Effects and Mechanisms of Fruit and Vegetable Juices on Cardiovascular Diseases
Abstract
:1. Introduction
2. Juices and Blood Pressure
3. Juices and Blood Lipids
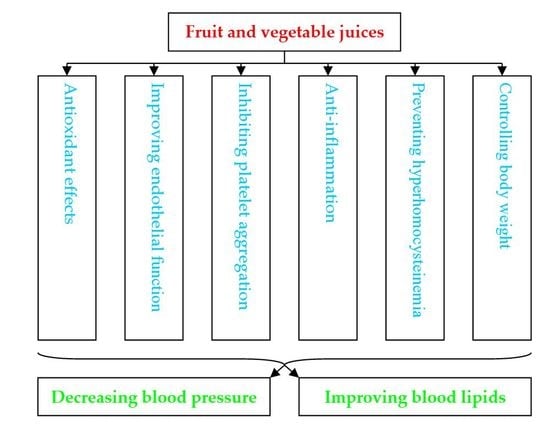
4. Mechanisms of Action of Juice on Cardiovascular Diseases
4.1. Antioxidant Effects
4.2. Improving Aspects of the Cardiovascular System
4.3. Inhibitory Effect on Platelet Aggregation
4.4. Anti-Inflammation
4.5. Preventing Hyperhomocysteinemia
4.6. Contributing to Body Weight Control
5. Possible Adverse Effects of Juices
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Deng, G.F.; Lin, X.; Xu, X.R.; Gao, L.L.; Xie, J.F.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Foods 2013, 5, 260–266. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Deng, G.F.; Shen, C.; Xu, X.R.; Kuang, R.D.; Guo, Y.J.; Zeng, L.S.; Gao, L.L.; Lin, X.; Xie, J.F.; Xia, E.Q.; et al. Potential of fruit wastes as natural resources of bioactive compounds. Int. J. Mol. Sci. 2012, 13, 8308–8323. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, B.T.; Xu, X.R.; Qin, X.S.; Gan, R.Y.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 wild fruits from South China. Molecules 2010, 15, 8602–8617. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.F.; Xu, X.R.; Guo, Y.J.; Xia, E.Q.; Li, S.; Wu, S.; Chen, F.; Ling, W.H.; Li, H.B. Determination of antioxidant property and their lipophilic and hydrophilic phenolic contents in cereal grains. J. Funct. Foods 2012, 4, 906–914. [Google Scholar] [CrossRef]
- Li, H.B.; Cheng, K.W.; Wong, C.C.; Fan, K.W.; Chen, F.; Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007, 102, 771–776. [Google Scholar] [CrossRef]
- Guo, Y.J.; Deng, G.F.; Xu, X.R.; Wu, S.; Li, S.; Xia, E.Q.; Li, F.; Chen, F.; Ling, W.H.; Li, H.B. Antioxidant capacities, phenolic compounds and polysaccharide contents of 49 edible macro-fungi. Food Funct. 2012, 3, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Li, H.B.; Xu, D.P.; Xu, X.R.; Chen, F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Foods 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Li, S.; Li, S.K.; Gan, R.Y.; Song, F.L.; Kuang, L.; Li, H.B. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind. Crops Prod. 2013, 51, 289–298. [Google Scholar] [CrossRef]
- Song, F.L.; Gan, R.Y.; Zhang, Y.; Xiao, Q.; Kuang, L.; Li, H.B. Total phenolic contents and antioxidant capacities of selected Chinese medicinal plants. Int. J. Mol. Sci. 2010, 11, 2362–2372. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, B.T.; Gan, R.Y.; Zhang, Y.; Xu, X.R.; Xia, E.Q.; Li, H.B. Total phenolic contents and antioxidant capacities of herbal and tea infusions. Int. J. Mol. Sci. 2011, 12, 2112–2124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Zhou, T.; Zheng, J.; Li, S.; Li, H.B. Dietary natural products for prevention and treatment of liver cancer. Nutrients 2016, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, S.; Li, H.B.; Deng, G.F.; Ling, W.H.; Wu, S.; Xu, X.R.; Chen, F. Antiproliferative activity of peels, pulps and seeds of 61 fruits. J. Funct. Foods 2013, 5, 1298–1309. [Google Scholar] [CrossRef]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, S.; Li, H.B.; Deng, G.F.; Ling, W.H.; Xu, X.R. Antiproliferative activities of tea and herbal infusions. Food Funct. 2013, 4, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Drossard, C.; Frohling, B.; Bolzenius, K.; Dietrich, H.; Kunz, C.; Kersting, M. Liking of anthocyanin-rich juices by children and adolescents. Appetite 2012, 58, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.L.; Nandal, U.; Pal, A.; Jain, S. Bioactive compounds and medicinal properties of fruit juices. Fruits 2014, 69, 391–412. [Google Scholar] [CrossRef]
