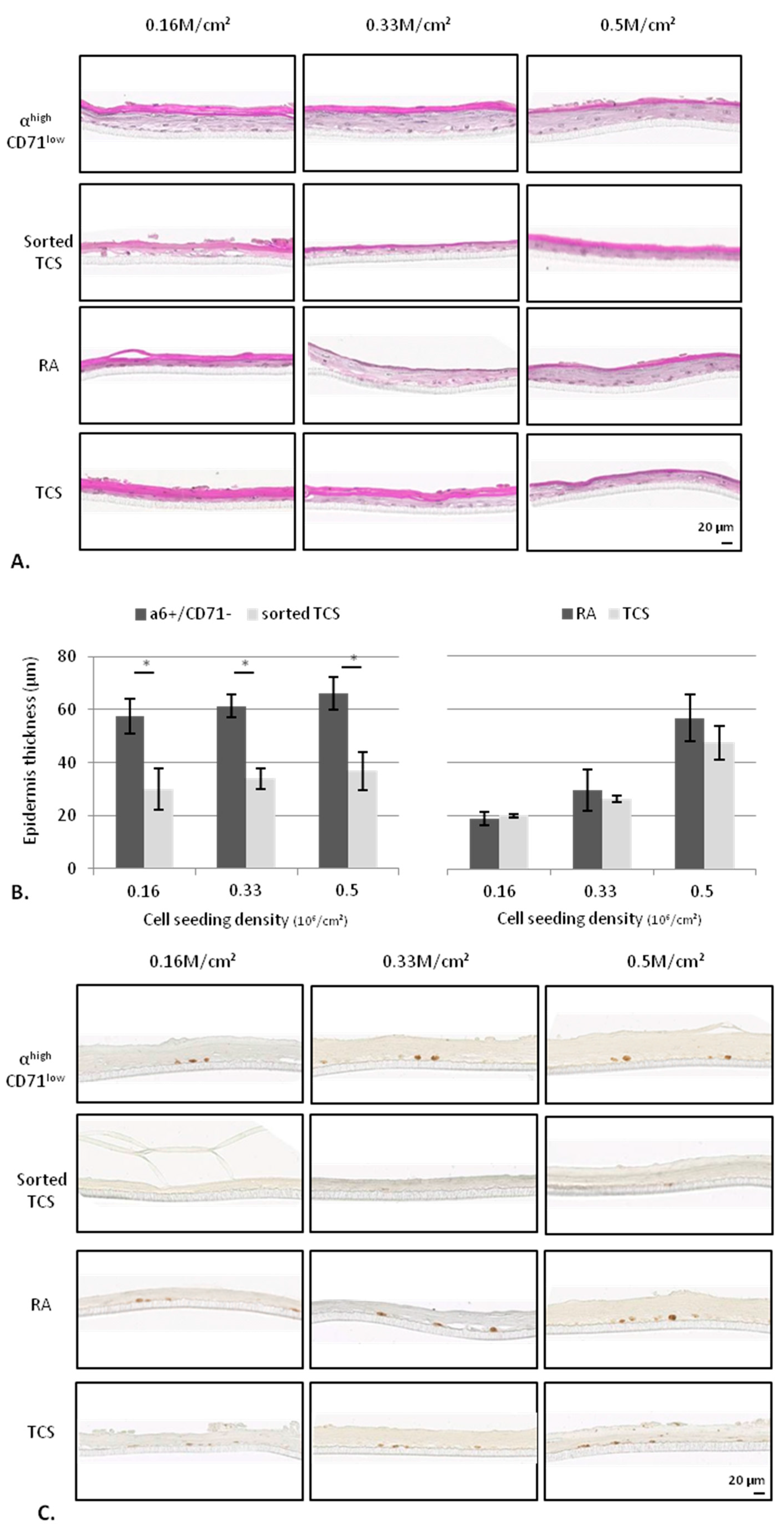
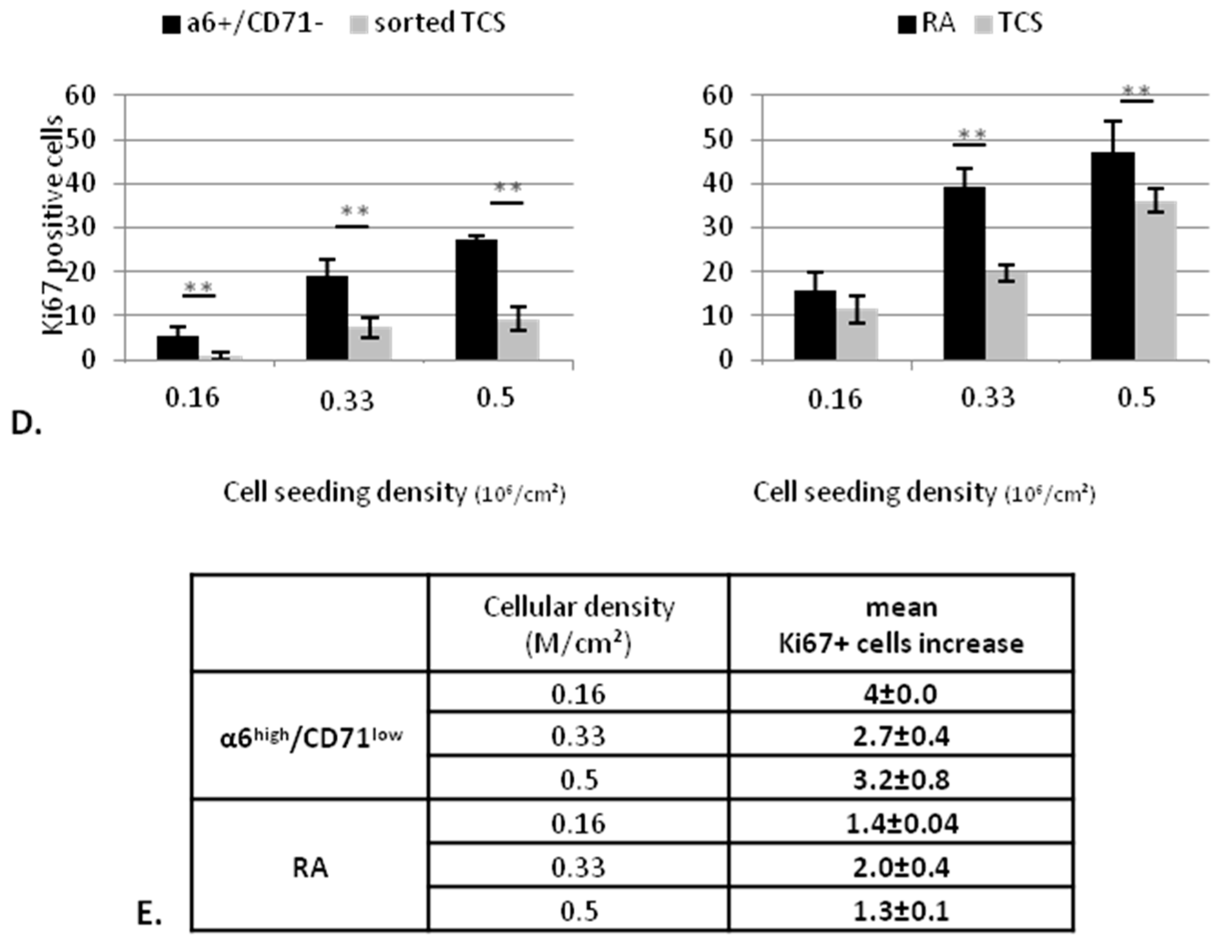
2.2.2. KSC Sorted by Flow Cytometry (α6high/CD71low) are More Potent for Reconstructing Pluristratified Human Epidermis than RA Cells
3. Discussion
4. Materials and Methods
4.1. Human Skin Samples
4.2. Keratinocyte Extraction and Culture
4.3. Colony Forming Unit and Colony Forming Efficiency
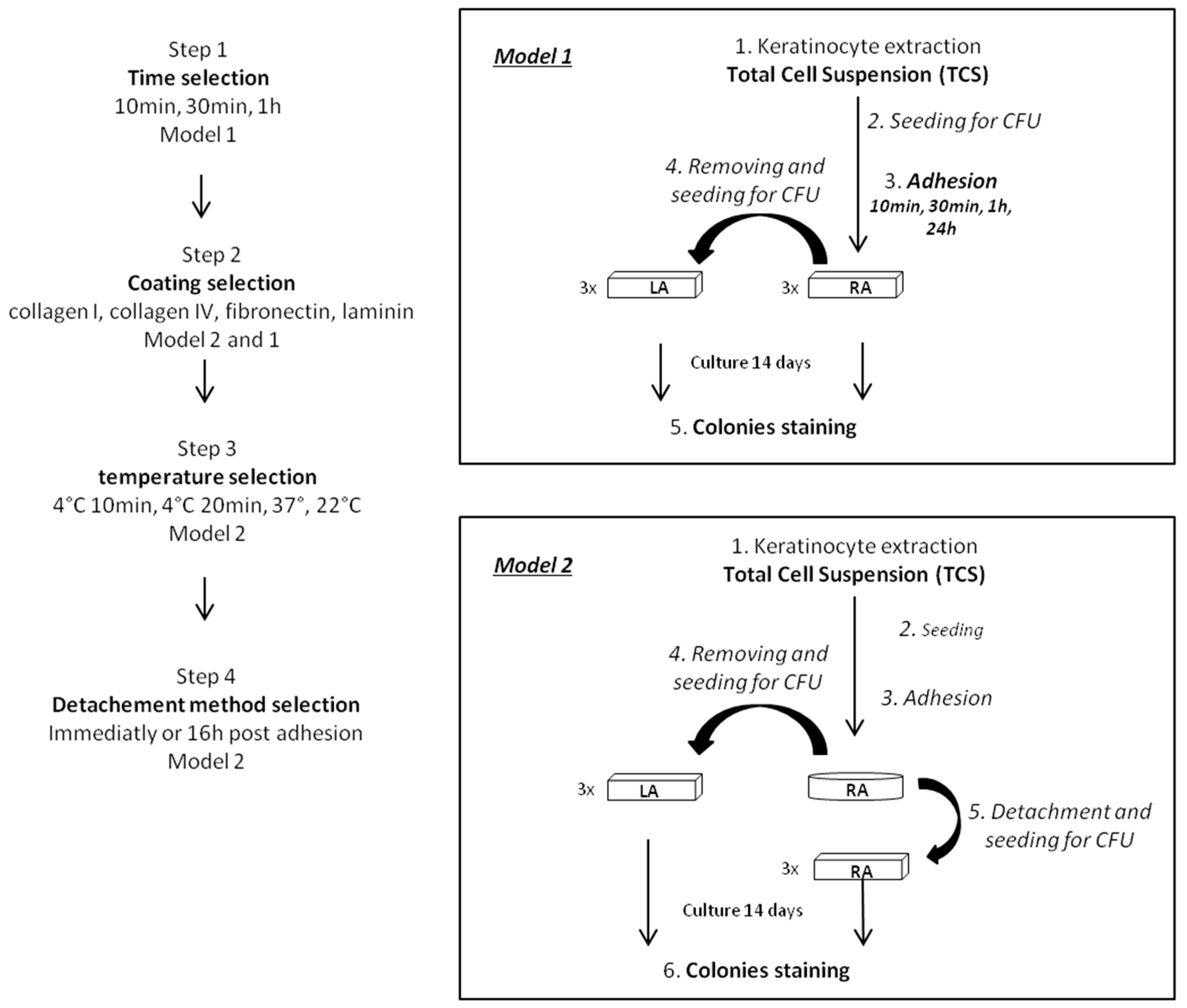
4.4. Experimental Protocol for Optimization of the Rapid Adhesion Method
Step 1: Optimization of the adhesion time using model 1
Step 2: Selection of coating using model 1 and 2
Step 3: Temperature selection using model 2
Step 4: Selection of the keratinocyte detachment method using model 2
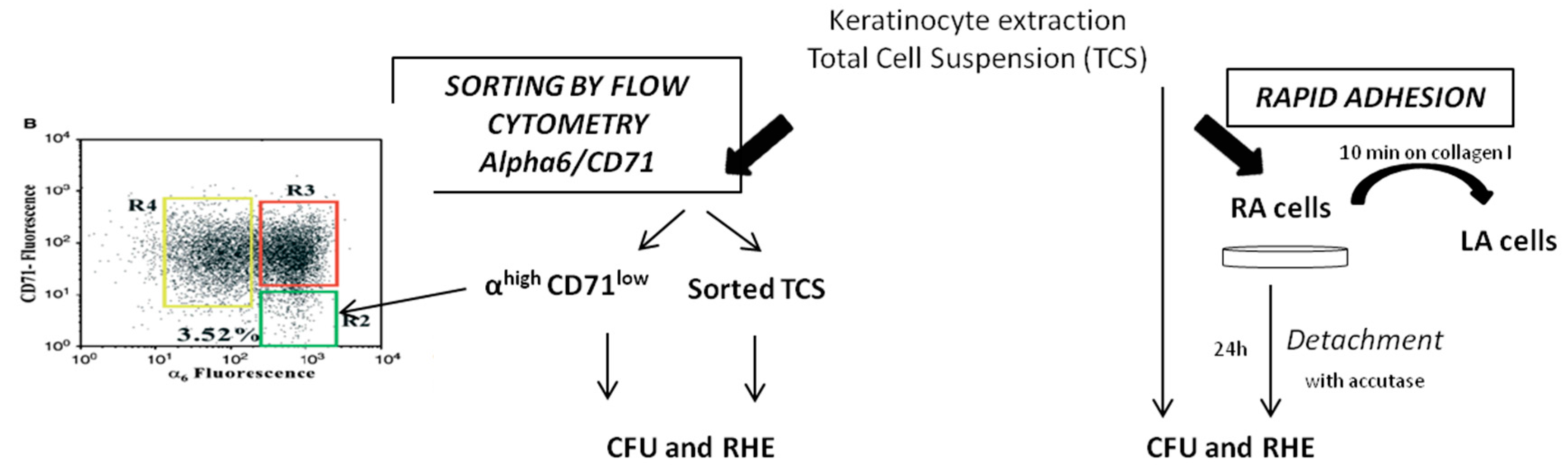
4.5. Experimental Procedure for Comparison of Optimized Rapid Adhesion Method to FACS Sorted α6high/CD71low Cells
4.5.1. RA Cell Isolation by Optimized Rapid Adhesion Method (Model 2)
4.5.2. KSC Isolation by Flow Cytometry
4.6. Epidermis Reconstruction
4.6.1. Preparation
4.6.2. Analysis by Histology and Immunohistological Analysis
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| KSC | keratinocyte stem cells |
| RA | rapid adherent cells |
| LA | low adherent cells |
| TCS | total cell suspension |
| RHE | reconstructed human epidermis |
| CFU | colony forming unit |
| CFE | colony forming efficiency |
Appendix A
References
- Giangreco, A.; Qin, M.; Pintar, J.E.; Watt, F.M. Epidermal stem cells are retained in vivo throughout skin aging. Aging Cell 2008, 7, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Barrandon, Y.; Green, H. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl. Acad. Sci. USA 1987, 84, 2302–2306. [Google Scholar] [CrossRef] [PubMed]
- Rochat, A.; Kobayashi, K.; Barrandon, Y. Location of stem cells of human hair follicles by clonal analysis. Cell 1994, 76, 1063–1073. [Google Scholar] [CrossRef]
- Cotsarelis, G.; Sun, T.T.; Lavker, R.M. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990, 61, 1329–1337. [Google Scholar] [CrossRef]
- Lavker, R.M.; Sun, T.T. Epidermal stem cells: Properties, markers, and location. Proc. Natl. Acad. Sci. USA 2000, 97, 13473–13475. [Google Scholar] [CrossRef] [PubMed]
