Role of Aquaporin 1 Signalling in Cancer Development and Progression
Abstract
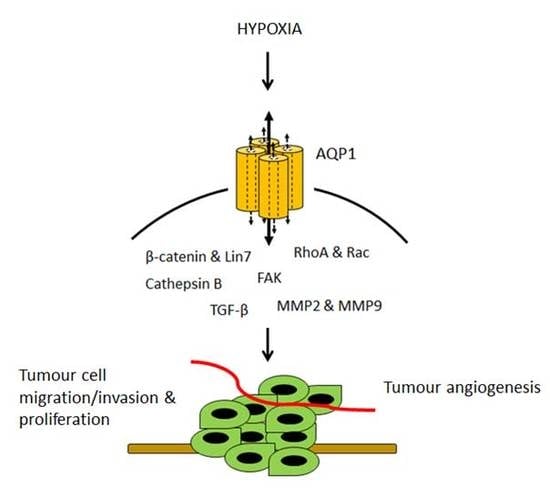
:1. Introduction
2. Aquaporin 1
3. Proposed Mechanisms Underlying AQP1-Enhanced Tumour Progression
3.1. AQP1-Modulated Tumour Cell Migration and Invasion
3.2. AQP1-Modulated Tumour Angiogenesis
3.3. AQP1-Modulated Tumour Proliferation
4. Hypoxia-Facilitated Glycolysis as an Inducer of AQP1 Expression in Tumour Cells
5. Regulation of AQP1 Activity
6. Downstream Effectors and Signalling Pathways in AQP1-Mediated Tumour Progression
6.1. β-Catenin and Lin-7
6.2. FAK
6.3. MMP2 and MMP9
6.4. RhoA and Rac
6.5. Cathepsin B
6.6. TGF-β
7. Perspectives
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AQP1 | Aquaporin 1 |
| AQPs | Aquaporins |
| BM-MSCs | Bone marrow-derived mesenchymal stem cells |
| CHIP28 | Channel forming integral membrane protein of 28 kDa |
| CRC | Colorectal cancer |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial-mesenchymal transition |
| HIF | Hypoxia-inducible transcription factor |
| HRE | Hypoxia response element |
| HRVEC | Human retinal vascular endothelial cell |
| HUVECs | Human umbilical vein endothelial cells |
| MMTV-PyVT | Mouse mammary tumor virus-driven polyoma virus middle T oncogene |
| MSC | Mesenchymal stem cells |
| NPA | Asparagine-proline-alanine |
| PKA | cAMP-dependent protein kinase |
| PKC | Protein kinase C |
| VEGF | Vascular endothelial growth factor |
| VEGF-A | Vascular endothelial growth factor-A |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
References
- World Health Organization. World Cancer Report 2014; International Agency for Research on Cancer: Lyon, France, 2014. [Google Scholar]
- Ryerson, A.B.; Eheman, C.R.; Altekruse, S.F.; Ward, J.W.; Jemal, A.; Sherman, R.L.; Henley, S.J.; Holtzman, D.; Lake, A.; Noone, A.M.; et al. Annual report to the nation on the status of cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer 2016, 122, 1312–1337. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Cancer in Australia: An Overview 2014; AIHW: Canberra, Australia, 2014. [Google Scholar]
- Agre, P.; King, L.S.; Yasui, M.; Guggino, W.B.; Ottersen, O.P.; Fujiyoshi, Y.; Engel, A.; Nielsen, S. Aquaporin water channels—From atomic structure to clinical medicine. J. Physiol. 2002, 542, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Ranieri, G.; Annese, T.; Nico, B. Aquaporins in cancer. Biochim. Biophys. Acta 2014, 1840, 1550–1553. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Saadoun, S. Key roles of aquaporins in tumor biology. Biochim. Biophys. Acta 2015, 1848, 2576–2583. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Aquaporins in clinical medicine. Annu. Rev. Med. 2012, 63, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Denker, B.M.; Smith, B.L.; Kuhajda, F.P.; Agre, P. Identification, purification, and partial characterization of a novel MR 28,000 integral membrane protein from erythrocytes and renal tubules. J. Biol. Chem. 1988, 263, 15634–15642. [Google Scholar] [PubMed]
- Raina, S.; Preston, G.M.; Guggino, W.B.; Agre, P. Molecular cloning and characterization of an aquaporin cdna from salivary, lacrimal, and respiratory tissues. J. Biol. Chem. 1995, 270, 1908–1912. [Google Scholar] [PubMed]
- Wang, J.; Feng, L.; Zhu, Z.; Zheng, M.; Wang, D.; Chen, Z.; Sun, H. Aquaporins as diagnostic and therapeutic targets in cancer: How far we are? J. Transl. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Yool, A.J.; Stamer, W.D.; Regan, J.W. Forskolin stimulation of water and cation permeability in aquaporin 1 water channels. Science 1996, 273, 1216–1218. [Google Scholar] [CrossRef] [PubMed]
- Anthony, T.L.; Brooks, H.L.; Boassa, D.; Leonov, S.; Yanochko, G.M.; Regan, J.W.; Yool, A.J. Cloned human aquaporin-1 is a cyclic gmp-gated ion channel. Mol. Pharmacol. 2000, 57, 576–588. [Google Scholar] [PubMed]