- Singh, G.M.; Micha, R.; Khatibzadeh, S.; Shi, P.L.; Lim, S.; Andrews, K.G.; Engell, R.E.; Ezzati, M.; Mozaffarian, D. Global, regional, and national consumption of sugar-sweetened beverages, fruit juices, and milk: A systematic assessment of beverage intake in 187 countries. PLoS ONE 2015, 10, e0124845. [Google Scholar] [CrossRef] [PubMed]
- Starek, M.; Guja, A.; Dabrowska, M.; Krzek, J. Assay of β-carotene in dietary supplements and fruit juices by TLC-densitometry. Food Anal. Methods 2015, 8, 1347–1355. [Google Scholar] [CrossRef]
- Peluso, I.; Villano, D.V.; Roberts, S.A.; Cesqui, E.; Raguzzini, A.; Borges, G.; Crozier, A.; Catasta, G.; Toti, E.; Serafini, M. Consumption of mixed fruit-juice drink and vitamin C reduces postprandial stress induced by a high fat meal in healthy overweight subjects. Curr. Pharm. Des. 2014, 20, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Roque, M.J.; Rojas-Grau, M.A.; Elez-Martinez, P.; Martin-Belloso, O. In vitro bioaccessibility of health-related compounds as affected by the formulation of fruit juice- and milk-based beverages. Food Res. Int. 2014, 62, 771–778. [Google Scholar] [CrossRef]
- Eriksen, A.; Tillin, T.; O’Connor, L.; Brage, S.; Hughes, A.; Mayet, J.; McKeigue, P.; Whincup, P.; Chaturvedi, N.; Forouhi, N.G. The impact of health behaviours on incident cardiovascular disease in europeans and south asians−A prospective analysis in the UK SABRE study. PLoS ONE 2015, 10, e0117364. [Google Scholar] [CrossRef] [PubMed]
- Okuda, N.; Miura, K.; Okayama, A.; Okamura, T.; Abbott, R.D.; Nishi, N.; Fujiyoshi, A.; Kita, Y.; Nakamura, Y.; Miyagawa, N.; et al. Fruit and vegetable intake and mortality from cardiovascular disease in Japan: A 24-year follow-up of the NIPPON DATA80 study. Eur. J. Clin. Nutr. 2015, 69, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Sikand, G.; Kris-Etherton, P.; Boulos, N.M. Impact of functional foods on prevention of cardiovascular disease and diabetes. Curr. Cardiol. Rep. 2015, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Trude, A.; Kharmats, A.; Jock, B.; Liu, D.; Lee, K.; Martins, P.A.; Pardilla, M.; Swartz, J.; Gittelsohn, J. Patterns of food consumption are associated with obesity, self-reported diabetes and cardiovascular disease in five american indian communities. Ecol. Food Nutr. 2015, 54, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.; Eckel, R.H.; Howard, B.V.; St Jeor, S.; Bazzarre, T.L. Lyon diet heart study—Benefits of a mediterranean-style, national cholesterol education program/American heart association step I dietary pattern on cardiovascular disease. Circulation 2001, 103, 1823–1825. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure (JNC 7): Is it really practical? Natl. Med. J. India 2004, 17, 227. [Google Scholar] [PubMed]
- Hallikainen, M.A.; Uusitupa, M.I. Effects of 2 low-fat stanol ester-containing margarines on serum cholesterol concentrations as part of a low-fat diet in hypercholesterolemic subjects. Am. J. Clin. Nutr. 1999, 69, 403–410. [Google Scholar] [PubMed]
- Hallikainen, M.A.; Sarkkinen, E.S.; Uusitupa, M.I. Effects of low-fat stanol ester enriched margarines on concentrations of serum carotenoids in subjects with elevated serum cholesterol concentrations. Eur. J. Clin. Nutr. 1999, 53, 966–969. [Google Scholar] [CrossRef] [PubMed]
