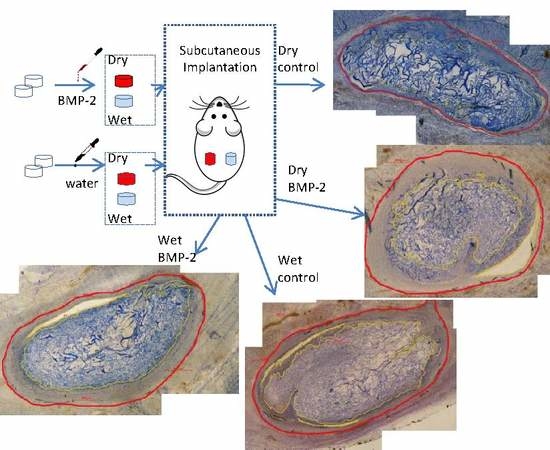
The Acute Inflammatory Response to Absorbed Collagen Sponge Is Not Enhanced by BMP-2
Abstract
:1. Introduction
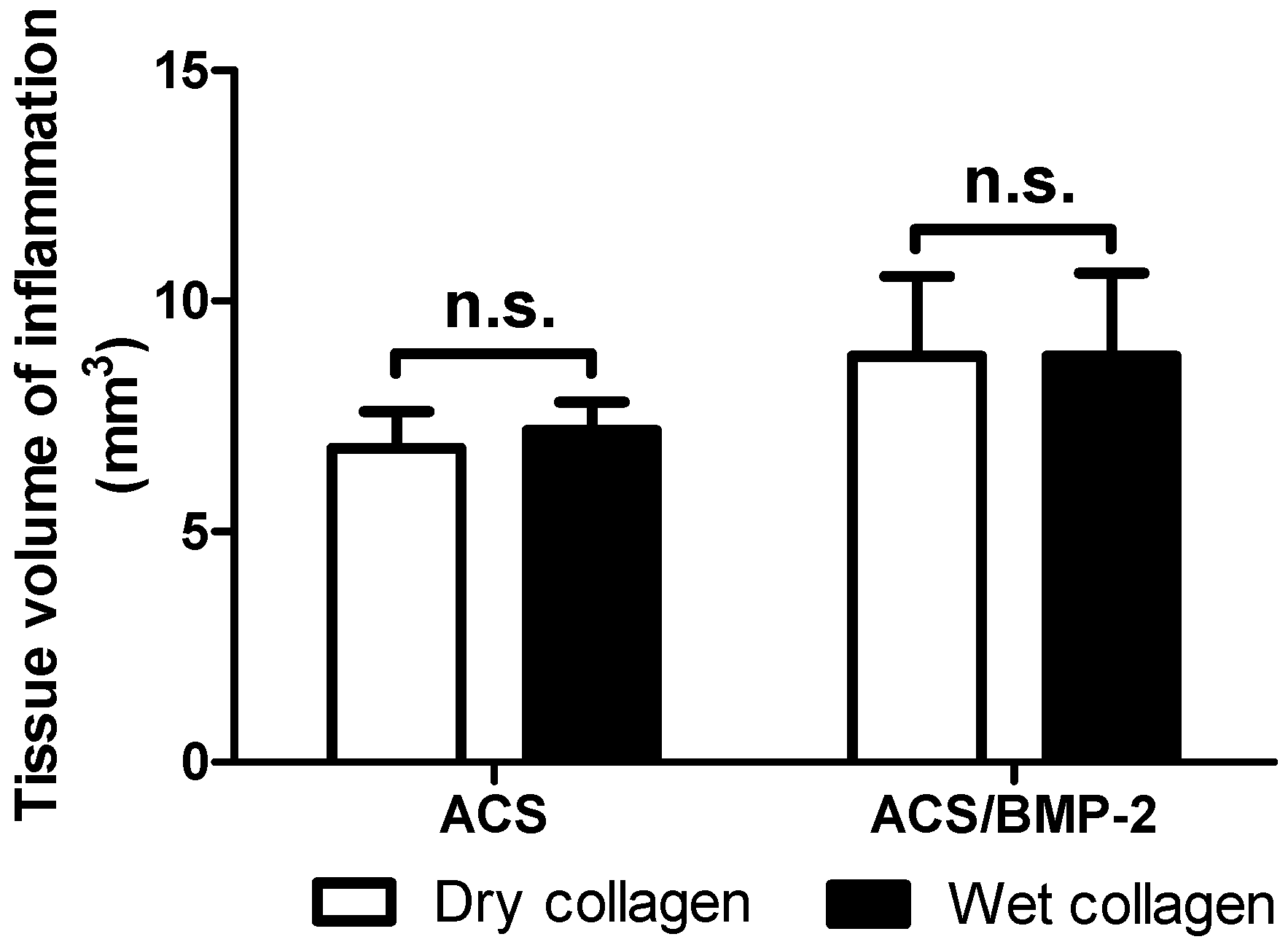
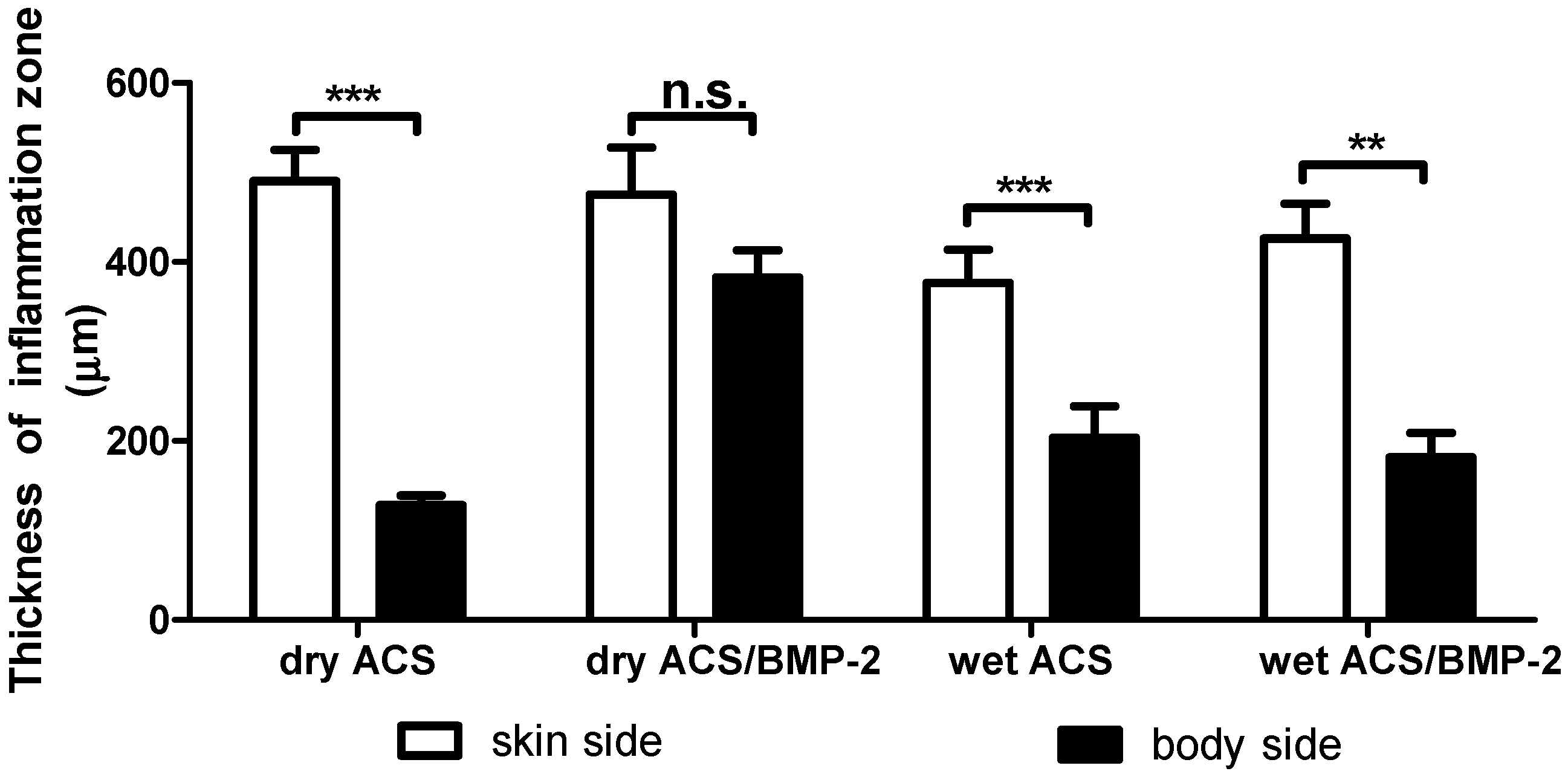
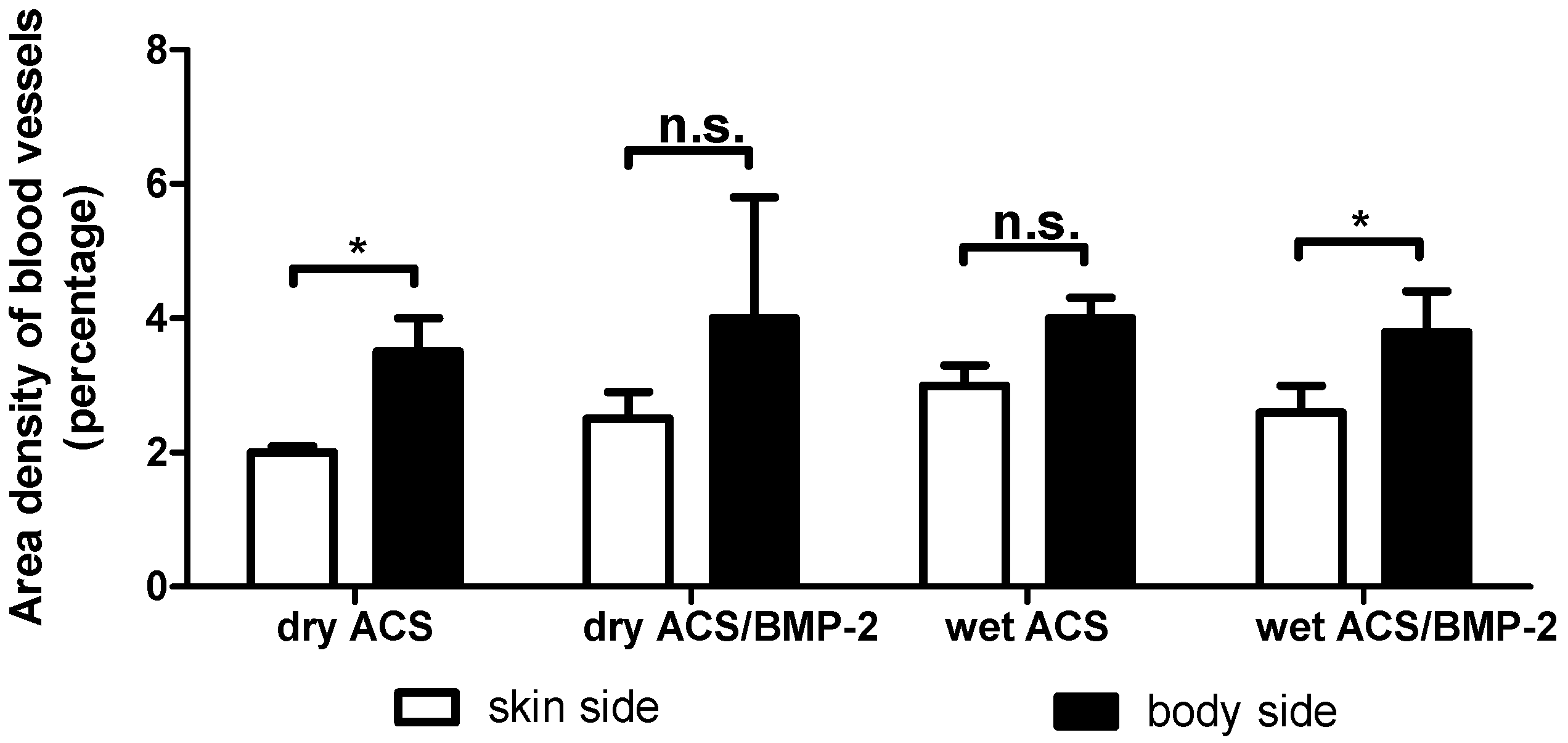
2. Results
3. Discussion
4. Materials and Methods
4.1. Animal Preparation
4.2. Animal Husbandry
4.3. Tissue Processing
4.4. Histomorphometry
4.4.1. Sample Volume and Volume of Inflammation
4.4.2. Thickness of Inflammation Volume
4.4.3. Blood Vessel Density
4.4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wozney, J.M.; Rosen, V.; Celeste, A.J.; Mitsock, L.M.; Whitters, M.J.; Kriz, R.W.; Hewick, R.M.; Wang, E.A. Novel regulators of bone formation: Molecular clones and activities. Science 1988, 242, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone morphogenetic proteins in tissue engineering: The road from the laboratory to the clinic, part I (basic concepts). J. Tissue Eng. Regen. Med. 2008, 2, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahill, K.S.; Chi, J.H.; Day, A.; Claus, E.B. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA 2009, 302, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Benglis, D.; Wang, M.Y.; Levi, A.D. A comprehensive review of the safety profile of bone morphogenetic protein in spine surgery. Neurosurgery 2008. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; la Chaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.K.; Moncayo, V.M.; Smitson, R.D.; Pierre-Jerome, C.; Terk, M.R. Recombinant human bone morphogenetic protein 2-induced heterotopic ossification of the retroperitoneum, psoas muscle, pelvis and abdominal wall following lumbar spinal fusion. Skelet. Radiol. 