- Boassa, D.; Yool, A.J. Single amino acids in the carboxyl terminal domain of aquaporin-1 contribute to cgmp-dependent ion channel activation. BMC Physiol. 2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Yool, A.J.; Schulten, K.; Tajkhorshid, E. Mechanism of gating and ion conductivity of a possible tetrameric pore in aquaporin-1. Structure 2006, 14, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Jain, R.K.; Witwer, B.; Brown, D. Water channel (aquaporin 1) expression and distribution in mammary carcinomas and glioblastomas. Microvasc. Res. 1999, 58, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Papadopoulos, M.C.; Davies, D.C.; Bell, B.A.; Krishna, S. Increased aquaporin 1 water channel expression in human brain tumours. Br. J. Cancer 2002, 87, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.; Soria, J.C.; Jang, S.J.; Lee, J.; Obaidul Hoque, M.; Sibony, M.; Trink, B.; Chang, Y.S.; Sidransky, D.; Mao, L. Involvement of aquaporins in colorectal carcinogenesis. Oncogene 2003, 22, 6699–6703. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.O.; Soria, J.C.; Woo, J.; Lee, T.; Lee, J.; Jang, S.J.; Upadhyay, S.; Trink, B.; Monitto, C.; Desmaze, C.; et al. Aquaporin 1 is overexpressed in lung cancer and stimulates NIH-3T3 cell proliferation and anchorage-independent growth. Am. J. Pathol. 2006, 168, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, B. Expression of aquaporin-1 in nasopharyngeal cancer tissues. J. Otolaryngol. Head Neck Surg. 2010, 39, 511–515. [Google Scholar] [PubMed]
- El Hindy, N.; Bankfalvi, A.; Herring, A.; Adamzik, M.; Lambertz, N.; Zhu, Y.; Siffert, W.; Sure, U.; Sandalcioglu, I.E. Correlation of aquaporin-1 water channel protein expression with tumor angiogenesis in human astrocytoma. Anticancer Res. 2013, 33, 609–613. [Google Scholar] [PubMed]
- Chen, R.; Shi, Y.; Amiduo, R.; Tuokan, T.; Suzuk, L. Expression and prognostic value of aquaporin 1, 3 in cervical carcinoma in women of uygur ethnicity from xinjiang, china. PLoS ONE 2014, 9, e98576. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, W.Y.; Ding, D.G. Expression of aquaporin 1 in bladder uroepithelial cell carcinoma and its relevance to recurrence. Asian Pac. J. Cancer Prev. 2015, 16, 3973–3976. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, X.; Wu, L.; Jiang, Z. Elevated AQP1 expression is associated with unfavorable oncologic outcome in patients with hilar cholangiocarcinoma. Technol. Cancer Res. Treat. 2016. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Yoon, G. Overexpression of aquaporin-1 is a prognostic factor for biochemical recurrence in prostate adenocarcinoma. Pathol. Oncol. Res. 2016, 23, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Shi, Y.F.; Chen, X.D.; Qi, W.J. The influence of aquaporin-1 and microvessel density on ovarian carcinogenesis and ascites formation. Int. J. Gynecol. Cancer 2006, 16, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Otterbach, F.; Callies, R.; Adamzik, M.; Kimmig, R.; Siffert, W.; Schmid, K.W.; Bankfalvi, A. Aquaporin 1 (AQP1) expression is a novel characteristic feature of a particularly aggressive subgroup of basal-like breast carcinomas. Breast Cancer Res. Treat. 2010, 120, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Hojo, S.; Sekine, S.; Sawada, S.; Okumura, T.; Nagata, T.; Shimada, Y.; Tsukada, K. Expression of aquaporin-1 is a poor prognostic factor for stage ii and iii colon cancer. Mol. Clin. Oncol. 