- Deurenberg-Yap, M.; Li, T.; Tan, W.L.; van Staveren, W.A.; Chew, S.K.; Deurenberg, P. Can dietary factors explain differences in serum cholesterol profiles among different ethnic groups (Chinese, Malays and Indians) in Singapore? Asia Pac. J. Clin. Nutr. 2001, 10, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xia, M.; Ma, J.; Hao, Y.T.; Liu, J.; Mou, H.; Cao, L.; Ling, W.H. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009, 90, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S.F.; Kazaks, A.G.; Holt, R.R.; Chen, H.J.; Winters, B.L.; Khoo, C.S.; Poston, W.; Haddock, C.K.; Reeves, R.S.; Foreyt, J.P.; et al. The use of a commercial vegetable juice as a practical means to increase vegetable intake: A randomized controlled trial. Nutr. J. 2010, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.Z.; Zhou, D.; Zhu, Y.M. The long-term prognosis of cardiovascular disease and all-cause mortality for metabolically healthy obesity: A systematic review and meta-analysis. J. Epidemiol. Community Health 2016, 70, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.M.; Li, X.L.; Zheng, L.; Chen, X.L.; Lan, Q.; Wu, H.; Ding, X.G.; Qian, D.G.; Shen, Y.X.; Yu, Z.R.; et al. Abdominal obesity is strongly associated with cardiovascular disease and its risk factors in elderly and very elderly community-dwelling Chinese. Sci. Rep. 2016, 6, 21521. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Dong, S.Y.; Wang, M.L.; Li, J.M.; Ren, C.L.; Gao, C.Q. Obesity and novel cardiovascular markers in a population without diabetes and cardiovascular disease in China. Prev. Med. 2016, 91, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ. Res. 2016, 118, 1786–1807. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.E. Does consumption of high-fructose corn syrup beverages cause obesity in children? Pediatr. Obes. 2013, 8, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; O’Connor, L.; Ye, Z.; Mursu, J.; Hayashino, Y.; Bhupathiraju, S.N.; Forouhi, N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. Br. J. Sports Med. 2016, 50, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Heazlewood, V.; Kotseva, K.; Turner, E.L.; Wood, D. Blood pressure control in patients with coronary heart disease is facilitated by fruit and vegetable intake. Eur. Heart J. 2011, 321, 108. [Google Scholar]
- Du, H.; Li, L.; Bennett, D.; Guo, Y.; Bian, Z.; Chen, J.; Key, T.; Collins, R.; Peto, R.; Chen, Z. Fresh fruit consumption, blood pressure and cardiovascular disease risk: A prospective cohort study of 0.5 million adults in the China kadoorie biobank. Eur. Heart J. 2014, 351, 725. [Google Scholar]
- Pienovi, L.; Lara, M.; Bustos, P.; Amigo, H. Fruit and vegetable intake, and blood pressure: A population research. Arch. Latinoam. Nutr. 2015, 65, 21–26. [Google Scholar] [PubMed]
- Reshef, N.; Hayari, Y.; Goren, C.; Boaz, M.; Madar, Z.; Knobler, H. Antihypertensive effect of sweetie fruit in patients with stage I hypertension. Am. J. Hypertens. 2005, 18, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Sahebkar, A.; Afshani, M.R.; Keshvari, M.; Haghjooyjavanmard, S.; Rafieian-Kopaei, M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-Inflammatory effects of pomegranate juice in hypertensive subjects. Phytother. Res. 2014, 28, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xing, A.H.; Chen, K.; Wang, B.; Zhou, R.; Chen, S.H.; Xu, H.X.; Mi, M.T. Effect of fruit juice on cholesterol and blood pressure in adults: A meta-analysis of 19 randomized controlled trials. PLoS ONE 2013, 8, e61420. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N.; et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008, 51, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Milsom, A.B.; Okorie, M.; Maleki-Toyserkani, S.; Akram, F.; Rehman, F.; Arghandawi, S.; Pearl, V.; Benjamin, N.; Loukogeorgakis, S.; et al. Inorganic nitrate supplementation lowers blood pressure in humans role for nitrite-derived NO. Hypertension 2010, 56, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, D.A.; Kaffa, N.; George, T.W.; Methven, L.; Lovegrove, J.A. