A Lipid Emulsion Reverses Toxic-Dose Bupivacaine-Induced Vasodilation during Tyrosine Phosphorylation-Evoked Contraction in Isolated Rat Aortae
Abstract
:1. Introduction
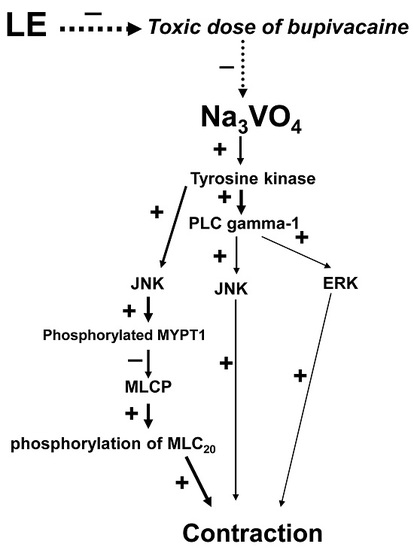
2. Results
3. Discussion
4. Materials and Methods
4.1. Preparation of Aortic Rings for Tension Measurements
4.2. Experimental Protocols
4.3. Cell Culture
4.4. Western Blot Analysis
4.5. Fura-2 Loading and Simultaneous Measurement of [Ca2+]i and Tension
4.6. Materials
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| JNK | c-Jun NH2-terminal kinase |
| MYPT1 | Myosin phosphatase target subunit 1 |
| PLC γ-1 | Phospholipase C γ-1 |
| ERK | Extracellular signal-regulated kinase |
| [Ca2+]i | Intracellular calcium level |
| RAVSMCs | Rat aortic vascular smooth muscle cells |
| CPI-17 | Phosphorylation-dependent inhibitory protein of myosin phosphatase |
| PDBu | Phorbol 12,13-dibutyrate |
References
- Weinberg, G.L. Lipid emulsion infusion: Resuscitation for local anesthetic and other drug overdose. Anesthesiology 2012, 117, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Ok, S.H.; Han, J.Y.; Lee, S.H.; Shin, I.W.; Lee, H.K.; Chung, Y.K.; Choi, M.J.; Sohn, J.T. Lipid emulsion-mediated reversal of toxic-dose aminoamide local anesthetic-induced vasodilation in isolated rat aorta. Korean J. Anesthesiol. 2013, 64, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Ok, S.H.; Sohn, J.T.; Baik, J.S.; Kim, J.G.; Park, S.S.; Sung, H.J.; Shin, M.K.; Kwon, Y.H.; Park, C.S.; Shin, I.W.; et al. Lipid emulsion reverses levobupivacaine-induced responses in isolated rat aortic vessels. Anesthesiology 2011, 114, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Akata, T. General anesthetics and vascular smooth muscle: Direct actions of general anesthetics on cellular mechanisms regulating vascular tone. Anesthesiology 2007, 106, 365–391. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.D.; Wijetunge, S. Role of tyrosine phosphorylation in excitation-contraction coupling in vascular smooth muscle. Acta Physiol. Scand. 1998, 164, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ogawa, K.; Tokinaga, Y.; Mizumoto, K.; Kakutani, T.; Hatano, Y. The inhibitory effects of isoflurane on protein tyrosine phosphorylation-modulated contraction of rat aortic smooth muscle. Anesthesiology 2004, 101, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Mizumoto, K.; Kakutani, T.; Hasegawa, A.; Ogawa, K.; Hatano, Y. Comparison of the effects of isoflurane and sevoflurane on protein tyrosine phosphorylation-mediated vascular contraction. Acta Anaesthesiol. Scand. 2005, 49, 852–868. [Google Scholar] [CrossRef] [PubMed]
- Yayama, K.; Sasahara, T.; Ohba, H.; Funasaka, A.; Okamoto, H. Orthovanadate-induced vasocontraction is mediated by the activation of Rho-kinase through Src-dependent transactivation of epidermal growth factor receptor. Pharmacol. Res. Perspect. 2014, 2, e00039. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Matsuzaki, M.; Sasahara, T.; Shin, M.; Yayama, K. Orthovanadate-induced vasoconstriction of rat mesenteric arteries is mediated by Rho kinase-dependent inhibition of myosin light chain phosphatase. Biol. Pharm. Bull. 2015, 38, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Abebe, W.; Agrawal, D.K. Role of tyrosine kinases in norepinephrine-induced contraction of vascular smooth muscle. J. Cardiovasc. Pharmacol. 1995, 26, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Akata, T. Cellular and molecular mechanisms regulating vascular tone. Part 2: Regulatory mechanisms modulating Ca2+ mobilization and/or myofilament Ca2+ sensitivity in vascular smooth muscle cells. J. Anesth. 