Protective Effects of Ferulic Acid against Chronic Cerebral Hypoperfusion-Induced Swallowing Dysfunction in Rats
Abstract
:1. Introduction
2. Results
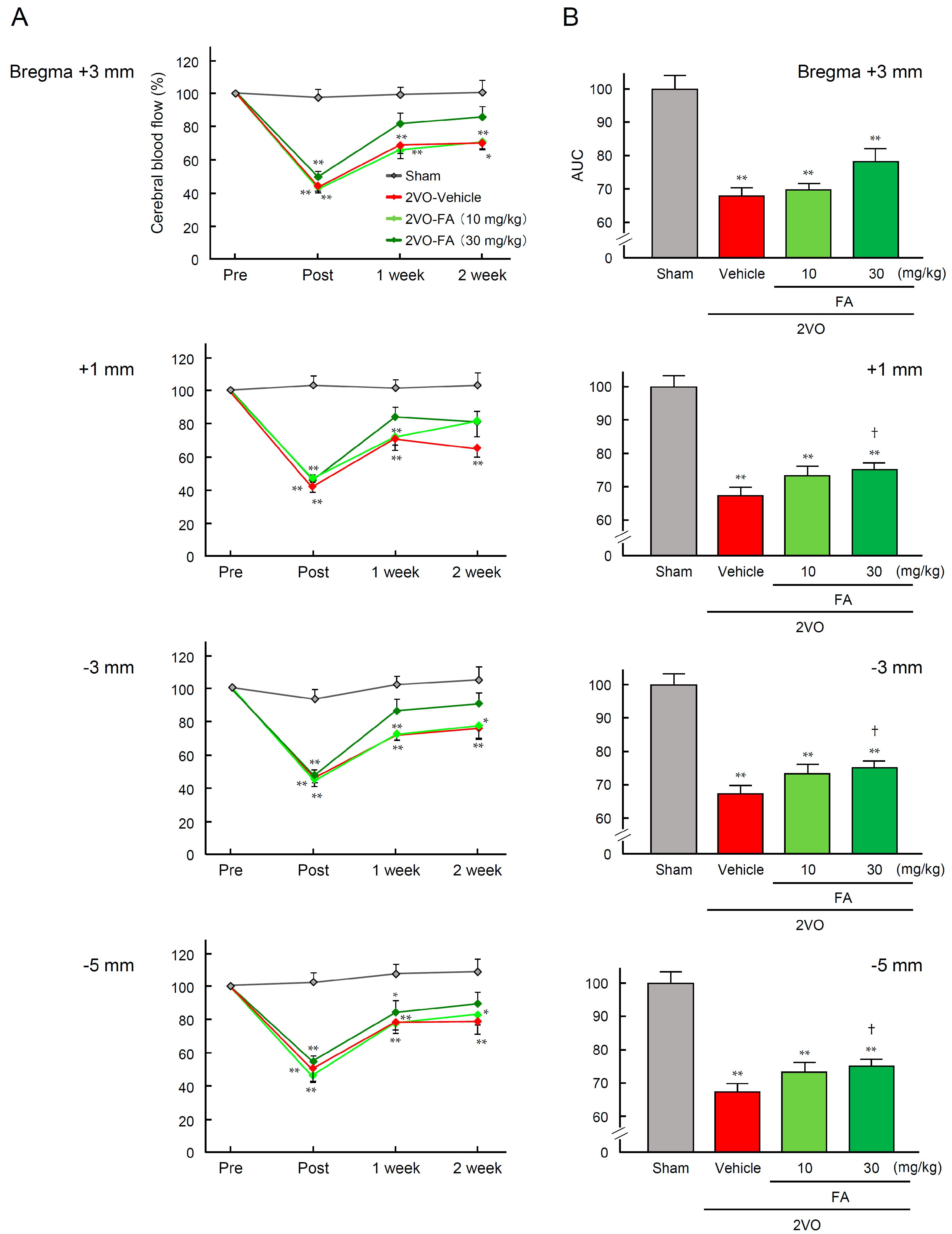
2.1. Physiological Characteristic Parameters and Cerebral Blood Flow
2.2. Ferulic Acid (FA) Suppresses Ligation of Bilateral Common Carotid Arteries (2VO)-Induced Swallowing Dysfunction
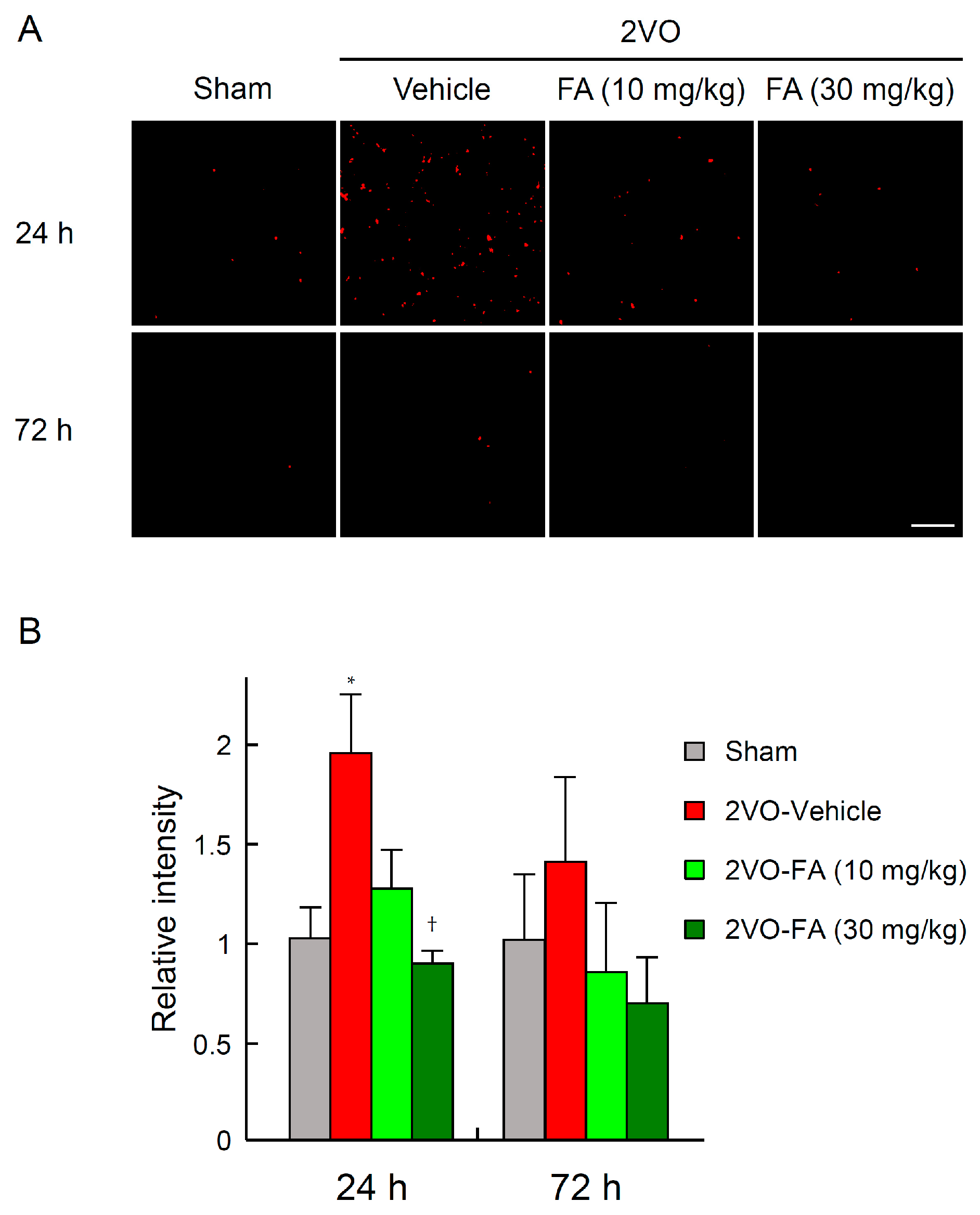
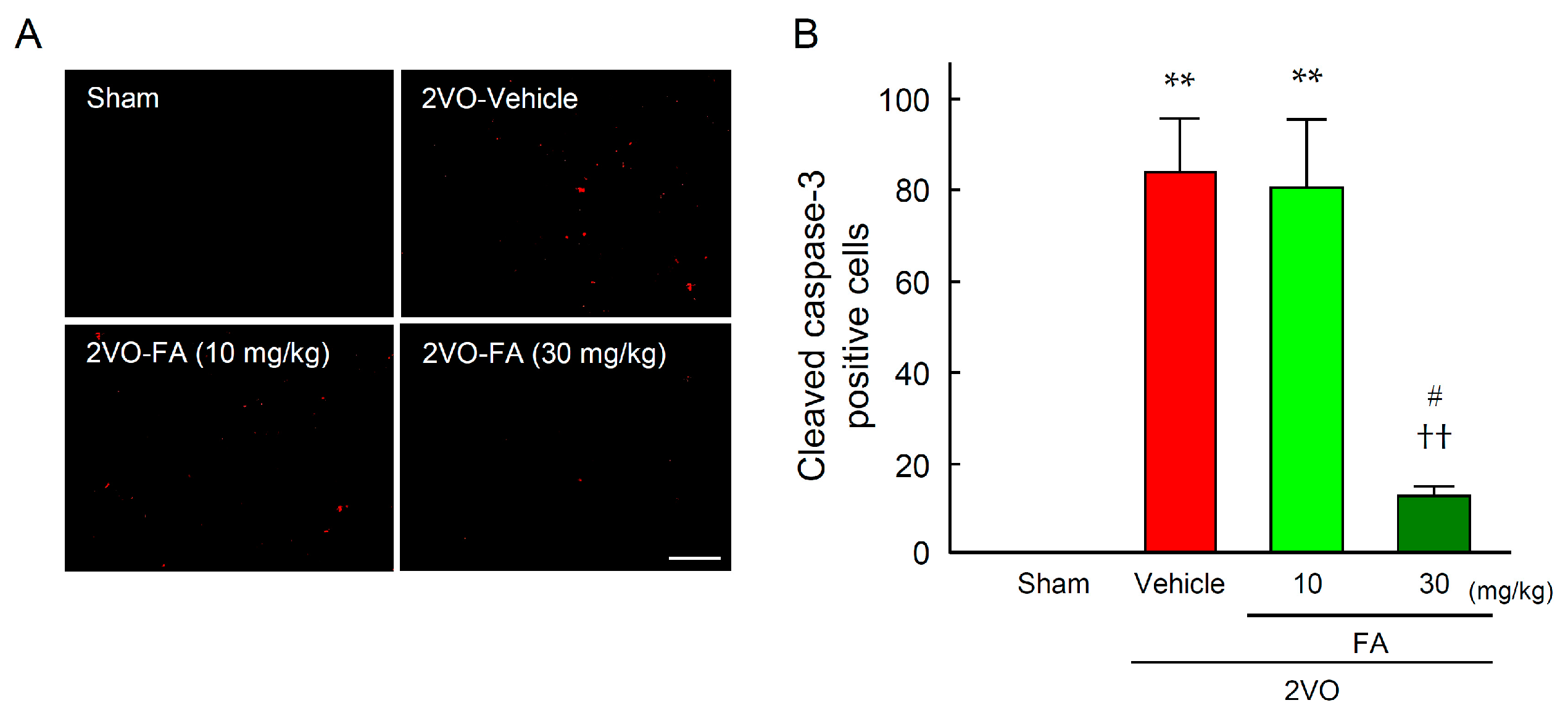
2.3. FA Ameliorates Oxidative Stress and Apoptotic Cell Death in the Striatum
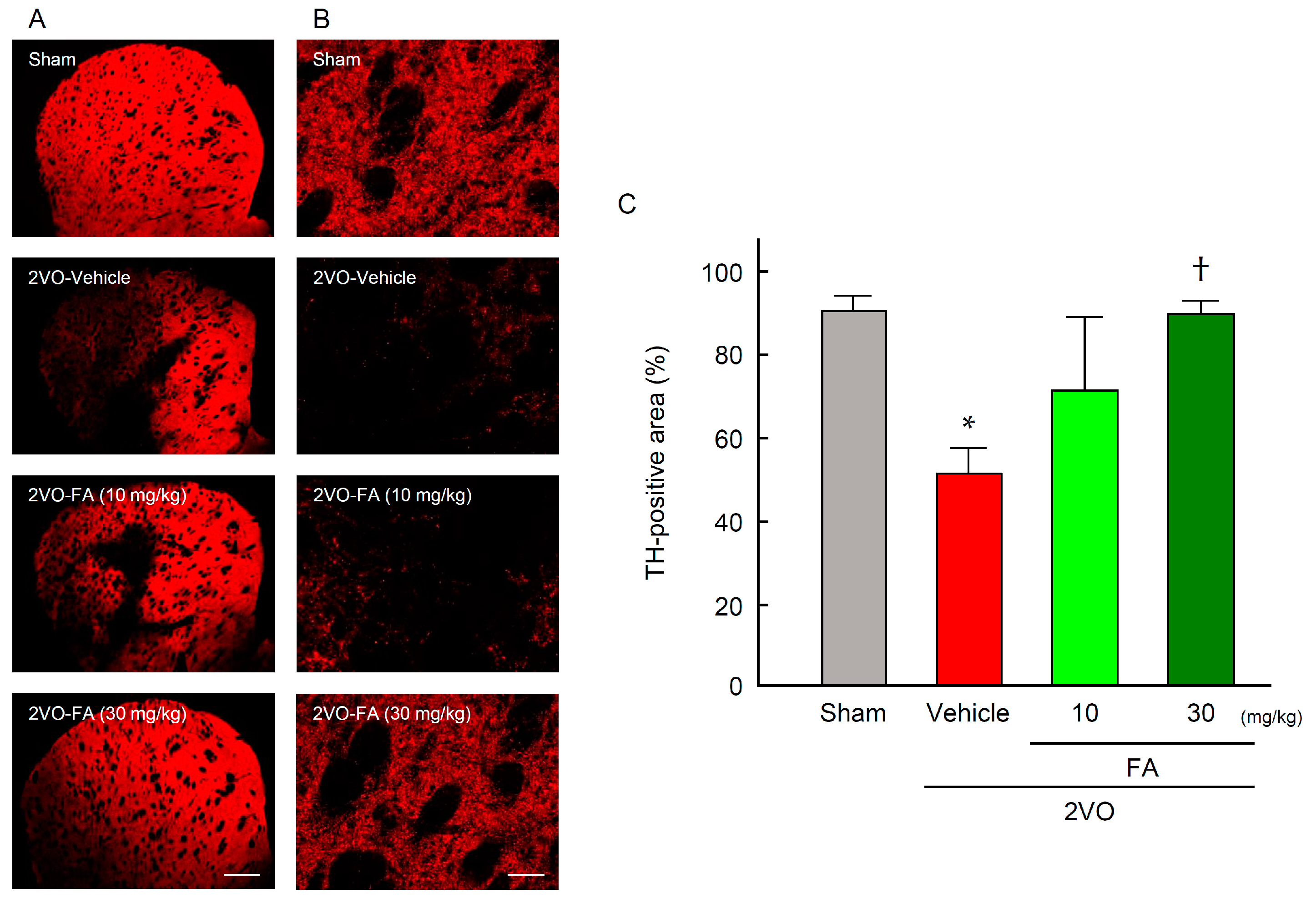
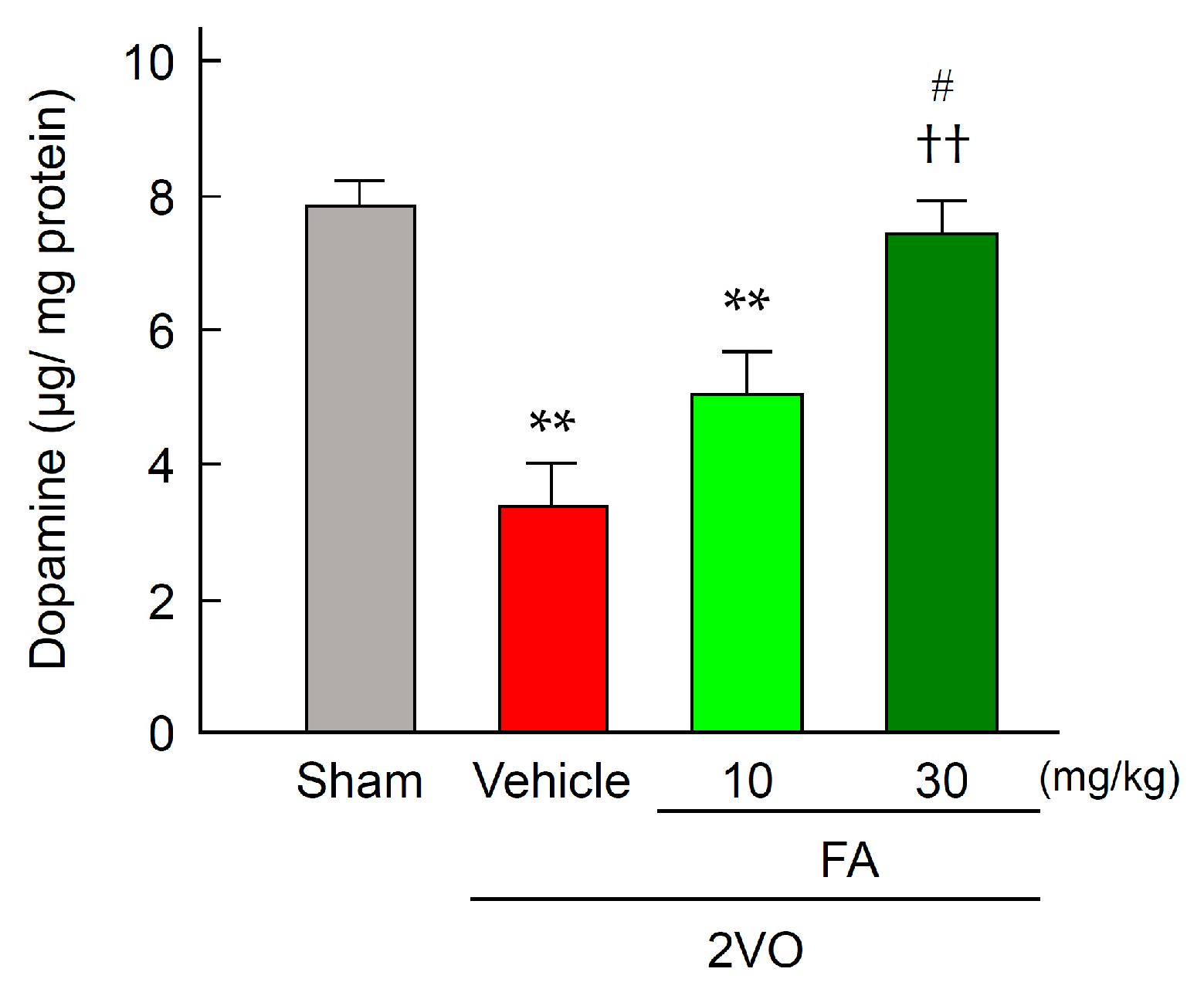
2.4. FA Increases Expression of TH and Dopamine Content in the Striatum
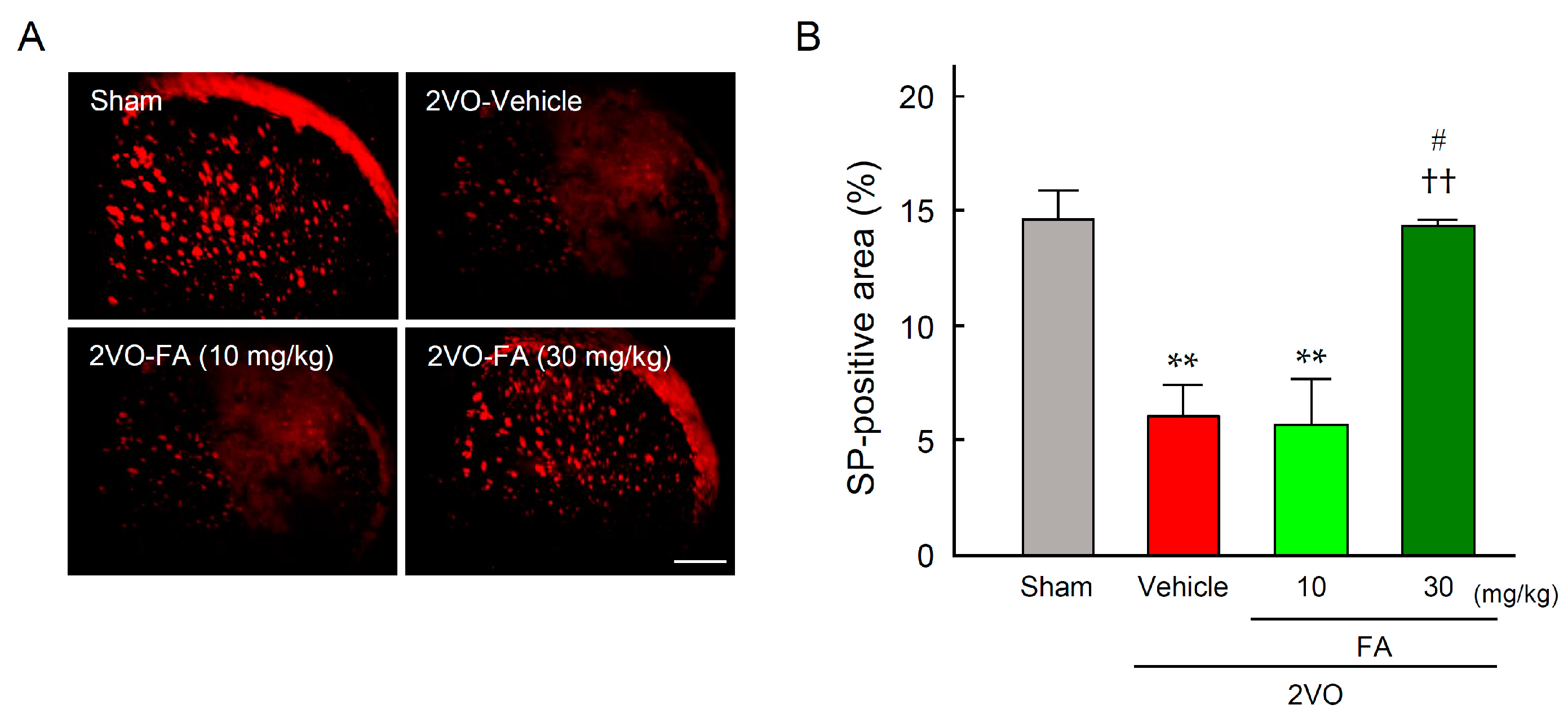
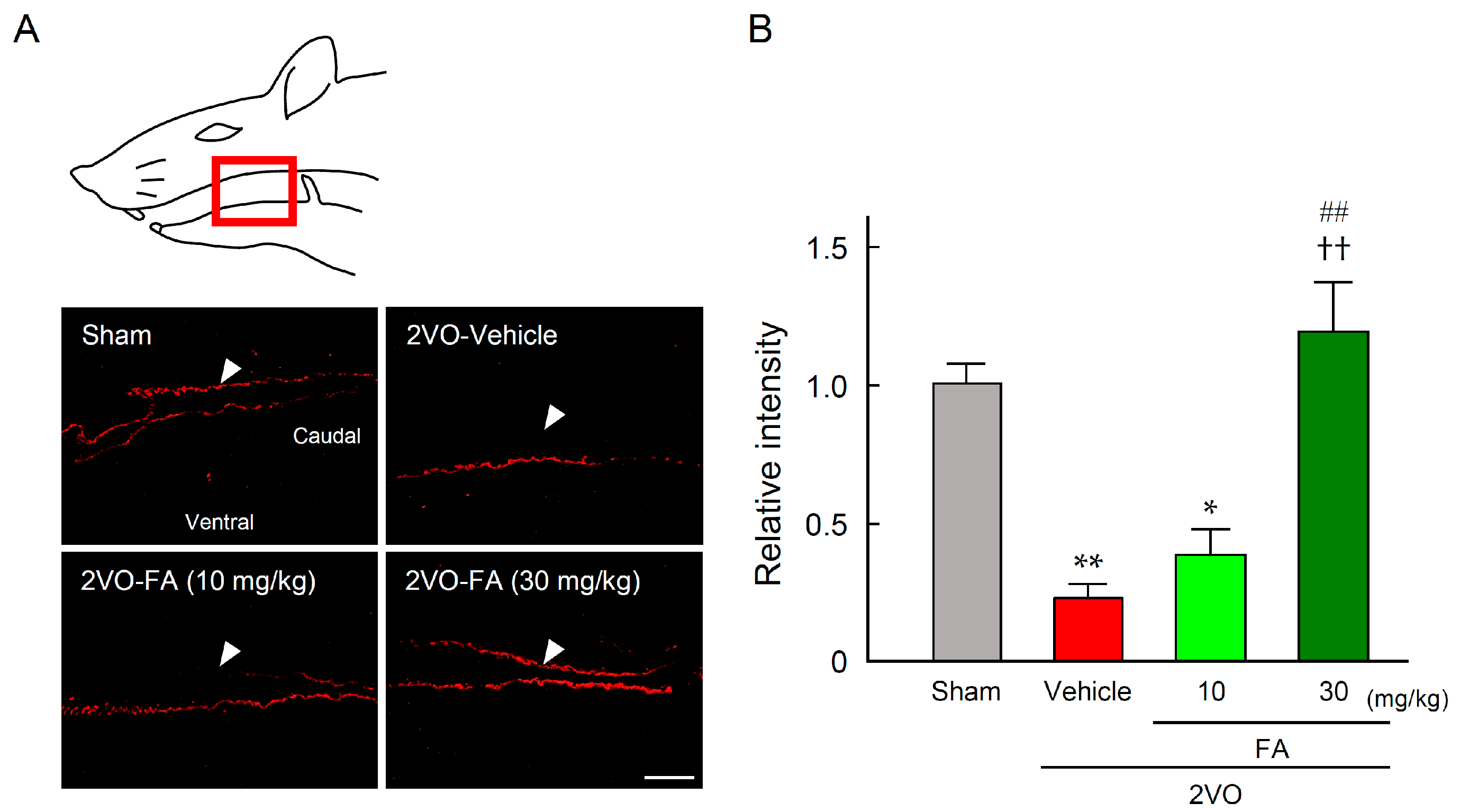
2.5. FA Maintains SP Expression in the Striatum and Laryngopharyngeal Region
3. Discussion
4. Materials and Methods
4.1. Animals and Treatments
4.2. 2VO Procedure
4.3. Measurement of CBF
4.4. Measurement of Swallowing
4.5. Measurement of Systemic Oxidative Stress
4.6. Evaluation of O2− Production by DHE Staining
4.7. Immunohistochemistry
4.8. Measurement of Dopamine Concentration
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| CBF | Cerebral blood flow |
| CREB | Cyclic AMP-responsive element binding protein |
| CPG | Swallowing central pattern generator |
| DHE | Dihydroethidium |
| d-ROMs | Diacron-reactive oxygen metabolites |
| EMG | Electromyogram |
| FA | Ferulic acid; 4-hydroxy-3-methoxycinnamic acid |
| ROS | Reactive oxygen species |
| SP | Substance P |
| TH | Tyrosine hydroxylase |
| TRPV1 | Transient receptor potential vanilloid type 1 |
| U. CARR | Carratelli units |
| 2VO | Ligation of bilateral common carotid arteries |