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. Br. J. Nutr. 2012, 108, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Coles, L.T.; Clifton, P.M. Effect of beetroot juice on lowering blood pressure in free-living, disease-free adults: A randomized, placebo-controlled trial. Nutr. J. 2012, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Potter, A.S.; Foroudi, S.; Stamatikos, A.; Patil, B.S.; Deyhim, F. Drinking carrot juice increases total antioxidant status and decreases lipid peroxidation in adults. Nutr. J. 2011, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Houston, M.C.; Cooil, B.; Olafsson, B.J.; Raggi, P. Juice powder concentrate and systemic blood pressure, progression of coronary artery calcium and antioxidant status in hypertensive subjects: A pilot study. Evid. Based Complement. Altern. 2007, 4, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Habauzit, V.; Verny, M.A.; Milenkovic, D.; Barber-Chamoux, N.; Mazur, A.; Dubray, C.; Morand, C. Flavanones protect from arterial stiffness in postmenopausal women consuming grapefruit juice for 6 mo: A randomized, controlled, crossover trial. Am. J. Clin. Nutr. 2015, 102, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Foroudi, S.; Potter, A.S.; Stamatikos, A.; Patil, B.S.; Deyhim, F. Drinking orange juice increases total antioxidant status and decreases lipid peroxidation in adults. J. Med. Food 2014, 17, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Ravn-Haren, G.; Dragsted, L.O.; Buch-Andersen, T.; Jensen, E.N.; Jensen, R.I.; Nemeth-Balogh, M.; Paulovicsova, B.; Bergstrom, A.; Wilcks, A.; Licht, T.R.; et al. Intake of whole apples or clear apple juice has contrasting effects on plasma lipids in healthy volunteers. Eur. J. Nutr. 2013, 52, 1875–1889. [Google Scholar] [CrossRef] [PubMed]
- Thaptimthong, T.; Kasemsuk, T.; Sibmooh, N.; Unchern, S. Platelet inhibitory effects of juices from Pachyrhizus erosus L. root and Psidium guajava L. fruit: A randomized controlled trial in healthy volunteers. BMC Complement. Altern. Med. 2016, 16, 269. [Google Scholar] [CrossRef] [PubMed]
- Kent, K.; Charlton, K.E.; Jenner, A.; Roodenrys, S. Acute reduction in blood pressure following consumption of anthocyanin-rich cherry juice may be dose-interval dependant: A pilot cross-over study. Int. J. Food Sci. Nutr. 2016, 67, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Tjelle, T.E.; Holtung, L.; Bohn, S.K.; Aaby, K.; Thoresen, M.; Wiik, S.A.; Paur, I.; Karlsen, A.S.; Retterstol, K.; Iversen, P.O.; et al. Polyphenol-rich juices reduce blood pressure measures in a randomised controlled trial in high normal and hypertensive volunteers. Br. J. Nutr. 2015, 114, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.R.; Han, J.H.; Lee, H.J.; Park, Y.K.; Kang, M.H. Purple grape juice supplementation in smokers and antioxidant status according to different types of GST polymorphisms. J. Clin. Biochem. Nutr. 2015, 56, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, M.; Winyard, P.G.; Aizawa, K.; Anning, C.; Shore, A.; Benjamin, N. Effect of dietary nitrate on blood pressure, endothelial function, and insulin sensitivity in type 2 diabetes. Free Radic. Biol. Med. 2013, 60, 89–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asgary, S.; Afshani, M.R.; Sahebkar, A.; Keshvari, M.; Taheri, M.; Jahanian, E.; Rafieian-Kopaei, M.; Malekian, F.; Sarrafzadegan, N. Improvement of hypertension, endothelial function and systemic inflammation following short-term supplementation with red beet (Beta vulgaris L.) juice: A randomized crossover pilot study. J. Hum. Hypertens. 