Effects of Postnatal Enriched Environment in a Model of Parkinson’s Disease in Adult Rats
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Behaviour
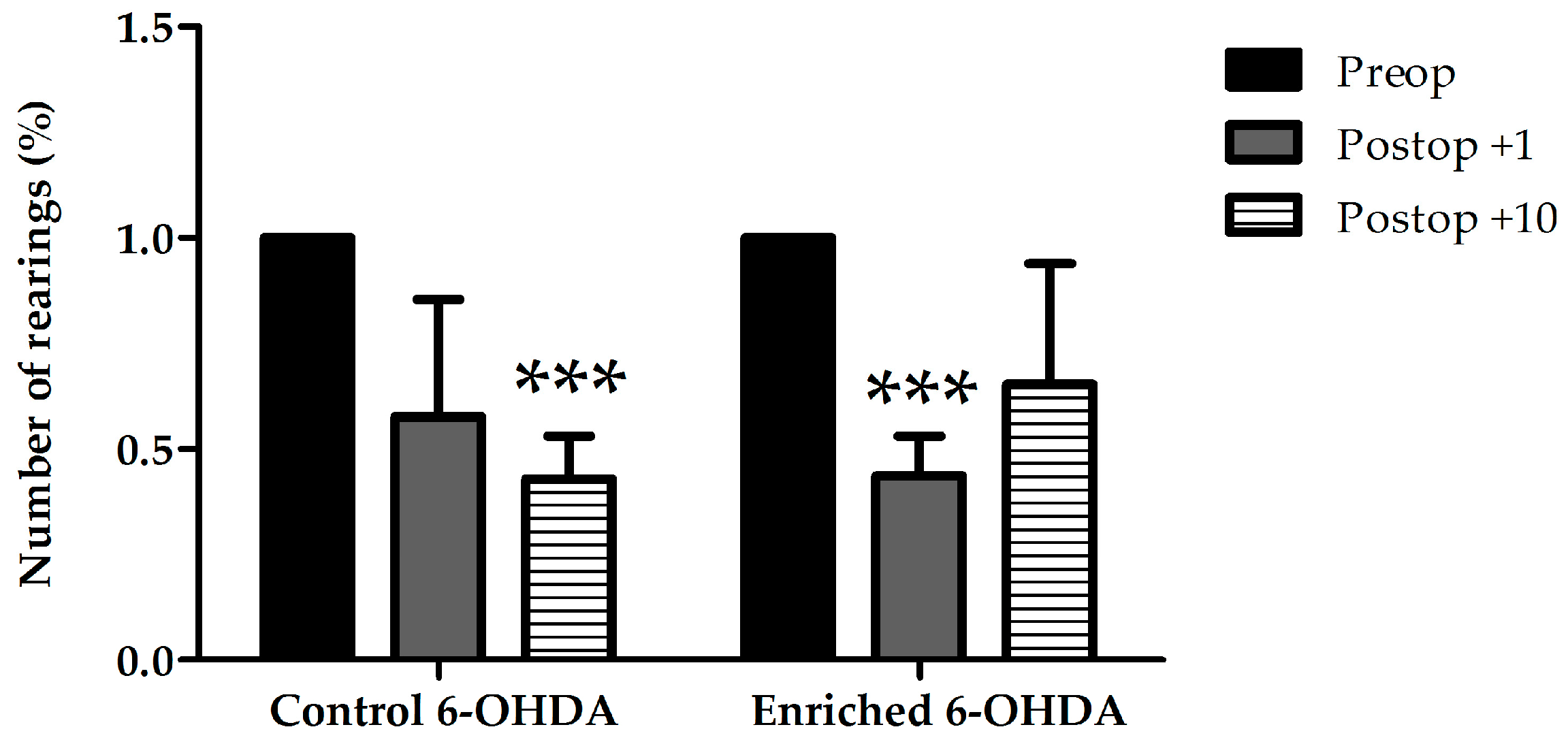
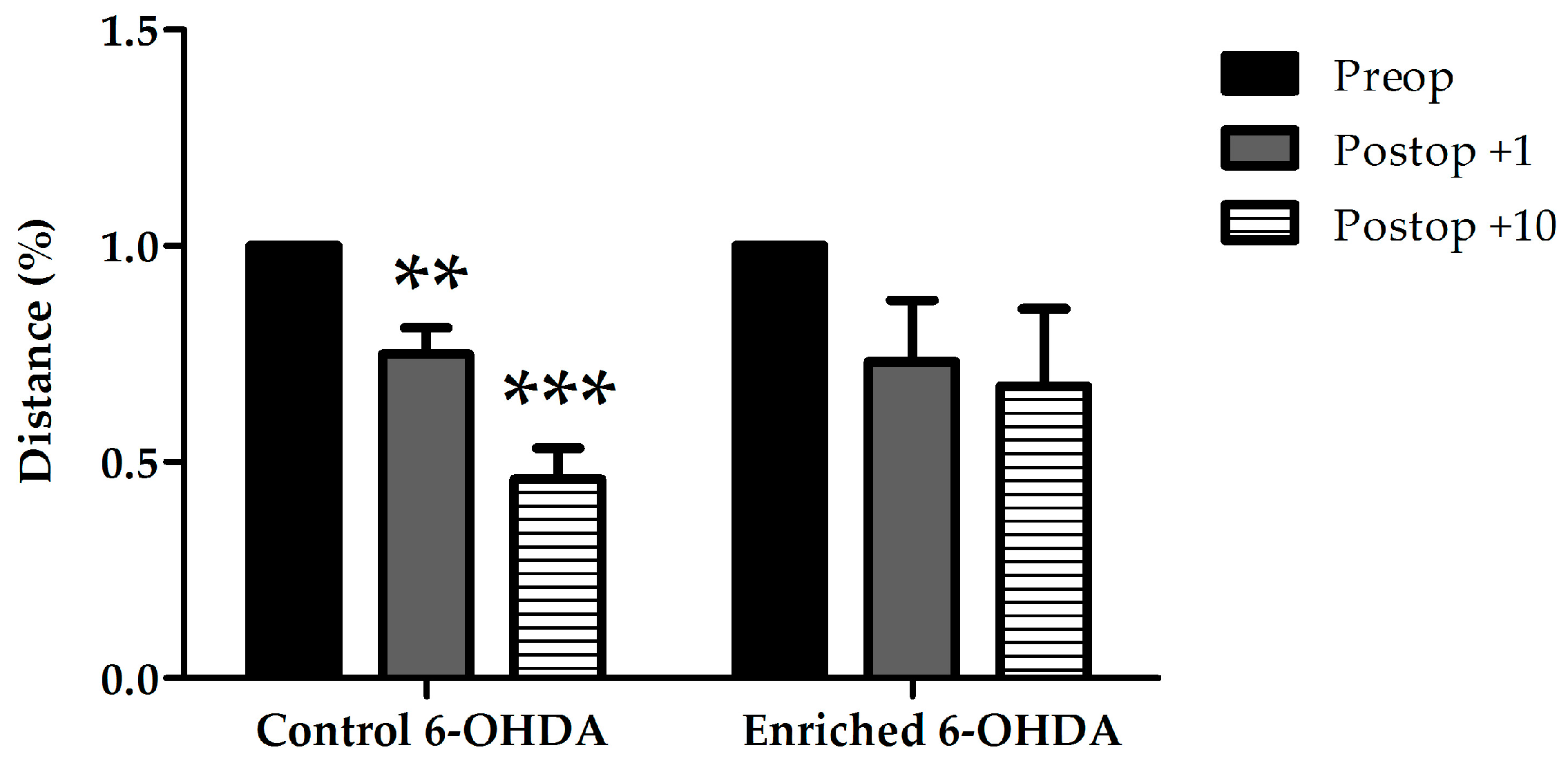
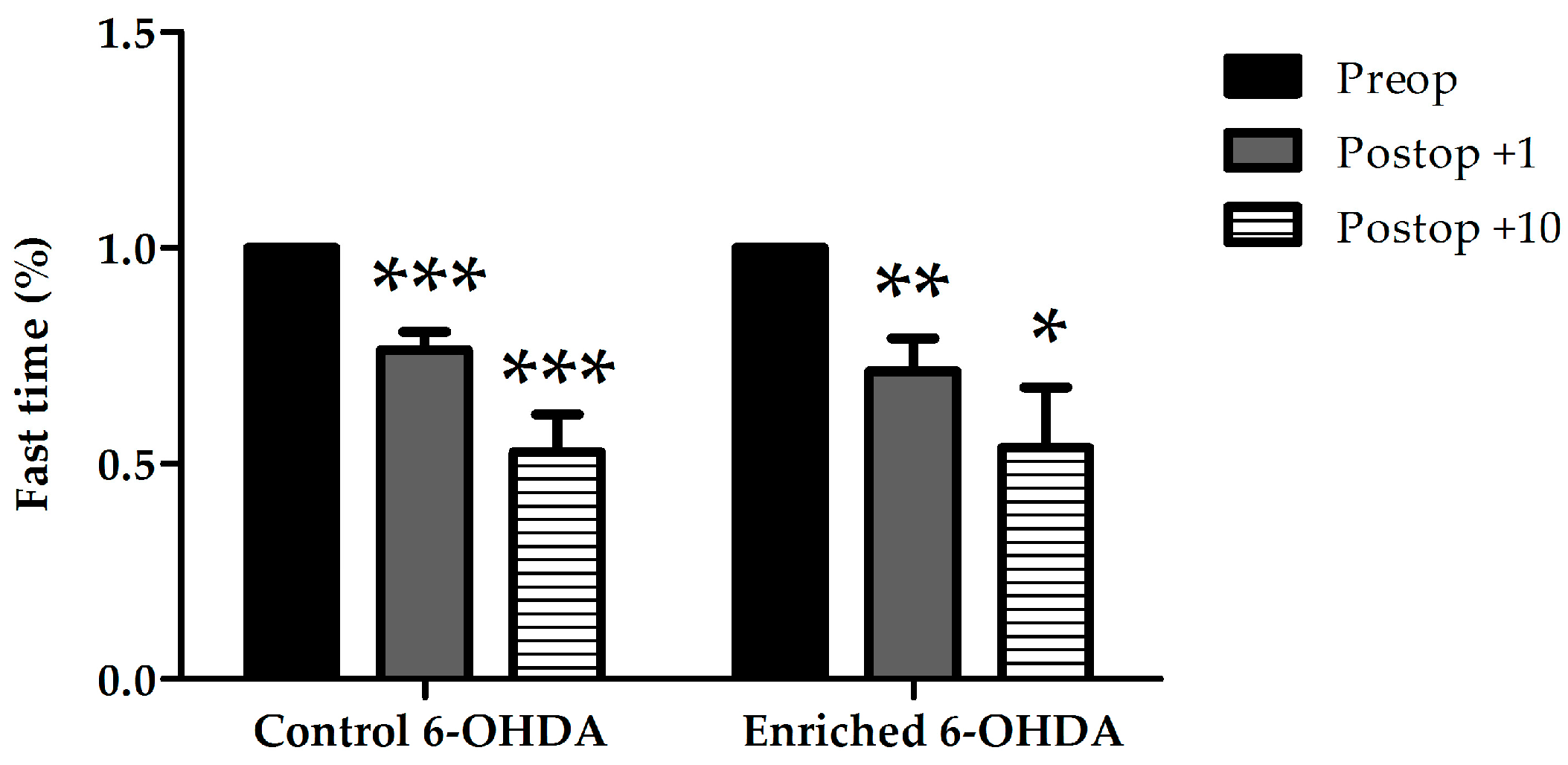
2.1.1. Examination of Hypokinetic Signs
2.1.2. Examination of Asymmetrical Signs
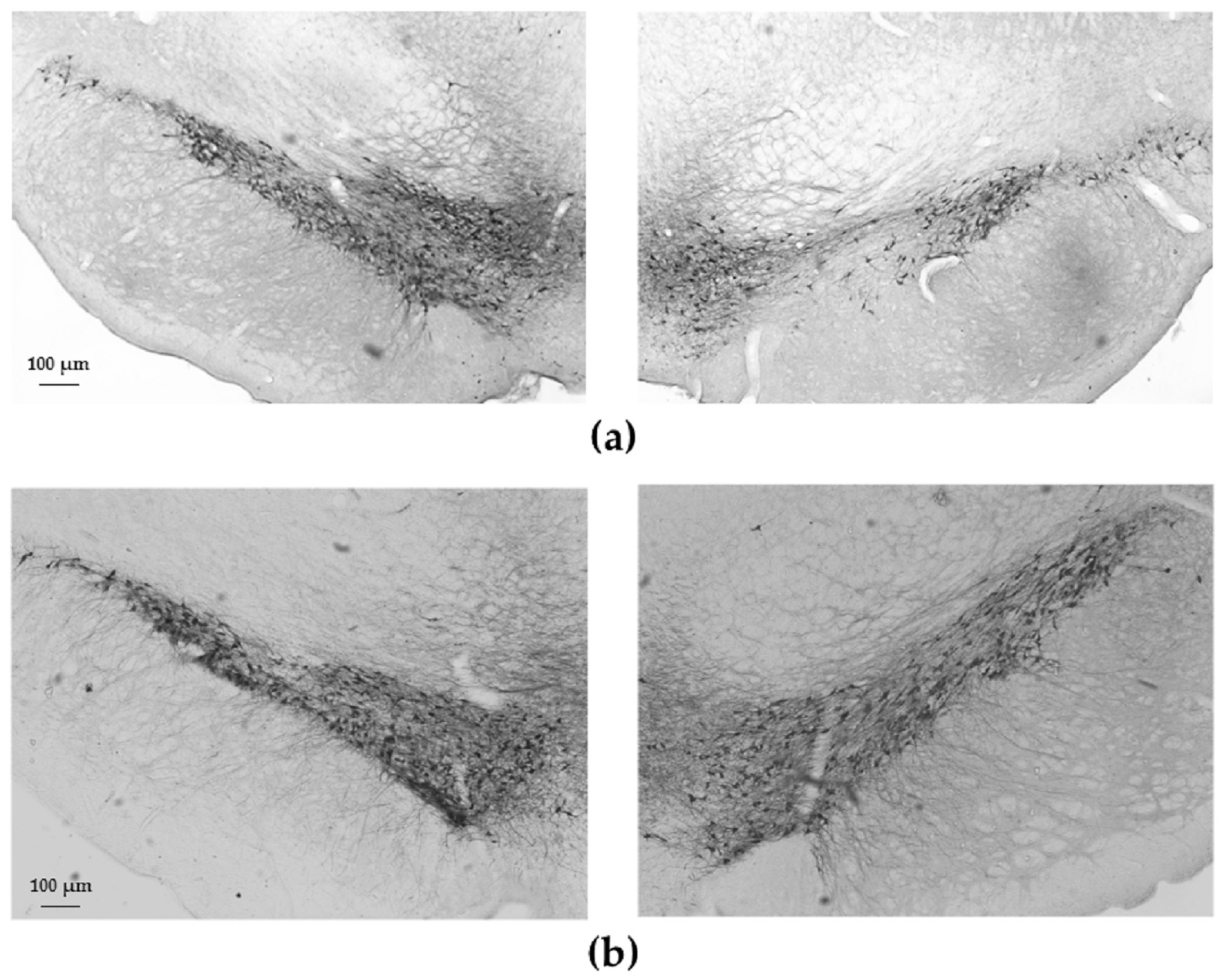
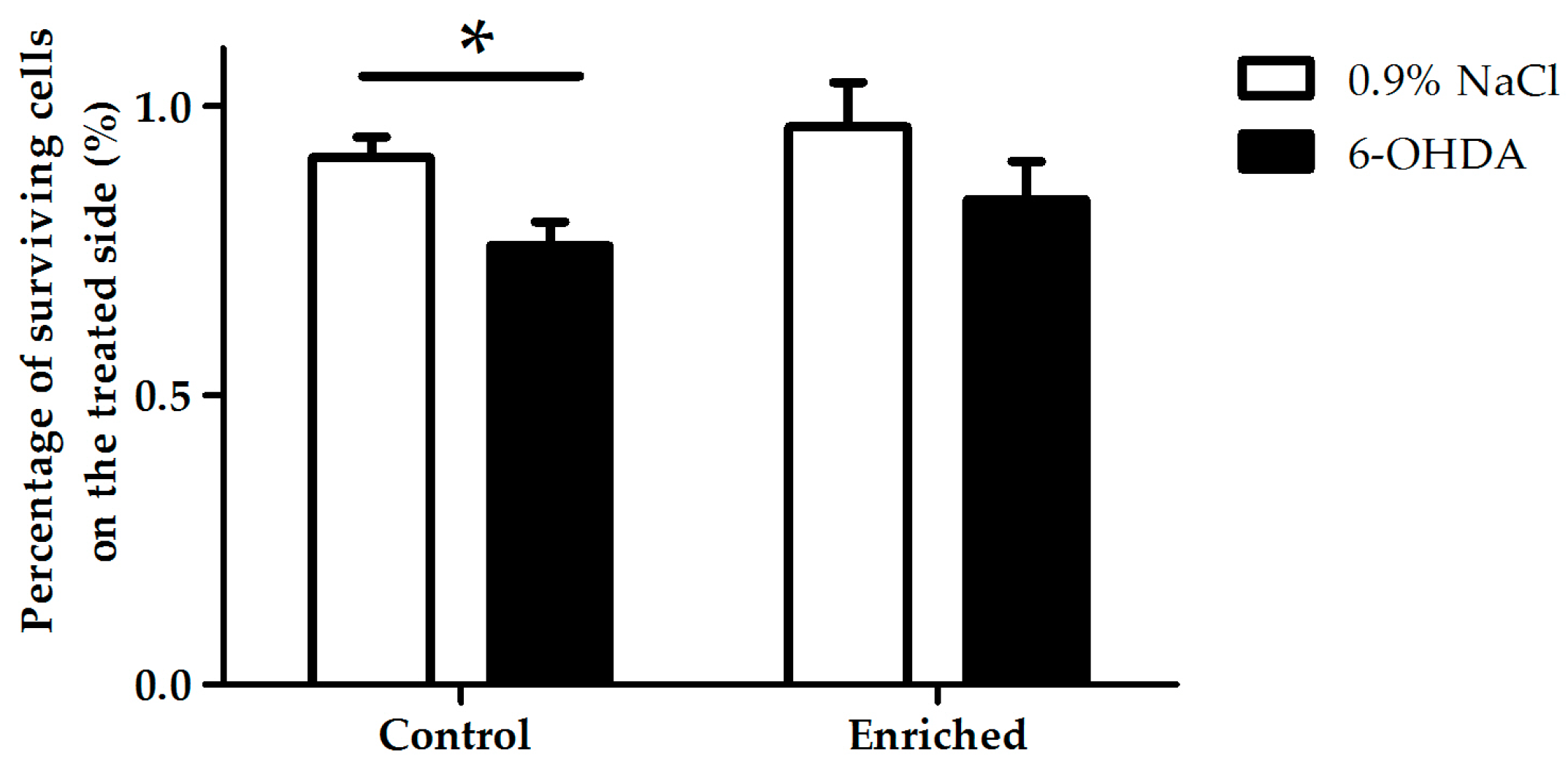
2.2. Tyrosine-Hydroxylase (TH)-Immunohistochemistry
3. Discussion
4. Materials and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Federoff, H.J. Nur(R1)turing a notion on the etiopathogenesis of Parkinson’s disease. Neurotox. Res. 2009, 16, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 2, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Sherer, T. The future of research in Parkinson disease. JAMA Neurol. 2014, 71, 1351–1352. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, A.; Carcaillon, L.; Kab, S.; Moisan, F. Epidemiology of Parkinson’s disease. Rev. Neurol. 2016, 17, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Hofer, A.; Gasser, T. New aspects of genetic contributions to Parkinson’s disease. J. Mol. Neurosci. 2004, 24, 417–424. [Google Scholar] [CrossRef]
- Damier, P.; Hirsch, E.C.; Agid, Y.; Graybiel, A.M. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 1999, 122, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Rochet, J.C.; Outeiro, T.F.; Conway, K.A.; Ding, T.T.; Volles, M.J.; Lashuel, H.A.; Bieganski, R.M.; Lindquist, S.L.; Lansbury, P.T. Interactions among alpha-synuclein, dopamine, and biomembranes: Some clues for understanding neurodegeneration in Parkinson’s disease. J. Mol. Neurosci. 2004, 23, 23–34. [Google Scholar] [CrossRef]
- Lee, S.J. Origins and effects of extracellular alpha-synuclein: Implications in Parkinson’s disease. J. Mol. Neurosci. 2008, 3, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Gerfen, C.R. Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci. 2000, 2, S64–S70. [Google Scholar] [CrossRef]