2010, 39, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Balseiro, S.; Nottmeier, E.W. Vertebral osteolysis originating from subchondral cyst end plate defects in transforaminal lumbar interbody fusion using rhBMP-2. Report of two cases. Spine J. 2010, 10, e6–e10. [Google Scholar] [CrossRef] [PubMed]
- Robin, B.N.; Chaput, C.D.; Zeitouni, S.; Rahm, M.D.; Zerris, V.A.; Sampson, H.W. Cytokine-mediated inflammatory reaction following posterior cervical decompression and fusion associated with recombinant human bone morphogenetic protein-2: A case study. Spine 2010, 35, E1350–E1354. [Google Scholar] [CrossRef] [PubMed]
- Garrett, M.P.; Kakarla, U.K.; Porter, R.W.; Sonntag, V.K. Formation of painful seroma and edema after the use of recombinant human bone morphogenetic protein-2 in posterolateral lumbar spine fusions. Neurosurgery 2010, 66, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Taghavi, C.E.; Song, K.J.; Sintuu, C.; Yoo, J.H.; Keorochana, G.; Tzeng, S.T.; Fei, Z.; Liao, J.C.; Wang, J.C. Inflammatory characteristics of rhBMP-2 in vitro and in an in vivo rodent model. Spine 2011, 36, E149–E154. [Google Scholar] [CrossRef] [PubMed]
- Shahlaie, K.; Kim, K.D. Occipitocervical fusion using recombinant human bone morphogenetic protein-2: Adverse effects due to tissue swelling and seroma. Spine 2008, 33, 2361–2366. [Google Scholar] [CrossRef] [PubMed]