2016, 30, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Liu, A.H.; Croft, K.D.; Ward, N.C.; Shinde, S.; Moodley, Y.; Lundberg, J.O.; Puddey, I.B.; Woodman, R.J.; Hodgson, J.M. Absence of an effect of high nitrate intake from beetroot juice on blood pressure in treated hypertensive individuals: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S.F.; Poston, W.; Reeves, R.S.; Kazaks, A.G.; Holt, R.R.; Keen, C.L.; Chen, H.J.; Haddock, C.K.; Winters, B.L.; Khoo, C.; et al. Weight loss in individuals with metabolic syndrome given DASH diet counseling when provided a low sodium vegetable juice: A randomized controlled trial. Nutr. J. 2010, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Aptekmann, N.P.; Cesar, T.B. Long-term orange juice consumption is associated with low LDL-cholesterol and apolipoprotein B in normal and moderately hypercholesterolemic subjects. Lipids Health Dis. 2013, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Krepa, E.; Klapcinska, B.; Podgorski, T.; Szade, B.; Tyl, K.; Hadzik, A. Effects of supplementation with acai (Euterpe oleracea Mart.) berry-based juice blend on the blood antioxidant defence capacity and lipid profile in junior hurdlers. A pilot study. Biol. Sport 2015, 32, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Maldonado, A.; Hidalgo, M.; Arteaga, P.; de Pascual-Teresa, S.; Nova, E. Effects of regular consumption of vitamin C-rich or polyphenol-rich apple juice on cardiometabolic markers in healthy adults: A randomized crossover trial. Eur. J. Nutr. 2014, 53, 1645–1657. [Google Scholar] [CrossRef] [PubMed]
- Duffey, K.J.; Sutherland, L.A. Adult consumers of cranberry juice cocktail have lower C-reactive protein levels compared with nonconsumers. Nutr. Res. 2015, 35, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Chang, Y.Y.; Huang, H.C.; Wu, Y.C.; Yang, M.D.; Chao, P.M. Tomato juice supplementation in young women reduces inflammatory adipokine levels independently of body fat reduction. Nutrition 2015, 31, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Aiso, I.; Inoue, H.; Seiyama, Y.; Kuwano, T. Compared with the intake of commercial vegetable juice, the intake of fresh fruit and komatsuna (Brassica rapa L. Var. Perviridis) juice mixture reduces serum cholesterol in middle-aged men: A randomized controlled pilot study. Lipids Health Dis. 2014, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Kelsay, J.L.; Behall, K.M.; Prather, E.S. Effect of fiber from fruits and vegetables on metabolic responses of human subjects I. Bowel transit time, number of defecations, fecal weight, urinary excretions of energy and nitrogen and apparent digestibilities of energy, nitrogen, and fat. Am. J. Clin. Nutr. 1978, 31, 1149–1153. [Google Scholar] [PubMed]
- Nowak, D.; Grabczewska, Z.; Goslinski, M.; Obonska, K.; Dabrowska, A.; Kubica, J. Effect of chokeberry juice consumption on antioxidant capacity, lipids profile and endothelial function in healthy people: A pilot study. Czech J. Food Sci. 2016, 34, 39–46. [Google Scholar] [CrossRef]
- Salazar-Lugo, R.; Barahona, A.; Ortiz, K.; Chavez, C.; Freire, P.; Mendez, J.; Bermeo, B.; Santamaria, M.; Salas, H.; Oleas, M. Effect of consumption of tree tomato juice (Cyphomandra betacea) on lipid profile and glucose concentrations in adults with hyperlipidemia, Ecuador. Arch. Latinoam. Nutr. 2016, 66, 121–128. [Google Scholar]