- Potten, C.S.; Morris, R.J. Epithelial stem cells in vivo. J. Cell Sci. 1988, 10, 45–62. [Google Scholar] [CrossRef]
- Moore, K.A.; Lemischka, I.R. Stem cells and their niches. Science 2006, 311, 1880–1885. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Simmons, P.J.; Kaur, P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc. Natl. Acad. Sci. USA 1998, 95, 3902–3907. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Morris, R.J.; Kaur, P. Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proc. Natl. Acad. Sci. USA 2000, 97, 10960–10965. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Stone, M.G.; Simpson, C.; Reynolds, L.E.; Marshall, J.F.; Hart, I.R.; Hodivala-Dilke, K.M.; Eady, R.A.J. Desmosomal proteins, including desmoglein 3, serve as novel negative markers for epidermal stem cell-containing population of keratinocytes. J. Cell. Sci. 2003, 116, 4239–4248. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Cho, H.-J.; Choi, H.-R.; Kwon, S.-B.; Park, K.-C. Isolation of human epidermal stem cells by adherence and the reconstruction of skin equivalents. Cell. Mol. Life Sci. 2004, 61, 2774–2781. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.D.; Perkins, V.C.; Henry, A.; Stephens, P.E.; Robinson, M.K.; Watt, F.M. A tumor-associated β1 integrin mutation that abrogates epithelial differentiation control. J. Cell Biol. 2003, 160, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.J.; Watt, F.M. β-catenin signalling modulates proliferative potential of human epidermal keratinocytes independently of intercellular adhesion. Development 1999, 126, 2285–2298. [Google Scholar] [PubMed]
- Jones, P.H.; Watt, F.M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 1993, 73, 713–724. [Google Scholar] [CrossRef]
- Watt, F.M.; Jones, P.H. Expression and function of the keratinocyte integrins. Dev. Suppl. 1993, 185–192. [Google Scholar]