- Kakkar, A.K.; Dahiya, N. Management of Parkinson’s disease: Current and future pharmacotherapy. Eur. J. Pharmacol. 2015, 75, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, F.; Wang, X. Novel anti-inflammatory and neuroprotective agents for Parkinson’s disease. CNS Neurol. Disord. Drug Targets 2010, 9, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Renaud, J.; Tamas, A.; Tizabi, Y.; Socias, S.B.; Del-Bel, E.; Raisman-Vozari, R. Novel tactics for neuroprotection in Parkinson’s disease: Role of antibiotics, polyphenols and neuropeptides. Prog. Neurobiol. 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Song, J.X.; Sze, S.C.; Ng, T.B.; Lee, C.K.; Leung, G.P.; Shaw, P.C.; Tong, Y.; Zhang, Y.B. Anti-Parkinsonian drug discovery from herbal medicines: What have we got from neurotoxic models? J. Ethnopharmacol. 2012, 139, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Stayte, S.; Vissel, B. Advances in non-dopaminergic treatments for Parkinson’s disease. Front. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Tamas, A.; Lengvari, I.; Toth, G.; Szalontay, L.; Lubics, A. Comparative study of the effects of PACAP in young, aging, and castrated males in a rat model of Parkinson’s disease. Ann. N. Y. Acad. Sci. 2006, 1070, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Tamas, A.; Lubics, A.; Lengvari, I.; Reglodi, D. Effects of age, gender, and gonadectomy on neurochemistry and behavior in animal models of Parkinson’s disease. Endocrine 2006, 29, 275–287. [Google Scholar] [CrossRef]
- Shih, I.F.; Liew, Z.; Krause, N.; Ritz, B. Lifetime occupational and leisure time physical activity and risk of Parkinson’s disease. Park. Relat. Disord. 2016, 28, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.F.; Yang, T.; Yu, S.X.; Huang, H.D.; Jiang, L.L.; Gu, J.W.; Kuang, Y.Q. Aerobic exercise for Parkinson’s disease: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e100503. [Google Scholar] [CrossRef] [PubMed]