- Aviram, M.; Dornfield, L.; Kaplan, M.; Coleman, R.; Gaitini, D.; Nitecki, S.; Hofman, A.; Rosenblat, M.; Volkova, N.; Presser, D.; et al. Pomegranate juice flavonoids inhibit low-density lipoprotein oxidation and cardiovascular diseases: Studies in atherosclerotic mice and in humans. Drug Exp. Clin. Res. 2002, 28, 49–62. [Google Scholar]
- Betanzos-Cabrera, G.; Guerrero-Solano, J.A.; Martinez-Perez, M.M.; Calderon-Ramos, Z.G.; Belefant-Miller, H.; Cancino-Diaz, J.C. Pomegranate juice increases levels of paraoxonasel (PON1) expression and enzymatic activity in streptozotocin-induced diabetic mice fed with a high-fat diet. Food Res. Int. 2011, 44, 1381–1385. [Google Scholar] [CrossRef]
- Diaz-Rubio, M.E.; Perez-Jimenez, J.; Martinez-Bartolome, M.A.; Alvarez, I.; Saura-Calixto, F. Regular consumption of an antioxidant-rich juice improves oxidative status and causes metabolome changes in healthy adults. Plant Food. Hum. Nutr. 2015, 70, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Ismail, A.; Al-Sheraji, S.H.; Azlan, A.; Hamid, A.A. Effects of Mangifera pajang Kostermans juice on plasma antioxidant status and liver and kidney function in normocholesterolemic subjects. J. Funct. Foods 2013, 5, 1900–1908. [Google Scholar] [CrossRef]
- Oliveira, P.S.; Saccon, T.D.; da Silva, T.M.; Costa, M.Z.; Dutra, F.; de Vasconcelos, A.; Lencina, C.L.; Stefanello, F.M.; Barschak, A.G. Green juice as a protector against reactive species in rats. Nutr. Hosp. 2013, 28, 1407–1412. [Google Scholar] [PubMed]
- Codoner-Franch, P.; Betoret, E.; Betoret, N.; Lopez-Jaen, A.B.; Valls-Belles, V.; Fito, P. Dried apples enriched with mandarin juice by vacuum impregnation improve antioxidant capacity and decrease inflammation in obese children. Nutr. Hosp. 2013, 28, 1177–1183. [Google Scholar] [PubMed]
- Huebbe, P.; Giller, K.; de Pascual-Teresa, S.; Arkenau, A.; Adolphi, B.; Portius, S.; Arkenau, C.N.; Rimbach, G. Effects of blackcurrant-based juice on atherosclerosis-related biomarkers in cultured macrophages and in human subjects after consumption of a high-energy meal. Br. J. Nutr. 2012, 108, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Miglio, C.; Peluso, I.; Raguzzini, A.; Villano, D.V.; Cesqui, E.; Catasta, G.; Toti, E.; Serafini, M. Fruit juice drinks prevent endogenous antioxidant response to high-fat meal ingestion. Br. J. Nutr. 2014, 111, 294–300. [Google Scholar] [PubMed]
- Al-Olayan, E.M.; El-Khadragy, M.F.; Metwally, D.M.; Moneim, A. Protective effects of pomegranate (Punica granatum) juice on testes against carbon tetrachloride intoxication in rats. BMC Complement. Altern. Med. 2014, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Rouanet, J.M.; Decorde, K.; del Rio, D.; Auger, C.; Borges, G.; Cristol, J.P.; Lean, M.; Crozier, A. Berry juices, teas, antioxidants and the prevention of atherosclerosis in hamsters. Food Chem. 2010, 118, 266–271. [Google Scholar] [CrossRef]
- Jeon, G.I.; Shin, M.J.; Lee, K.H.; Park, E. Effect of onion juice supplementation on antioxidant status in participants with mild hypercholesterolemia. Food Sci. Biotechnol. 2013, 22S, 227–231. [Google Scholar] [CrossRef]
- Poudyal, H.; Panchal, S.; Brown, L. Comparison of purple carrot juice and beta-carotene in a high-carbohydrate, high-fat diet-fed rat model of the metabolic syndrome. Br. J. Nutr. 2010, 104, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Kivimaki, A.S.; Ehlers, P.I.; Turpeinen, A.M.; Vapaatalo, H.; Korpela, R. Lingonberry juice improves endothelium-dependent vasodilatation of mesenteric arteries in spontaneously hypertensive rats in a long-term intervention. J. Funct. Foods 2011, 3, 267–274. [Google Scholar] [CrossRef]