- Rihn, J.A.; Patel, R.; Makda, J.; Hong, J.; Anderson, D.G.; Vaccaro, A.R.; Hilibrand, A.S.; Albert, T.J. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J. 2009, 9, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; James, A.W.; Zara, J.N.; Asatrian, G.; Khadarian, K.; Zhang, J.B.; Ho, S.; Kim, H.J.; Ting, K.; Soo, C. BMP2-induced inflammation can be suppressed by the osteoinductive growth factor NELL-1. Tissue Eng. Part A 2013, 19, 2390–2401. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Liu, Y.; Iizuka, T.; Hunziker, E.B. The effect of a slow mode of BMP-2 delivery on the inflammatory response provoked by bone-defect-filling polymeric scaffolds. Biomaterials 2010, 31, 7485–7493. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Kim, S.E.; Park, J.Y.; Kundu, J.; Kim, S.W.; Kang, S.S.; Cho, D.W. Three-dimensional printing of rhbmp-2-loaded scaffolds with long-term delivery for enhanced bone regeneration in a rabbit diaphyseal defect. Tissue Eng. Part A 2014, 20, 1980–1992. [Google Scholar] [CrossRef] [PubMed]
- Geiger, M.; Li, R.H.; Friess, W. Collagen sponges for bone regeneration with rhBMP-2. Adv. Drug Deliv. Rev. 2003, 55, 1613–1629. [Google Scholar] [CrossRef] [PubMed]
- Hagi, T.T.; Wu, G.; Liu, Y.; Hunziker, E.B. Cell-mediated BMP-2 liberation promotes bone formation in a mechanically unstable implant environment. Bone 2010, 46, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where we have been and where we are going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Meyerson, H.; Anderson, J.M. Quantitative in vivo cytokine analysis at synthetic biomaterial implant sites. J. Biomed. Mater. Res. A 2009, 89, 152–159. [Google Scholar] [PubMed]