- Stingl, J.; Eirew, P.; Ricketson, I.; Shackleton, M.; Vaillant, F.; Choi, D.; Li, H.I.; Eaves, C.J. Purification and unique properties of mammary epithelial stem cells. Nature 2006, 439, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.M.; Fujiwara, H. Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Han, S.; Chang, S.; Cho, J. Increase of integrin α6+ p63+ cells after ultraviolet B irradiation in normal human keratinocytes. Dermatol. Rep. 2099, 1, e2. [Google Scholar] [CrossRef] [PubMed]
- Harfouche, G.; Vaigot, P.; Rachidi, W.; Rigaud, O.; Moratille, S.; Marie, M.; Lemaitre, G.; Fortunel, N.O.; Martin, M.T. Fibroblast growth factor type 2 signaling is critical for DNA repair in human keratinocyte stem cells. Stem Cells 2010, 28, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Rachidi, W.; Harfourche, G.; Lemaitre, G.; Amiot, F.; Vaigot, P.; Martin, M.T. Sensing radiosensitivity of human epidermal stem cells. Radiother. Oncol. 2007, 83, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Fortunel, N.O.; Chadli, L.; Bourreau, E.; Cadio, E.; Vaigot, P.; Marie, M.; Deshayes, N.; Rathman-Josserand, M.; Leclaire, J.; Martin, M.T. Cellular adhesion on collagen: A simple method to select human basal keratinocytes which preserves their high growth capacity. Eur. J. Dermatol. 2011, 21, 12–20. [Google Scholar] [PubMed]
- Pikuła, M.; Kondej, K.; Jaśkiewicz, J.; Skokowski, J.; Trzonkowski, P. Flow cytometric sorting and analysis of human epidermal stem cell candidates. Cell Biol. Int. 2010, 34, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Bata-Csorgo, Z.; Hammerberg, C.; Voorhees, J.J.; Cooper, K.D. Kinetics and regulation of human keratinocyte stem cell growth in short-term primary ex vivo culture. Cooperative growth factors from psoriatic lesional T lymphocytes stimulate proliferation among psoriatic uninvolved, but not normal, stem keratinocytes. J. Clin. Investig. 1995, 95, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, H.; Kaur, P. In vivo transplantation assay at limiting dilution to identify the intrinsic tissue reconstitutive capacity of keratinocyte stem cells and their progeny. Methods Mol. Biol. 2013, 989, 165–182. [Google Scholar] [PubMed]