2013, 1, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.W.; Kim, J.G.; Lee, S.J.; Chae, Y.S.; Jeong, J.Y.; Yoon, G.S.; Park, S.Y.; Kim, H.J.; Park, J.S.; Choi, G.S.; et al. Expression of aquaporin-1, aquaporin-3, and aquaporin-5 correlates with nodal metastasis in colon cancer. Oncology 2015, 88, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Monzani, E.; Shtil, A.A.; La Porta, C.A. The water channels, new druggable targets to combat cancer cell survival, invasiveness and metastasis. Curr. Drug Targets 2007, 8, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Hara-Chikuma, M.; Papadopoulos, M.C. Aquaporins—New players in cancer biology. J. Mol. Med. 2008, 86, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Nico, B.; Ribatti, D. Aquaporins in tumor growth and angiogenesis. Cancer Lett. 2010, 294, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Yool, A.J.; Brown, E.A.; Flynn, G.A. Roles for novel pharmacological blockers of aquaporins in the treatment of brain oedema and cancer. Clin. Exp. Pharmacol. Physiol. 2010, 37, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Nico, B.; Ribatti, D. Role of aquaporins in cell migration and edema formation in human brain tumors. Exp. Cell Res. 2011, 317, 2391–2396. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.J.; Tsujita, M.; Nakada, T. Aquaporins in drug discovery and pharmacotherapy. Mol. Asp. Med. 2012, 33, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Barrett-Jolley, R. Aquaporin water channels in the mammary gland: From physiology to pathophysiology and neoplasia. J. Mammary Gland Biol. Neoplasia 2014, 19, 91–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaraju, G.P.; Basha, R.; Rajitha, B.; Alese, O.B.; Alam, A.; Pattnaik, S.; El-Rayes, B. Aquaporins: Their role in gastrointestinal malignancies. Cancer Lett. 2016, 373, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Mehlen, P.; Puisieux, A. Metastasis: A question of life or death. Nat. Rev. Cancer 2006, 6, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Verkman, A.S. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. 2006, 20, 1892–1894. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y. Aquaporin-1 activity of plasma membrane affects HT20 colon cancer cell migration. IUBMB Life 2009, 61, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Oster, G.F.; Perelson, A.S. The physics of cell motility. J. Cell Sci. Suppl. 1987, 8, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Condeelis, J.; Bresnick, A.; Demma, M.; Dharmawardhane, S.; Eddy, R.; Hall, A.L.; Sauterer, R.; Warren, V. Mechanisms of amoeboid chemotaxis: An evaluation of the cortical expansion model. Dev. Genet. 1990, 11, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Verkman, A.S. Aquaporin water channels in the nervous system. Nat. Rev. Neurosci. 2013, 14, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; O’Hara, S.P.; Huang, B.Q.; Splinter, P.L.; Nelson, J.B.; LaRusso, N.F. Localized glucose and water influx facilitates cryptosporidium parvum cellular invasion by means of modulation of host-cell membrane protrusion. Proc. Natl. Acad. Sci. USA 2005, 102, 6338–6343. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Saadoun, S.; Verkman, A.S. Aquaporins and cell migration. Pflug. Arch. 2008, 456, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Stroka, K.M.; Jiang, H.; Chen, S.H.; Tong, Z.; Wirtz, D.; Sun, S.X.; Konstantopoulos, K. Water permeation drives tumor cell migration in confined microenvironments. Cell 2014, 157, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.; Fabian, A.; Hanley, P.J.; Stock, C. Role of ion channels and transporters in cell migration. Physiol. Rev. 2012, 92, 1865–1913. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Seeger, P.; Schuricht, B.; Alper, S.L.; Schwab, A. Polarization of Na+/H+ and Cl−/HCO3− exchangers in migrating renal epithelial cells. J. Gen. Physiol. 2000, 115, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Huttenlocher, A. Cell polarization mechanisms during directed cell migration. Nat. Cell Biol. 2005, 7, 336–337. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.; Stock, C. Ion channels and transporters in tumour cell migration and invasion. Philos. Trans. R Soc. Lond. B Biol. Sci. 2014, 369, 20130102. [Google Scholar] [CrossRef] [PubMed]