References
- Hankey, G.J. Long-term outcome after ischaemic stroke/transient ischaemic attack. Cerebrovasc. Dis. 2003, 16, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Martino, R.; Foley, N.; Bhogal, S.; Diamant, N.; Speechley, M.; Teasell, R. Dysphagia after stroke: Incidence, diagnosis, and pulmonary complications. Stroke 2005, 36, 2756–2763. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.L.; Roffe, C.; Beavan, J.; Blackett, B.; Fairfield, C.A.; Hamdy, S.; Havard, D.; McFarlane, M.; McLauglin, C.; Randall, M.; et al. Post-stroke dysphagia: A review and design considerations for future trials. Int. J. Stroke 2016, 11, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Ertekin, C.; Aydogdu, I. Neurophysiology of swallowing. Clin. Neurophysiol. 2003, 114, 2226–2244. [Google Scholar] [CrossRef]
- Cruz-Sanchez, F.F.; Cardozo, A.; Castejon, C.; Tolosa, E.; Rossi, M.L. Aging and the nigro-striatal pathway. J. Neural Transm. Suppl. 1997, 51, 9–25. [Google Scholar] [PubMed]
- Graybiel, A.M. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990, 13, 244–254. [Google Scholar] [CrossRef]
- Jin, Y.; Sekizawa, K.; Fukushima, T.; Morikawa, M.; Nakazawa, H.; Sasaki, H. Capsaicin desensitization inhibits swallowing reflex in guinea pigs. Am. J. Respir. Crit. Care Med. 1994, 149, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Miyamoto, N.; Tanaka, R.; Mochizuki, H.; Hattori, N.; Urabe, T. Activation of tyrosine hydroxylase prevents pneumonia in a rat chronic cerebral hypoperfusion model. Neuroscience 2009, 158, 665–672. [Google Scholar] [CrossRef]