- Lamotte, G.; Rafferty, M.R.; Prodoehl, J.; Kohrt, W.M.; Comella, C.L.; Simuni, T.; Corcos, D.M. Effects of endurance exercise training on the motor and non-motor features of Parkinson’s disease: A review. J. Park. Dis. 2015, 5, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Do, H. The effects of early experience on problem solving at maturity. Am. Psychol. 1947, 2, 306–307. [Google Scholar]
- Sale, A.; Cenni, M.C.; Ciucci, F.; Putignano, E.; Chierzi, S.; Maffei, L. Maternal enrichment during pregnancy accelerates retinal development of the fetus. PLoS ONE 2007, 2, e1160. [Google Scholar] [CrossRef] [PubMed]
- Ortuzar, N.; Argandoña, E.G.; Bengoetxea, H.; Lafuente, J.V. Combination of intracortically administered VEGF and environmental enrichment enhances brain protection in developing rats. J. Neural. Transm. (Vienna) 2011, 118, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Altman, J. Are new neurons formed in the brains of adult mammals? Science 1962, 135, 1127–1128. [Google Scholar] [CrossRef] [PubMed]
- Szeligo, F.; Leblond, C.P. Response of the three main types of glial cells of cortex and corpus callosum in rats handled during suckling or exposed to enriched, control and impoverished environments following weaning. J. Comp. Neurol. 1977, 172, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Black, J.E.; Isaacs, K.R.; Anderson, B.J.; Alcantara, A.A.; Greenough, W.T. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc. Natl. Acad. Sci. USA 1990, 87, 5568–5572. [Google Scholar] [CrossRef] [PubMed]
- Kleim, J.A.; Lussnig, E.; Schwarz, E.R.; Comery, T.A.; Greenough, W.T. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J. Neurosci. 1996, 16, 4529–4535. [Google Scholar] [PubMed]
- Horvath, G.; Reglodi, D.; Vadasz, G.; Farkas, J.; Kiss, P. Exposure to enriched environment decreases neurobehavioral deficits induced by neonatal glutamate toxicity. Int. J. Mol. Sci. 2013, 14, 19054–19066. [Google Scholar] [CrossRef] [PubMed]