- Deckers, M.M.; van Bezooijen, R.L.; van der Horst, G.; Hoogendam, J.; van Der Bent, C.; Papapoulos, S.E.; Lowik, C.W. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology 2002, 143, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Perez, V.A.; Alastalo, T.P.; Wu, J.C.; Axelrod, J.D.; Cooke, J.P.; Amieva, M.; Rabinovitch, M. Bone morphogenetic protein 2 induces pulmonary angiogenesis via Wnt-β-catenin and Wnt-RhoA-Rac1 pathways. J. Cell Biol. 2009, 184, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Raida, M.; Clement, J.H.; Leek, R.D.; Ameri, K.; Bicknell, R.; Niederwieser, D.; Harris, A.L. Bone morphogenetic protein 2 (BMP-2) and induction of tumor angiogenesis. J. Cancer Res. Clin. Oncol. 2005, 131, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Bagaria, V. Bone morphogenic protein: Current state of field and the road ahead. J. Orthop. 2005, 2, e3. [Google Scholar]
- McKay, W.F.; Peckham, S.M.; Badura, J.M. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (infuse bone graft). Int. Orthop. 2007, 31, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Luginbuehl, V.; Meinel, L.; Merkle, H.P.; Gander, B. Localized delivery of growth factors for bone repair. Eur. J. Pharm. Biopharm. 2004, 58, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Govender, S.; Csimma, C.; Genant, H.K.; Valentin-Opran, A.; Amit, Y.; Arbel, R.; Aro, H.; Atar, D.; Bishay, M.; Borner, M.G.; et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: A prospective, controlled, randomized study of four hundred and fifty patients. J. Bone Joint Surg. Am. 2002, 84, 2123–2134. [Google Scholar] [CrossRef] [PubMed]