- Itoh, M.; Meguro, K.; Fujiwara, T.; Hatazawa, J.; Iwata, R.; Ishiwata, K.; Takahashi, T.; Ido, T.; Sasaki, H. Assessment of dopamine metabolism in brain of patients with dementia by means of 18F-fluorodopa and PET. Ann. Nucl. Med. 1994, 8, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Ohrui, T.; Sekizawa, K.; Sasaki, H. Sputum substance P in aspiration pneumonia. Lancet 1995, 345, 1447. [Google Scholar] [CrossRef]
- Suttrup, I.; Warnecke, T. Dysphagia in parkinson’s disease. Dysphagia 2016, 31, 24–32. [Google Scholar] [CrossRef]
- Takizawa, C.; Gemmell, E.; Kenworthy, J.; Speyer, R. A systematic review of the prevalence of oropharyngeal dysphagia in stroke, Parkinson’s disease, Alzheimer’s disease, head injury, and pneumonia. Dysphagia 2016, 31, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Nakagawa, T.; Sekizawa, K.; Arai, H.; Sasaki, H. Levodopa and swallowing reflex. Lancet 1996, 348, 1320–1321. [Google Scholar] [CrossRef]
- Arai, T.; Sekizawa, K.; Yoshimi, N.; Toshima, S.; Fujiwara, H. Cabergoline and silent aspiration in elderly patients with stroke. J. Am. Geriatr. Soc. 2003, 51, 1815–1816. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Isono, C.; Sakamoto, H.; Ueno, S.; Kusunoki, S.; Nakamura, Y. Rotigotine transdermal patch improves swallowing in dysphagic patients with Parkinson’s disease. Dysphagia 2015, 30, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Abe, A.; Nishiyama, Y.; Hagiwara, H.; Okubo, S.; Ueda, M.; Katsura, K.; Katayama, Y. Administration of cilostazol, an antiplatelet, to patients with acute-stage cerebral infarction and its effects on plasma substance P level and latent time of swallowing reflex. J. Nippon. Med. Sch. 2013, 80, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Osawa, A.; Maeshima, S.; Tanahashi, N. Efficacy of