- Szabadfi, K.; Atlasz, T.; Horvath, G.; Kiss, P.; Hamza, L.; Farkas, J.; Tamas, A.; Lubics, A.; Gabriel, R.; Reglodi, D. Early postnatal enriched environment decreases retinal degeneration induced by monosodium glutamate treatment in rats. Brain Res. 2009, 1259, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Kiss, P.; Szabadfi, K.; Horvath, G.; Tamas, A.; Farkas, J.; Gabriel, R.; Reglodi, D. Gender-dependent effects of enriched environment and social isolation in ischemic retinal lesion in adult rats. Int. J. Mol. Sci. 2013, 14, 16111–16123. [Google Scholar] [CrossRef] [PubMed]
- Nithianantharajah, J.; Hannan, A.J. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 2006, 7, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Jagmag, S.A.; Tripathi, N.; Shukla, S.D.; Maiti, S.; Khurana, S. Evaluation of models of Parkinson’s disease. Front. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Manning-Bog, A.B.; Langston, J.W. Model fusion, the next phase in developing animal models for Parkinson’s disease. Neurotox. Res. 2007, 1, 219–240. [Google Scholar] [CrossRef]
- Segura-Aguilar, J.; Kostrzewa, R.M. Neurotoxin mechanisms and processes relevant to Parkinson’s disease: An update. Neurotox. Res. 2015, 27, 328–354. [Google Scholar] [CrossRef] [PubMed]
- Bezard, E.; Dovero, S.; Belin, D.; Duconger, S.; Jackson-Lewis, V.; Przedborski, S.; Piazza, P.V.; Gross, C.E.; Jaber, M. Enriched environment confers resistance to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and cocaine: Involvement of dopamine transporter and trophic factors. J. Neurosci. 2003, 23, 10999–11007. [Google Scholar] [PubMed]
- Faherty, C.J.; Raviie Shepherd, K.; Herasimtschuk, A.; Smeyne, R.J. Environmental enrichment in adulthood eliminates neuronal death in experimental Parkinsonism. Mol. Brain Res. 2005, 134, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Jadavji, N.M.; Kolb, B.; Metz, G.A. Enriched environment improves motor function in