- Stock, C.; Schwab, A. Ion channels and transporters in metastasis. Biochim. Biophys. Acta 2015, 1848, 2638–2646. [Google Scholar] [CrossRef] [PubMed]
- Kourghi, M.; Pei, J.V.; De Ieso, M.L.; Flynn, G.; Yool, A.J. Bumetanide derivatives AqB007 and AqB011 selectively block the aquaporin-1 ion channel conductance and slow cancer cell migration. Mol. Pharmacol. 2016, 89, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Pelagalli, A.; Nardelli, A.; Fontanella, R.; Zannetti, A. Inhibition of AQP1 hampers osteosarcoma and hepatocellular carcinoma progression mediated by bone marrow-derived mesenchymal stem cells. Int. J. Mol. Sci. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.; Kim, J.K.; Shiozawa, Y.; Wang, J.; Mishra, A.; Joseph, J.; Berry, J.E.; McGee, S.; Lee, E.; Sun, H.; et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat. Commun. 2013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lee, Y.W.; Rui, Y.F.; Cheng, T.Y.; Jiang, X.H.; Li, G. Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Res. Ther. 2013. [Google Scholar] [CrossRef] [PubMed]
- Zetter, B.R. Angiogenesis and tumor metastasis. Annu. Rev. Med. 1998, 49, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Munaron, L. Systems biology of ion channels and transporters in tumor angiogenesis: An omics view. Biochim. Biophys. Acta 2015, 1848, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Papadopoulos, M.C.; Hara-Chikuma, M.; Verkman, A.S. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 2005, 434, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Nicchia, G.P.; Stigliano, C.; Sparaneo, A.; Rossi, A.; Frigeri, A.; Svelto, M. Inhibition of aquaporin-1 dependent angiogenesis impairs tumour growth in a mouse model of melanoma. J. Mol. Med. 2013, 91, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Esteva-Font, C.; Jin, B.J.; Verkman, A.S. Aquaporin-1 gene deletion reduces breast tumor growth and lung metastasis in tumor-producing MMTV-PyVT mice. FASEB J. 2014, 28, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Monzani, E.; Bazzotti, R.; Perego, C.; La Porta, C.A. AQP1 is not only a water channel: It contributes to cell migration through lin7/β-catenin. PLoS ONE 2009, 4, e6167. [Google Scholar] [CrossRef] [PubMed]
- Clapp, C.; Martinez de la Escalera, G. Aquaporin-1: A novel promoter of tumor angiogenesis. Trends Endocrinol. Metab. 2006, 17, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Dorward, H.S.; Du, A.; Bruhn, M.A.; Wrin, J.; Pei, J.V.; Evdokiou, A.; Price, T.J.; Yool, A.J.; Hardingham, J.E. Pharmacological blockade of aquaporin-1 water channel by AqB013 restricts migration and invasiveness of colon cancer cells and prevents endothelial tube formation in vitro. J. Exp. Clin. Cancer Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Galan-Cobo, A.; Sanchez-Silva, R.; Serna, A.; Abreu-Rodriguez, I.; Munoz-Cabello, A.M.; Echevarria, M. Cellular overexpression of aquaporins slows down the natural HIF-2α degradation during prolonged hypoxia. Gene 2013, 522, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, S.; Liu, J.; Shi, Y.; Wang, J.; Chen, D.; Luo, L.; Qian, Y.; Huang, X.; Wang, H. RNAi-mediated silencing of AQP1 expression inhibited the proliferation, invasion and tumorigenesis of osteosarcoma cells. Cancer Biol. Ther. 2015, 16, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Klebe, S.; Griggs, K.; Cheng, Y.; Driml, J.; Henderson, D.W.; Reid, G. Blockade of aquaporin 1 inhibits proliferation, motility, and metastatic potential of mesothelioma in vitro but not in an in vivo model. Dis. Markers 2015, 2015, 286719. [Google Scholar] [CrossRef] [PubMed]