cilostazol in preventing aspiration pneumonia in acute cerebral infarction. J. Stroke Cerebrovasc. Dis. 2013, 22, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Zhang, N.; Liu, M.; Tanaka, R.; Mizuno, Y.; Urabe, T. Cilostazol protects against brain white matter damage and cognitive impairment in a rat model of chronic cerebral hypoperfusion. Stroke 2006, 37, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Ardiansyah; Ohsaki, Y.; Shirakawa, H.; Koseki, T.; Komai, M. Novel effects of a single administration of ferulic acid on the regulation of blood pressure and the hepatic lipid metabolic profile in stroke-prone spontaneously hypertensive rats. J. Agric. Food Chem. 2008, 56, 2825–2830. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.H.; Kim, S.R.; Hwang, I.K.; Ha, T.Y. Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/db mice. J. Agric. Food Chem. 2007, 55, 9800–9804. [Google Scholar] [CrossRef] [PubMed]
- Ramar, M.; Manikandan, B.; Raman, T.; Priyadarsini, A.; Palanisamy, S.; Velayudam, M.; Munusamy, A.; Marimuthu Prabhu, N.; Vaseeharan, B. Protective effect of ferulic acid and resveratrol against alloxan-induced diabetes in mice. Eur. J. Pharmacol. 2012, 690, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Cai, Y.Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2010, 62, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Cassano, R.; Ferrarelli, T.; Barone, E.; Picci, N.; Mancuso, C. Trans-ferulic acid-based solid lipid nanoparticles and their antioxidant effect in rat brain microsomes. Colloids Surf. B Biointerfaces 2013, 109, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Kanski, J.; Aksenova, M.; Stoyanova, A.; Butterfield, D.A. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: Structure-activity studies. J. Nutr. Biochem. 2002, 13, 273–281. [Google Scholar] [CrossRef]