intact and unilateral dopamine-depleted rats. Neuroscience 2006, 140, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Steiner, B.; Winter, C.; Hosman, K.; Siebert, E.; Kempermann, G.; Petrus, D.S.; Kupsch, A. Enriched environment induces cellular plasticity in the adult substantia nigra and improves motor behavior function in the 6-OHDA rat model of Parkinson’s disease. Exp. Neurol. 2006, 199, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Anastasia, A.; Torre, L.; de Erausquin, G.A.; Masco, D.H. Enriched environment protects the nigrostriatal dopaminergic system and induces astroglial reaction in the 6-OHDA rat model of Parkinson’s disease. J. Neurochem. 2009, 109, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.; Garnier, Y. Pathophysiology of perinatal brain damage. Brain Res. Brain Res. Rev. 1999, 30, 107–134. [Google Scholar] [CrossRef]
- Simon, N.P. Long-term neurodevelopmental outcome of asphyxiated newborns. Clin. Perinatol. 1999, 26, 767–778. [Google Scholar] [PubMed]
- Kiss, P.; Vadasz, G.; Kiss-Illes, B.; Horvath, G.; Tamas, A.; Reglodi, D.; Koppan, M. Environmental enrichment decreases asphyxia-induced neurobehavioral developmental delay in neonatal rats. Int. J. Mol. Sci. 2013, 14, 22258–22273. [Google Scholar] [CrossRef] [PubMed]
- Koehl, M.; Lemaire, V.; Vallee, M.; Abrous, N.; Piazza, P.V.; Mayo, W.; Maccari, S.; Le Moal, M. Long term neurodevelopmental and behavioral effects of perinatal life events in rats. Neurotox. Res. 2001, 3, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Montaron, M.F.; Koehl, M.; Lemaire, V.; Drapeau, E.; Abrous, D.N.; Le Moal, M. Environmentally induced long-term structural changes: Cues for functional orientation and vulnerabilities. Neurotox. Res. 2004, 6, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Rainaldi, M.A.; Perlman, J.M. Pathophysiology of birth asphyxia. Clin. Perinatol. 2016, 43, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Placha, K.; Luptakova, D.; Baciak, L.; Ujhazy, E.; Juranek, I. Neonatal brain injury as a consequence of insufficient cerebral oxygenation. Neuro. Endocrinol. Lett. 2016, 37, 79–96. [Google Scholar] [PubMed]