2007, 21, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Ok, S.H.; Kwon, S.C.; Lee, S.H.; Baik, J.; Kang, S.; Oh, J.; Sohn, J.T. Lipid emulsion inhibits vasodilation induced by a toxic dose of bupivacaine by suppressing bupivacaine-induced PKC and CPI-17 dephosphorylation but has no effect on vasodilation induced by a toxic dose of mepivacaine. Korean J. Pain 2016, 29, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Ok, S.H.; Byon, H.J.; Kwon, S.C.; Park, J.; Lee, Y.; Hwang, Y.; Baik, J.; Choi, M.J.; Sohn, J.T. Lipid emulsion inhibits vasodilation induced by a toxic dose of bupivacaine via attenuated dephosphorylation of myosin phosphatase target subunit 1 in isolated rat aorta. Int. J. Med. Sci. 2015, 12, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Ok, S.H.; Bae, S.I.; Kwon, S.C.; Park, J.C.; Kim, W.C.; Park, K.E.; Shin, I.W.; Lee, H.K.; Chung, Y.K.; Choi, M.J.; et al. Bupivacaine-induced vasodilation is mediated by decreased calcium sensitization in isolated endothelium-denuded rat aortas precontracted with phenylephrine. Korean J. Pain 2014, 27, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Appel, S.; Vetterkind, S.; Gangopadhyay, S.S.; Morgan, K.G. Smooth muscle signalling pathways in health and disease. J. Cell Mol. Med. 2008, 12, 2165–2180. [Google Scholar] [CrossRef] [PubMed]
- Casati, A.; Putzu, M. Bupivacaine, levobupivacaine and ropivacaine: Are they clinically different? Best Pract. Res. Clin. Anaesthesiol. 2005, 19, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Heard, K.; Foran, M.; Koyfman, A. Intravenous lipid emulsion in the emergency department: A systematic review of recent literature. J. Emerg. Med. 2015, 48, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Zausig, Y.A.; Zink, W.; Keil, M.; Sinner, B.; Barwing, J.; Wiese, C.H.; Graf, B.M. Lipid emulsion improves recovery from bupivacaine-induced cardiac arrest, but not from ropivacaine- or mepivacaine-induced cardiac arrest. Anesth. Analg. 2009, 109, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Aumeier, C.; Kasdorf, B.; Gruber, M.; Busse, H.; Wiese, C.H.; Zink, W.; Graf, B.M.; Zausig, Y.A. Lipid emulsion pretreatment has different effects on mepivacaine and bupivacaine cardiac toxicity in an isolated rat heart model. Br. J. Anaesth. 2014, 112, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Heavner, J.E. Local anesthetics. Curr. Opin. Anaesthesiol. 2007, 20, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Zausig, Y.A.; Ruf, S.; Rudakova, E.; Gruber, M.; Graf, B.M.; Volk, T. Lipid rescue reverses the bupivacaine-induced block of the fast Na+ current (INa) in cardiomyocytes of the rat left ventricle. Anesthesiology 2014, 120, 724–736. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.M.; Xian, H.; Bacaner, M. Long-chain fatty acids activate calcium channels in ventricular myocytes. Proc. Natl. Acad. Sci. USA 1992, 89, 6452–6456. [Google Scholar] [CrossRef] [PubMed]
- Partownavid, P.; Sharma, S.; Li, J.; Umar, S.; Rahman, S.; Eghbali, M. Involvement of opioid receptors in the lipid rescue of bupivacaine-induced cardiotoxicity. Anesth. Analg. 2015, 121, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Mayet, J.; Hughes, A. Cardiac and vascular pathophysiology in hypertension. Heart 2003, 89, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Ok, S.H.; Lee, S.H.; Yu, J.; Park, J.; Shin, I.W.; Lee, Y.; Cho, H.; Choi, M.J.; Baik, J.; Hong, J.M.; et al. Lipid emulsion attenuates acetylcholine-induced relaxation in isolated rat aorta. Biomed. Res. Int. 2015, 2015, 871545. [Google Scholar] [CrossRef] [PubMed]