- Alam, M.A.; Sernia, C.; Brown, L. Ferulic acid improves cardiovascular and kidney structure and function in hypertensive rats. J. Cardiovasc. Pharmacol. 2013, 61, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Hayashida, H.; Murata, M.; Takamatsu, J. Effect of ferulic acid and angelica archangelica extract on behavioral and psychological symptoms of dementia in frontotemporal lobar degeneration and dementia with lewy bodies. Geriatr. Gerontol. Int. 2011, 11, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Su, S.Y.; Tang, N.Y.; Ho, T.Y.; Chiang, S.Y.; Hsieh, C.L. Ferulic acid provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mrna expression in rats. Brain Res. 2008, 1209, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Koh, P.O. Ferulic acid modulates nitric oxide synthase expression in focal cerebral ischemia. Lab. Anim. Res. 2012, 28, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Wang, T.; Jiang, N.; Yu, P.; Chong, Y.; Fu, F. Ferulic acid ameliorates nerve injury induced by cerebral ischemia in rats. Exp. Ther. Med. 2015, 9, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.J.; Hu, J.F.; Wang, Y.H.; Chen, X.Y.; Zhou, R.; Chen, N.H. Osthole improves chronic cerebral hypoperfusion induced cognitive deficits and neuronal damage in hippocampus. Eur. J. Pharmacol. 2010, 636, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Yamamoto, M.; Jokura, H.; Fujii, A.; Tokimitsu, I.; Hase, T.; Saito, I. Ferulic acid restores endothelium-dependent vasodilation in aortas of spontaneously hypertensive rats. Am. J. Hypertens. 