- Zara, J.N.; Siu, R.K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J.; et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng. Part A 2011, 17, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, H.J.; Bendtsen, T.F.; Korbo, L.; Marcussen, N.; Moller, A.; Nielsen, K.; Nyengaard, J.R.; Pakkenberg, B.; Sorensen, F.B.; Vesterby, A.; et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 1988, 96, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Schenk, R.K.; Olah, A.J.; Herrmann, W. Preparation of Calcified Tissues for Light Microscopy. In Methods of Calcified Tissue Preparation; Dickson, G.R., Ed.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1984; pp. 1–56. [Google Scholar]
- Cruz-Orive, L.M.; Weibel, E.R. Recent stereological methods for cell biology: A brief survey. Am. J. Physiol. 1990, 258, L148–L156. [Google Scholar] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Wismeijer, D.; Hunziker, E.B.; Wu, G. The Acute Inflammatory Response to Absorbed Collagen Sponge Is Not Enhanced by BMP-2. Int. J. Mol. Sci. 2017, 18, 498. https://doi.org/10.3390/ijms18030498
Huang H, Wismeijer D, Hunziker EB, Wu G. The Acute Inflammatory Response to Absorbed Collagen Sponge Is Not Enhanced by BMP-2. International Journal of Molecular Sciences. 2017; 18(3):498. https://doi.org/10.3390/ijms18030498
Chicago/Turabian StyleHuang, Hairong, Daniel Wismeijer, Ernst B. Hunziker, and Gang Wu. 2017. "The Acute Inflammatory Response to Absorbed Collagen Sponge Is Not Enhanced by BMP-2" International Journal of Molecular Sciences 18, no. 3: 498. https://doi.org/10.3390/ijms18030498