- Baik, J.S.; Sohn, J.T.; Ok, S.H.; Kim, J.G.; Sung, H.J.; Park, S.S.; Park, J.Y.; Hwang, E.M.; Chung, Y.K. Levobupivacaine-induced contraction of isolated rat aorta is calcium dependent. Can. J. Physiol. Pharmacol. 2011, 89, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.J.; Choi, M.J.; Ok, S.H.; Lee, S.H.; Hwang, I.J.; Kim, H.S.; Chang, K.C.; Shin, I.W.; Lee, H.K.; Park, K.E.; et al. Mepivacaine-induced contraction is attenuated by endothelial nitric oxide release in isolated rat aorta. Can. J. Physiol. Pharmacol. 2012, 90, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Ok, S.H.; Han, J.Y.; Sung, H.J.; Yang, S.M.; Park, J.; Kwon, S.C.; Choi, M.J.; Sohn, J.T. Ropivacaine-induced contraction is attenuated by both endothelial nitric oxide and voltage-dependent potassium channels in isolated rat aortae. BioMed Res. Int. 2013, 2013, 565271. [Google Scholar] [CrossRef] [PubMed]
- Ok, S.H.; Park, C.S.; Kim, H.J.; Lee, S.H.; Choi, B.H.; Eun, S.Y.; Kim, K.N.; Yang, S.M.; Shin, I.W.; Choi, M.J.; et al. Effect of two lipid emulsions on reversing high-dose levobupivacaine-induced reduced vasoconstriction in the rat aortas. Cardiovasc. Toxicol. 2013, 13, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Papapetropoulos, A.; Fulton, D.; Lin, M.I.; Fontana, J.; McCabe, T.J.; Zoellner, S.; García-Cardeña, G.; Zhou, Z.; Gratton, J.P.; Sessa, W.C. Vanadate is a potent activator of endothelial nitric-oxide synthase: Evidence for the role of the serine/threonine kinase Akt and the 90-kDa heat shock protein. Mol. Pharmacol. 2004, 65, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.M.; Ahn, D.S.; Seok, H.S.; Jeong, Y.; Kang, B.S. Effects of vanadate on vascular contractility and membrane potential in the rabbit aorta. Yonsei Med. J. 1992, 33, 14–23. [Google Scholar] [CrossRef]
- Santos, A.C.; DeArmas, P.I. Systemic toxicity of levobupivacaine, bupivacaine, and ropivacaine during continuous intravenous infusion to nonpregnant and pregnant ewes. Anesthesiology 2001, 95, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Ok, S.H.; Kwon, S.C.; Baik, J.; Hong, J.M.; Oh, J.; Han, J.Y.; Sohn, J.T. Dexmedetomidine-induced contraction involves CPI-17 phosphorylation in isolated rat aortas. Int. J. Mol. Sci. 2016, 17, 663. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 7, 248–254. [Google Scholar] [CrossRef]
- Karaki, H.; Ozaki, H.; Hori, M.; Mitsui-Saito, M.; Amano, K.; Harada, K.; Miyamoto, S.; Nakazawa, H.; Won, K.J.; Sato, K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol. Rev. 1997, 49, 157–230. [Google Scholar] [PubMed]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [PubMed]








© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ok, S.-H.; Lee, S.H.; Kwon, S.-C.; Choi, M.H.; Shin, I.-W.; Kang, S.; Park, M.; Hong, J.-M.; Sohn, J.-T. A Lipid Emulsion Reverses Toxic-Dose Bupivacaine-Induced Vasodilation during Tyrosine Phosphorylation-Evoked Contraction in Isolated Rat Aortae. Int. J. Mol. Sci. 2017, 18, 394. https://doi.org/10.3390/ijms18020394
Ok S-H, Lee SH, Kwon S-C, Choi MH, Shin I-W, Kang S, Park M, Hong J-M, Sohn J-T. A Lipid Emulsion Reverses Toxic-Dose Bupivacaine-Induced Vasodilation during Tyrosine Phosphorylation-Evoked Contraction in Isolated Rat Aortae. International Journal of Molecular Sciences. 2017; 18(2):394. https://doi.org/10.3390/ijms18020394
Chicago/Turabian StyleOk, Seong-Ho, Soo Hee Lee, Seong-Chun Kwon, Mun Hwan Choi, Il-Woo Shin, Sebin Kang, Miyeong Park, Jeong-Min Hong, and Ju-Tae Sohn. 2017. "A Lipid Emulsion Reverses Toxic-Dose Bupivacaine-Induced Vasodilation during Tyrosine Phosphorylation-Evoked Contraction in Isolated Rat Aortae" International Journal of Molecular Sciences 18, no. 2: 394. https://doi.org/10.3390/ijms18020394
APA StyleOk, S.-H., Lee, S. H., Kwon, S.-C., Choi, M. H., Shin, I.-W., Kang, S., Park, M., Hong, J.-M., & Sohn, J.-T. (2017). A Lipid Emulsion Reverses Toxic-Dose Bupivacaine-Induced Vasodilation during Tyrosine Phosphorylation-Evoked Contraction in Isolated Rat Aortae. International Journal of Molecular Sciences, 18(2), 394. https://doi.org/10.3390/ijms18020394