2007, 20, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, J.; Kojima, N.; Saeki, K.; Ishihara, M.; Takayama, M. Perindopril increases the swallowing reflex by inhibiting substance P degradation and tyrosine hydroxylase activation in a rat model of dysphagia. Eur. J. Pharmacol. 2015, 746, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Kitada, Y.; Yahagi, R.; Okuda-Akabane, K. Effect of stimulation of the laryngopharynx with water and salt solutions on voluntary swallowing in humans: Characteristics of water receptors in the laryngopharyngeal mucosa. Chem. Sens. 2010, 35, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, T.; Udemgba, C.; Inoue, M.; Canning, B.J. Laryngeal and tracheal afferent nerve stimulation evokes swallowing in anaesthetized guinea pigs. J. Physiol. 2013, 591, 4667–4679. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, T.; Takahashi, H.; Ebihara, S.; Okazaki, T.; Sasaki, T.; Watando, A.; Nemoto, M.; Sasaki, H. Capsaicin troche for swallowing dysfunction in older people. J. Am. Geriatr. Soc. 2005, 53, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Yasuda, Y.; Takaya, T.; Toshima, S.; Kashiki, Y.; Yoshimii, N.; Fujiwara, H.; Nakayama, K.; Yamaya, M.; Sasaki, H. Angiotensin-converting enzyme inhibitors angiotensin II receptor antagonists, and symptomless dysphagia. Chest 2000, 117, 1819–1820. [Google Scholar] [CrossRef] [PubMed]
- Sivam, S.P.; Cox, J. Postnatal administration of D1 dopamine agonist reverses neonatal dopaminergic lesion-induced changes in striatal enkephalin and substance P systems. Brain Res. 2006, 1073–1074, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.X.; Sekizawa, K.; Ohrui, T.; Nakayama, K.; Sasaki, H. Dopamine D1 receptor antagonist inhibits swallowing reflex in guinea pigs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998, 274, R76–R80. [Google Scholar]