- Campanille, V.; Saraceno, G.E.; Riviere, S.; Logica, T.; Kölliker, R.; Capani, F.; Castilla, R. Long lasting cerebellar alterations after perinatal asphyxia in rats. Brain Res. Bull. 2015, 116, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.Y.; He, X.Y.; Li, Q.; Mo, Y.X.; Liang, K. Neurobehavioral function of neonatal mice following excitotoxic brain damage. Zhongguo Dang Dai Er Ke Za Zhi 2009, 11, 191–193. [Google Scholar] [PubMed]
- Lantz, C.L.; Sipe, G.O.; Wong, E.L.; Majewska, A.K.; Medina, A.E. Effects of developmental alcohol exposure on potentiation and depression of visual cortex responses. Alcohol. Clin. Exp. Res. 2015, 39, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Eriksson, P.; Fredriksson, A.; Buratovic, S.; Viberg, H. Developmental neurotoxic effects of two pesticides: Behavior and biomolecular studies on chlorpyrifos and carbaryl. Toxicol. Appl. Pharmacol. 2015, 288, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Brolese, G.; Lunardi, P.; de Souza, D.F.; Lopes, F.M.; Leite, M.C.; Gonçalves, C.A. Pre- and postnatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal glutamate uptake in adolescent offspring. PLoS ONE 2015, 10, e0127845. [Google Scholar] [CrossRef] [PubMed]
- Lajud, N.; Torner, L. Early life stress and hippocampal neurogenesis in the neonate: Sexual dimorphism, long term consequences and possible mediators. Front. Mol. Neurosci. 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Naninck, E.F.; Hoeijmakers, L.; Kakava-Georgiadou, N.; Meesters, A.; Lazic, S.E.; Lucassen, P.J.; Korosi, A. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus 2015, 25, 309–328. [Google Scholar] [CrossRef] [PubMed]