- Dong, R.; Liu, X.; Liu, Y.; Deng, Z.; Nie, X.; Wang, X.; Jin, Y. Enrichment of epidermal stem cells by rapid adherence and analysis of the reciprocal interaction of epidermal stem cells with neighboring cells using an organotypic system. Cell Biol. Int. 2007, 31, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rivera, A.; Pavón-Rodríguez, A.; Jiménez-Acosta, F.; Poblet, E.; Braun, K.M.; Cormenzana, P.; Ciria, J.P.; Larretxea, R.; Cárdenas, J.M.; Izeta, A. Functional characterization of highly adherent CD34+ keratinocytes isolated from human skin. Exp. Dermatol. 2010, 19, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Pouliot, N.; Saunders, N.A.; Kaur, P. Laminin 10/11: An alternative adhesive ligand for epidermal keratinocytes with a functional role in promoting proliferation and migration. Exp. Dermatol. 2002, 11, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Spichkina, O.G.; Kalmykova, N.V.; Kukhareva, L.V.; Voronkina, I.V.; Blinova, M.I.; Pinaev, G.P. Isolation of human basal keratinocytes by selective adhesion to extracellular matrix proteins. Tsitologiia 2006, 48, 841–847. [Google Scholar] [PubMed]
- Xie, J.L.; Li, T.Z.; Qi, S.H.; Huang, B.; Chen, X.G.; Chen, J.D. A study of using tissue-engineered skin reconstructed by candidate epidermal stem cells to cover the nude mice with full-thickness skin defect. J. Plast. Reconstr. Aesthet. Surg. 2007, 60, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Li, A. Adhesive properties of human basal epidermal cells: An analysis of keratinocyte stem cells, transit amplifying cells, and postmitotic differentiating cells. J. Investig. Dermatol. 2000, 114, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Miao, C.; Guo, W.; Jia, L.; Zhou, J.; Ma, B.; Peng, S.; Liu, S.; Cao, Y.; Duan, E. Enrichment of putative human epidermal stem cells based on cell size and collagen type IV adhesiveness. Cell Res. 2008, 18, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Radu, E.; Simionescu, O.; Regalia, T.; Dumitrescu, D.; Popescu, L.M. Stem cells p63(+) in keratinocyte cultures from human adult skin. J. Cell. Mol. Med. 2002, 6, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Wang, Z.; Huang, B.; Ge, J.; Lu, R.; Zhang, K.; Fan, Z.; Lu, L.; Peng, Z.; Cui, G. Putative epidermal stem cell convert into corneal epithelium-like cell under corneal tissue in vitro. Sci. China C Life Sci. 2007, 50, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Schubert, S.; Lehmann, J.; Kalfon, L.; Slor, H.; Falik-Zaccai, T.C.; Emmert, S. Clinical utility gene card for: Xeroderma pigmentosum. Eur. J. Hum. Genet. 