- Shinohara, Y. Antiplatelet cilostazol is effective in the prevention of pneumonia in ischemic stroke patients in the chronic stage. Cerebrovasc. Dis. 2006, 22, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Tang, N.Y.; Kao, S.T.; Hsieh, C.L. Ferulic acid administered at various time points protects against cerebral infarction by activating p38 MAPK/p90RSK/CREB/Bcl-2 anti-apoptotic signaling in the subacute phase of cerebral ischemia-reperfusion injury in rats. PLoS ONE 2016, 11, e0155748. [Google Scholar] [CrossRef] [PubMed]
- Kajii, Y.; Shingai, T.; Kitagawa, J.; Takahashi, Y.; Taguchi, Y.; Noda, T.; Yamada, Y. Sour taste stimulation facilitates reflex swallowing from the pharynx and larynx in the rat. Physiol. Behav. 2002, 77, 321–325. [Google Scholar] [CrossRef]
- Xuan, M.; Okazaki, M.; Iwata, N.; Asano, S.; Kamiuchi, S.; Matsuzaki, H.; Sakamoto, T.; Miyano, Y.; Iizuka, H.; Hibino, Y. Chronic treatment with a water-soluble extract from the culture medium of ganoderma lucidum mycelia prevents apoptosis and necroptosis in hypoxia/ischemia-induced injury of type 2 diabetic mouse brain. Evid. Based Complement. Altern. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Mamiya, T.; Lu, L.; Mouri, A.; Niwa, M.; Kim, H.C.; Zou, L.B.; Nagai, T.; Yamada, K.; Ikejima, T.; et al. Silibinin attenuates cognitive deficits and decreases of dopamine and serotonin induced by repeated methamphetamine treatment. Behav. Brain Res. 2010, 207, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watoson, C. The Rat Brain in Stereotaxic Coordinates, 5th ed.; Academic Press: New York, NY, USA, 2005. [Google Scholar]