- Galan-Cobo, A.; Ramirez-Lorca, R.; Echevarria, M. Role of aquaporins in cell proliferation: What else beyond water permeability? Channels 2016, 10, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Kunzelmann, K. Ion channels and cancer. J. Membr. Biol. 2005, 205, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.F.; Hoffmann, E.K.; Novak, I. Cell volume regulation in epithelial physiology and cancer. Front. Physiol. 2013, 4, 233. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Edwards, N.A.; Proescholdt, M.A.; Oldfield, E.H.; Merrill, M.J. Regulation and function of aquaporin-1 in glioma cells. Neoplasia 2007, 9, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Potter, C.P.; Harris, A.L. Diagnostic, prognostic and therapeutic implications of carbonic anhydrases in cancer. Br. J. Cancer 2003, 89, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Foufelle, F.; Girard, J.; Ferre, P. Glucose regulation of gene expression. Curr. Opin. Clin. Nutr. Metab. Care 1998, 1, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Dentin, R.; Denechaud, P.D.; Benhamed, F.; Girard, J.; Postic, C. Hepatic gene regulation by glucose and polyunsaturated fatty acids: A role for ChREBP. J. Nutr. 2006, 136, 1145–1149. [Google Scholar] [PubMed]
- Kim, J.W.; Zeller, K.I.; Wang, Y.; Jegga, A.G.; Aronow, B.J.; O’Donnell, K.A.; Dang, C.V. Evaluation of Myc E-box phylogenetic footprints in glycolytic genes by chromatin immunoprecipitation assays. Mol. Cell. Biol. 2004, 24, 5923–5936. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Rodriguez, I.; Sanchez Silva, R.; Martins, A.P.; Soveral, G.; Toledo-Aral, J.J.; Lopez-Barneo, J.; Echevarria, M. Functional and transcriptional induction of aquaporin-1 gene by hypoxia; analysis of promoter and role of HIF-1α. PLoS ONE 2011, 6, e28385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, A.; Sakurai, K.; Kaneko, K.; Ogino, J.; Yagui, K.; Ishikawa, K.; Ishibashi, T.; Matsumoto, T.; Yokote, K.; Saito, Y. The role of the hypoxia-inducible factor 1 binding site in the induction of aquaporin-1 mRNA expression by hypoxia. DNA Cell Biol. 2011, 30, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Yu, S.; Xiao, L.; Zhang, J.; Liu, C.; Lu, Y.; Liu, C. Correlation between the expression of aquaporin 1 and hypoxia-inducible factor 1 in breast cancer tissues. J. Huazhong Univ. Sci. Technolog Med. Sci. 2008, 28, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Tie, L.; Lu, N.; Pan, X.Y.; Pan, Y.; An, Y.; Gao, J.W.; Lin, Y.H.; Yu, H.M.; Li, X.J. Hypoxia-induced up-regulation of aquaporin-1 protein in prostate cancer cells in a p38-dependent manner. Cell. Physiol. Biochem. 2012, 29, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Umenishi, F.; Schrier, R.W. Hypertonicity-induced aquaporin-1 (AQP1) expression is mediated by the activation of mapk pathways and hypertonicity-responsive element in the AQP1 gene. J. Biol. Chem. 2003, 278, 15765–15770. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, Y.; Xu, Z.; Wang, H.; Zhao, Z.; Li, Y.; Yang, P.; Wei, X. Involvement of COX-2/PGE2 signalling in hypoxia-induced angiogenic response in endothelial cells. J. Cell. Mol. Med. 2012, 16, 1840–1855. [Google Scholar] [CrossRef] [PubMed]
- Beavo, J.A.; Brunton, L.L. Cyclic nucleotide research—Still expanding after half a century. Nat. Rev. Mol. Cell Biol. 2002, 3, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.V.; Han, Z.; Wax, M.B. Regulation of water channel activity of aquaporin 1 by arginine vasopressin and atrial natriuretic peptide. Biochem. Biophys. Res. Commun. 1997, 238, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Jenq, W.; Mathieson, I.M.; Ihara, W.; Ramirez, G. Aquaporin-1: An osmoinducible water channel in cultured mlMCD-3 cells. Biochem. Biophys. Res. Commun. 1998, 245, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Patil, R.V. Protein kinase A-dependent phosphorylation of aquaporin-1. Biochem. Biophys. Res. Commun. 2000, 273, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Kennelly, P.J.; Krebs, E.G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J. Biol. Chem. 1991, 266, 15555–15558. [Google Scholar] [PubMed]