- Farkas, J.; Reglodi, D.; Gaszner, B.; Szogyi, D.; Horvath, G.; Lubics, A.; Tamas, A.; Frank, F.; Besirevic, D.; Kiss, P. Effects of maternal separation on the neurobehavioral development of newborn Wistar rats. Brain Res. Bull. 2009, 79, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Neigh, G.N.; Gillespie, C.F.; Nemeroff, C.B. The neurobiological toll of child abuse and neglect. Trauma Violence Abuse 2009, 10, 389–410. [Google Scholar] [CrossRef] [PubMed]
- Alhajji, L.; Nemeroff, C.B. Personalized medicine and mood disorders. Psychiatr. Clin. N. Am. 2015, 38, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Westfall, N.C.; Nemeroff, C.B. The preeminence of early life trauma as a risk factor for worsened long-term health outcomes in women. Curr. Psychiatry Rep. 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.B. Functional outcome in rats transferred to an enriched environment 15 days after focal brain ischemia. Stroke 1996, 27, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Rönnback, A.; Dahlqvist, P.; Svensson, P.A.; Jernas, M.; Carlsson, B.; Carlsson, L.M.; Olsson, T. Gene expression profiling of the rat hippocampus one month after focal cerebral ischemia followed by enriched environment. Neurosci. Lett. 2005, 385, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, J.; Sun, H.; Zhang, L.; Liu, H.; Zeng, X.; Yang, Y.; Yao, Z. An enriched environment reverses the synaptic plasticity deficit induced by chronic cerebral hypoperfusion. Neurosci. Lett. 2011, 502, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Soeda, F.; Tanaka, A.; Shirasaki, T.; Takahama, K. An enriched environment mitigates the brain-disruptive effects of prenatal diethylstilbestrol exposure in mice. Neuroscience 2010, 169, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S.; Lee, M.H.; Anderson, D.W.; Zuck, L.; Lidsky, T.I. Enriched environment during development is protective against lead-induced neurotoxicity. Brain Res. 2001, 896, 48–55. [Google Scholar] [CrossRef]