2014, 22. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.H.; Harper, S.; Watt, F.M. Stem cell patterning and fate in human epidermis. Cell 1995, 80, 83–93. [Google Scholar] [CrossRef]
- Barton, S.P.; Marks, R. Changes in suspensions of human keratinocytes due to trypsin. Arch. Dermatol. Res. 1981, 271, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, M. Functional correlation between cell adhesive properties and some cell surface proteins. J. Cell Biol. 1977, 75, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Aumailley, M.; Bruckner-Tuderman, L.; Carter, W.G.; Deutzmann, R.; Edgar, D.; Ekblom, P.; Engel, J.; Engvall, E.; Hohenester, E.; Jones, J.C.R.; et al. A simplified laminin nomenclature. Matrix Biol. 2005, 24, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Yurchenco, P.D.; Cheng, Y.S.; Colognato, H. Laminin forms an independent network in basement membranes. J. Cell Biol. 1992, 117, 1119–1133. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-R.; Byun, S.-Y.; Kwon, S.-H.; Park, K.-C. Niche interactions in epidermal stem cells. World J. Stem Cells 2015, 7, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-R.; Nam, K.-M.; Park, S.-J.; Kim, D.-S.; Huh, C.-H.; Park, W.-Y.; Park, K.-C. Suppression of miR135b increases the proliferative potential of normal human keratinocytes. J. Investig. Dermatol. 2014, 134, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Sagvolden, G.; Giaever, I.; Pettersen, E.O.; Feder, J. Cell adhesion force microscopy. Proc. Natl. Acad. Sci. USA 1999, 96, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Lomakina, E.B.; Waugh, R.E. Micromechanical tests of adhesion dynamics between neutrophils and immobilized ICAM-1. Biophys. J. 2004, 86, 1223–1233. [Google Scholar] [CrossRef]