- Pearson, R.B.; Kemp, B.E. Protein kinase phosphorylation site sequences and consensus specificity motifs: Tabulations. Methods Enzymol. 1991, 200, 62–81. [Google Scholar] [PubMed]
- Patil, R.V.; Yang, X.; Saito, I.; Coca-Prados, M.; Wax, M.B. Cloning of a novel cdna homologous to CHIP28 water channel from ocular ciliary epithelium. Biochem. Biophys. Res. Commun. 1994, 204, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zitron, E.; Homme, M.; Kihm, L.; Morath, C.; Scherer, D.; Hegge, S.; Thomas, D.; Schmitt, C.P.; Zeier, M.; et al. Aquaporin-1 channel function is positively regulated by protein kinase c. J. Biol. Chem. 2007, 282, 20933–20940. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Rui, Y.; Xu, L.; Wan, C.; Jiang, X.; Li, G. AQP1 enhances migration of bone marrow mesenchymal stem cells through regulation of fak and β-catenin. Stem Cells Dev. 2014, 23, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Morin, P.J. Β-catenin signaling and cancer. Bioessays 1999, 21, 1021–1030. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/β-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Cong, F.; Zhang, J.; Pao, W.; Zhou, P.; Varmus, H. A protein knockdown strategy to study the function of β-catenin in tumorigenesis. BMC Mol. Biol. 2003, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- He, T.C.; Sparks, A.B.; Rago, C.; Hermeking, H.; Zawel, L.; da Costa, L.T.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Identification of c-MYC as a target of the APC pathway. Science 1998, 281, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Mann, B.; Gelos, M.; Siedow, A.; Hanski, M.L.; Gratchev, A.; Ilyas, M.; Bodmer, W.F.; Moyer, M.P.; Riecken, E.O.; Buhr, H.J.; et al. Target genes of β-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc. Natl. Acad. Sci. USA 1999, 96, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Tetsu, O.; McCormick, F. β-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999, 398, 422–426. [Google Scholar] [PubMed]
- Yun, X.; Jiang, H.; Lai, N.; Shimoda, L. The C-terminal tail of aquaporin 1 modulates β-catenin expression in pulmonary arterial smooth muscle cells (1175.2). FASEB J. 2014, 28, 1175.2. [Google Scholar]
- Polette, M.; Mestdagt, M.; Bindels, S.; Nawrocki-Raby, B.; Hunziker, W.; Foidart, J.M.; Birembaut, P.; Gilles, C. β-catenin and ZO-1: Shuttle molecules involved in tumor invasion-associated epithelial-mesenchymal transition processes. Cells Tissues Organs 2007, 185, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.H.; Nelson, W.J.; Weis, W.I. Three-dimensional structure of the armadillo repeat region of β-catenin. Cell 1997, 90, 871–882. [Google Scholar] [CrossRef]
- Sulzmaier, F.J.; Jean, C.; Schlaepfer, D.D. Fak in cancer: Mechanistic findings and clinical applications. Nat. Rev. Cancer 2014, 14, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.L.; Chen, L.C.; Shen, T.L. Emerging roles of focal adhesion kinase in cancer. BioMed Res. Int. 2015, 2015, 690690. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Dong, J. Aquaporin 1 promotes the proliferation and migration of lung cancer cell in vitro. Oncol. Rep. 2015, 34, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Wolf, K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, E.L.; Hotary, K.; Bradford, C.; Weiss, S.J. Role of membrane type 1-matrix metalloproteinase and gelatinase a in head and neck squamous cell carcinoma invasion in vitro. Otolaryngol. Head Neck Surg. 1999, 121, 337–343. [Google Scholar] [CrossRef]
- Hofmann, U.B.; Westphal, J.R.; Van Muijen, G.N.; Ruiter, D.J. Matrix metalloproteinases in human melanoma. J. Investig. Dermatol. 2000, 115, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Koblinski, J.E.; Ahram, M.; Sloane, B.F. Unraveling the role of proteases in cancer. Clin. Chim. Acta 2000, 291, 113–135. [Google Scholar] [CrossRef]