- Horvath, G.; Reglodi, D.; Farkas, J.; Vadasz, G.; Mammel, B.; Kvarik, T.; Bodzai, G.; Kiss-Illes, B.; Farkas, D.; Matkovits, A.; et al. Perinatal positive and negative influences on the early neurobehavioral reflex and motor development. Adv. Neurobiol. 2015, 10, 149–167. [Google Scholar] [PubMed]
- Kovesdi, E.; Gyorgy, A.B.; Kwon, S.K.; Wingo, D.L.; Kamnaksh, A.; Long, J.B.; Kasper, C.E.; Agoston, D.V. The effect of enriched environment on the outcome of traumatic brain injury; a behavioral, proteomics, and histological study. Front. Neurosci. 2011, 5. [Google Scholar] [CrossRef] [PubMed]
- Young, J.; Pionk, T.; Hiatt, I.; Geeck, K.; Smith, J.S. Environmental enrichment aides in functional recovery following unilateral controlled cortical impact of the forelimb sensorimotor area however intranasal administration of nerve growth factor does not. Brain Res. Bull. 2015, 115, 17–22. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jungling, A.; Reglodi, D.; Karadi, Z.N.; Horvath, G.; Farkas, J.; Gaszner, B.; Tamas, A. Effects of Postnatal Enriched Environment in a Model of Parkinson’s Disease in Adult Rats. Int. J. Mol. Sci. 2017, 18, 406. https://doi.org/10.3390/ijms18020406
Jungling A, Reglodi D, Karadi ZN, Horvath G, Farkas J, Gaszner B, Tamas A. Effects of Postnatal Enriched Environment in a Model of Parkinson’s Disease in Adult Rats. International Journal of Molecular Sciences. 2017; 18(2):406. https://doi.org/10.3390/ijms18020406
Chicago/Turabian StyleJungling, Adel, Dora Reglodi, Zsofia Nozomi Karadi, Gabor Horvath, Jozsef Farkas, Balazs Gaszner, and Andrea Tamas. 2017. "Effects of Postnatal Enriched Environment in a Model of Parkinson’s Disease in Adult Rats" International Journal of Molecular Sciences 18, no. 2: 406. https://doi.org/10.3390/ijms18020406
APA StyleJungling, A., Reglodi, D., Karadi, Z. N., Horvath, G., Farkas, J., Gaszner, B., & Tamas, A. (2017). Effects of Postnatal Enriched Environment in a Model of Parkinson’s Disease in Adult Rats. International Journal of Molecular Sciences, 18(2), 406. https://doi.org/10.3390/ijms18020406