- Juliano, R.L.; Gagalang, E. The adhension of Chinese hamster cells. I. Effects of temperature, metabolic inhibitors and proteolytic dissection of cell surface macromolecules. J. Cell. Physiol. 1977, 92, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L.; Gagalang, E. The effect of membrane-fluidizing agents on the adhesion of CHO cells. J. Cell. Physiol. 1979, 98, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Campanero, M.R.; Pulido, R.; Ursa, M.A.; Rodríguez-Moya, M.; de Landázuri, M.O.; Sánchez-Madrid, F. An alternative leukocyte homotypic adhesion mechanism, LFA-1/ICAM-1-independent, triggered through the human VLA-4 integrin. J. Cell Biol. 1990, 110, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Rico, F.; Chu, C.; Abdulreda, M.H.; Qin, Y.; Moy, V.T. Temperature modulation of integrin-mediated cell adhesion. Biophys. J. 2010, 99, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.J.; Ito, T.; Okada, T.S.; Ohnishi, S.I. A correlation between membrane fluidity and the critical temperature for cell adhesion. J. Cell Biol. 1976, 71, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Cuvelier, D.; Théry, M.; Chu, Y.-S.; Dufour, S.; Thiéry, J.-P.; Bornens, M.; Nassoy, P.; Mahadevan, L. The universal dynamics of cell spreading. Curr. Biol. 2007, 17, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Jetha, K.A.; Egginton, S.; Nash, G.B. Changes in the integrin-mediated adhesion of human neutrophils in the cold and after rewarming. Biorheology 2007, 44, 37–49. [Google Scholar] [PubMed]
- Moya, F.; Silbert, D.F.; Glaser, L. The relation of temperature and lipid composition to cell adhesion. Biochim. Biophys. Acta 1979, 550, 485–499. [Google Scholar] [CrossRef]
- Chen, S.; So, E.C.; Strome, S.E.; Zhang, X. Impact of detachment methods on M2 macrophage phenotype and function. J. Immunol. Methods 2015, 426, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Ma, Q.-H.; Duan, J.-J.; Wu, H.-B.; Zhao, X.-L.; Yu, S.-C.; Bian, X.-W. Optimized dissociation protocol for isolating human glioma stem cells from tumorspheres via fluorescence-activated cell sorting. Cancer Lett. 2016, 377, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, X.; Hu, C.; Wang, P.; Li, X. Rho kinase inhibitor Y-27632 and Accutase dramatically increase mouse embryonic stem cell derivation. In Vitro Cell. Dev. Biol. Anim. 2012, 48, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Liu, T.