- Maekawa, K.; Sato, H.; Furukawa, M.; Yoshizaki, T. Inhibition of cervical lymph node metastasis by marimastat (BB-2516) in an orthotopic oral squamous cell carcinoma implantation model. Clin. Exp. Metastasis 2002, 19, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Sein, T.T.; Thant, A.A.; Hiraiwa, Y.; Amin, A.R.; Sohara, Y.; Liu, Y.; Matsuda, S.; Yamamoto, T.; Hamaguchi, M. A role for fak in the concanavalin a-dependent secretion of matrix metalloproteinase-2 and -9. Oncogene 2000, 19, 5539–5542. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Crampton, S.P.; Hughes, C.C. Wnt signaling induces matrix metalloproteinase expression and regulates t cell transmigration. Immunity 2007, 26, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Parri, M.; Chiarugi, P. Rac and Rho GTPases in cancer cell motility control. Cell Commun. Signal. 2010, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, D.; Kurisu, S.; Takenawa, T. Involvement of Rac and Rho signaling in cancer cell motility in 3D substrates. Oncogene 2009, 28, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Rozhin, J.; Sameni, M.; Ziegler, G.; Sloane, B.F. Pericellular pH affects distribution and secretion of cathepsin B in malignant cells. Cancer Res. 1994, 54, 6517–6525. [Google Scholar] [PubMed]
- Aggarwal, N.; Sloane, B.F. Cathepsin B: Multiple roles in cancer. Proteom. Clin. Appl. 2014, 8, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Tummalapalli, P.; Spomar, D.; Gondi, C.S.; Olivero, W.C.; Gujrati, M.; Dinh, D.H.; Rao, J.S. RNAi-mediated abrogation of cathepsin B and MMP-9 gene expression in a malignant meningioma cell line leads to decreased tumor growth, invasion and angiogenesis. Int. J. Oncol. 2007, 31, 1039–1050. [Google Scholar] [PubMed]
- Gogineni, V.R.; Gupta, R.; Nalla, A.K.; Velpula, K.K.; Rao, J.S. uPAR and cathepsin B shRNA impedes TGF-β1-driven proliferation and invasion of meningioma cells in a XIAP-dependent pathway. Cell Death Dis. 2012, 3, e439. [Google Scholar] [CrossRef] [PubMed]
- Rao Malla, R.; Gopinath, S.; Alapati, K.; Gorantla, B.; Gondi, C.S.; Rao, J.S. Knockdown of cathepsin B and upar inhibits CD151 and α3β1 integrin-mediated cell adhesion and invasion in glioma. Mol. Carcinog. 2013, 52, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Droga-Mazovec, G.; Bojic, L.; Petelin, A.; Ivanova, S.; Romih, R.; Repnik, U.; Salvesen, G.S.; Stoka, V.; Turk, V.; Turk, B. Cysteine cathepsins trigger caspase-dependent cell death through cleavage of bid and antiapoptotic Bcl-2 homologues. J. Biol. Chem. 2008, 283, 19140–19150. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; Doll, J.A.; Bauer, J.; Jung, B.; Munshi, H.G.; Bartholin, L.; Pasche, B.; Lee, C.; Grippo, P.J. TGF-β: Duality of function between tumor prevention and carcinogenesis. J. Natl. Cancer Inst. 2014, 106, djt369. [Google Scholar] [CrossRef] [PubMed]
- Quasar Collaborative Group; Gray, R.; Barnwell, J.; McConkey, C.; Hills, R.K.; Williams, N.S.; Kerr, D.J. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet 2007, 370, 2020–2029. [Google Scholar] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomita, Y.; Dorward, H.; Yool, A.J.; Smith, E.; Townsend, A.R.; Price, T.J.; Hardingham, J.E. Role of Aquaporin 1 Signalling in Cancer Development and Progression. Int. J. Mol. Sci. 2017, 18, 299. https://doi.org/10.3390/ijms18020299
Tomita Y, Dorward H, Yool AJ, Smith E, Townsend AR, Price TJ, Hardingham JE. Role of Aquaporin 1 Signalling in Cancer Development and Progression. International Journal of Molecular Sciences. 2017; 18(2):299. https://doi.org/10.3390/ijms18020299
Chicago/Turabian StyleTomita, Yoko, Hilary Dorward, Andrea J. Yool, Eric Smith, Amanda R. Townsend, Timothy J. Price, and Jennifer E. Hardingham. 2017. "Role of Aquaporin 1 Signalling in Cancer Development and Progression" International Journal of Molecular Sciences 18, no. 2: 299. https://doi.org/10.3390/ijms18020299