| Group | Relative Body Weights (%) | Survival Rates (%) |
|---|---|---|
| Sham | 115.2 ± 1.2 | 100 (12/12) |
| 2VO-Vehicle | 99.2 ± 2.2 | 72.2 (13/18) |
| 2VO-FA (10 mg/kg) | 98.8 ± 1.6 | 62.5 (10/16) |
| 2VO-FA (30 mg/kg) | 99.1 ± 2.6 | 64.7 (11/17) |
| Group | Oxidative Stress (U. CARR) | ||
|---|---|---|---|
| 24 h | 72 h | 14 days | |
| Sham | 196.5 ± 23.2 | 267.3 ± 19.7 | 229.2 ± 19.3 |
| 2VO-Vehicle | 483.0 ± 13.1 ** | 551.3 ± 99.0 * | 395.6 ± 25.5 ** |
| 2VO-FA (10 mg/kg) | 415.5 ± 29.0 ** | 455.8 ± 48.3 | 249.3 ± 42.8 †† |
| 2VO-FA (30 mg/kg) | 283.0 ± 25.5 ††,## | 248.5 ± 29.8 † | 263.1 ± 22.3 † |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asano, T.; Matsuzaki, H.; Iwata, N.; Xuan, M.; Kamiuchi, S.; Hibino, Y.; Sakamoto, T.; Okazaki, M. Protective Effects of Ferulic Acid against Chronic Cerebral Hypoperfusion-Induced Swallowing Dysfunction in Rats. Int. J. Mol. Sci. 2017, 18, 550. https://doi.org/10.3390/ijms18030550
Asano T, Matsuzaki H, Iwata N, Xuan M, Kamiuchi S, Hibino Y, Sakamoto T, Okazaki M. Protective Effects of Ferulic Acid against Chronic Cerebral Hypoperfusion-Induced Swallowing Dysfunction in Rats. International Journal of Molecular Sciences. 2017; 18(3):550. https://doi.org/10.3390/ijms18030550
Chicago/Turabian StyleAsano, Takashi, Hirokazu Matsuzaki, Naohiro Iwata, Meiyan Xuan, Shinya Kamiuchi, Yasuhide Hibino, Takeshi Sakamoto, and Mari Okazaki. 2017. "Protective Effects of Ferulic Acid against Chronic Cerebral Hypoperfusion-Induced Swallowing Dysfunction in Rats" International Journal of Molecular Sciences 18, no. 3: 550. https://doi.org/10.3390/ijms18030550