-Q.; Dai, M.-S.; Ge, D.; Li, X.-Q.; Ma, X.-H.; Cui, Z.-F. Comparison of different culture modes for long-term expansion of neural stem cells. Cytotechnology 2006, 52, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cho, J.Y.; Lee, S.Y.; Lee, K.-W.; Lee, J.; Song, J.-Y. Vanillin protects human keratinocyte stem cells against ultraviolet B irradiation. Food Chem. Toxicol. 2014, 63, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Shin, Y.-K.; Song, J.-Y.; Lee, K.-W. Protective mechanism of morin against ultraviolet B-induced cellular senescence in human keratinocyte stem cells. Int. J. Radiat. Biol. 2014, 90, 20–28. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metral, E.; Bechetoille, N.; Demarne, F.; Rachidi, W.; Damour, O. α6 Integrin (α6high)/Transferrin Receptor (CD71)low Keratinocyte Stem Cells Are More Potent for Generating Reconstructed Skin Epidermis Than Rapid Adherent Cells. Int. J. Mol. Sci. 2017, 18, 282. https://doi.org/10.3390/ijms18020282
Metral E, Bechetoille N, Demarne F, Rachidi W, Damour O. α6 Integrin (α6high)/Transferrin Receptor (CD71)low Keratinocyte Stem Cells Are More Potent for Generating Reconstructed Skin Epidermis Than Rapid Adherent Cells. International Journal of Molecular Sciences. 2017; 18(2):282. https://doi.org/10.3390/ijms18020282
Chicago/Turabian StyleMetral, Elodie, Nicolas Bechetoille, Frédéric Demarne, Walid Rachidi, and Odile Damour. 2017. "α6 Integrin (α6high)/Transferrin Receptor (CD71)low Keratinocyte Stem Cells Are More Potent for Generating Reconstructed Skin Epidermis Than Rapid Adherent Cells" International Journal of Molecular Sciences 18, no. 2: 282. https://doi.org/10.3390/ijms18020282
APA StyleMetral, E., Bechetoille, N., Demarne, F., Rachidi, W., & Damour, O. (2017). α6 Integrin (α6high)/Transferrin Receptor (CD71)low Keratinocyte Stem Cells Are More Potent for Generating Reconstructed Skin Epidermis Than Rapid Adherent Cells. International Journal of Molecular Sciences, 18(2), 282. https://doi.org/10.3390/ijms18020282