- Flammer, A.J.; Martin, E.A.; Gossl, M.; Widmer, R.J.; Lennon, R.J.; Sexton, J.A.; Loeffler, D.; Khosla, S.; Lerman, L.O.; Lerman, A. Polyphenol-rich cranberry juice has a neutral effect on endothelial function but decreases the fraction of osteocalcin-expressing endothelial progenitor cells. Eur. J. Nutr. 2013, 52, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Auger, C.; Pollet, B.; Arnold, C.; Marx, C.; Schini-Kerth, V.B. Great heterogeneity of commercial fruit juices to induce endothelium-dependent relaxations in isolated porcine coronary arteries: Role of the phenolic content and composition. J. Med. Food 2015, 18, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Rosafio, G.; Arcoleo, G.; Mattina, A.; Canino, B.; Montana, M.; Verga, S.; Rini, G. Effects of red orange juice intake on endothelial function and inflammatory markers in adult subjects with increased cardiovascular risk. Am. J. Clin. Nutr. 2012, 95, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, H.; Takekoshi, S.; Gato, N.; Utatsu, H.; Motley, E.D.; Eguchi, K.; Fitzgerald, T.G.; Mifune, M.; Frank, G.D.; Eguchi, S. Fruit-juice concentrate of Asian plum inhibits growth signals of vascular smooth muscle cells induced by angiotensin II. Life Sci. 2002, 72, 659–667. [Google Scholar] [CrossRef]
- Siasos, G.; Tousoulis, D.; Kokkou, E.; Oikonomou, E.; Kollia, M.E.; Verveniotis, A.; Gouliopoulos, N.; Zisimos, K.; Plastiras, A.; Maniatis, K.; et al. Favorable effects of concord grape juice on endothelial function and arterial stiffness in healthy smokers. Am. J. Hypertens. 2014, 27, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Ray, S.; Craigie, A.M.; Kennedy, G.; Hill, A.; Barton, K.L.; Broughton, J.; Belch, J. Lowering of oxidative stress improves endothelial function in healthy subjects with habitually low intake of fruit and vegetables: A randomized controlled trial of antioxidant- and polyphenol-rich blackcurrant juice. Free Radic. Biol. Med. 2014, 72, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Noratto, G.; Martino, H.; Simbo, S.; Byrne, D.; Mertens-Talcott, S.U. Consumption of polyphenol-rich peach and plum juice prevents risk factors for obesity-related metabolic disorders and cardiovascular disease in Zucker rats. J. Nutr. Biochem. 2015, 26, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, A.; Asayama, R.; Mogi, M.; Nakaoka, H.; Kan-No, H.; Tsukuda, K.; Chisaka, T.; Wang, X.L.; Bai, H.Y.; Shan, B.S.; et al. Drinking citrus fruit juice inhibits vascular remodeling in cuff-induced vascular injury mouse model. PLoS ONE 2015, 10, e0117616. [Google Scholar] [CrossRef] [PubMed]
- Ramli, N.S.; Brown, L.; Ismail, P.; Rahmat, A. Effects of red pitaya juice supplementation on cardiovascular and hepatic changes in high-carbohydrate, high-fat diet-induced metabolic syndrome rats. BMC Complement. Altern. Med. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Eno, A.E.; Owo, O.I.; Itam, E.H.; Konya, R.S. Blood pressure depression by the fruit juice of Carica papaya L. in renal and DOCA-induced hypertension in the rat. Phytother. Res. 2000, 14, 235–239. [Google Scholar] [CrossRef]
- Keevil, J.G.; Osman, H.E.; Reed, J.D.; Folts, J.D. Grape juice, but not orange juice or grapefruit juice, inhibits human platelet aggregation. J. Nutr. 2000, 130, 53–56. [Google Scholar] [PubMed]
- Mattiello, T.; Trifiro, E.; Jotti, G.S.; Pulcinelli, F.M. Effects of pomegranate juice and extract polyphenols on platelet function. J. Med. Food 2009, 12, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Napoleone, E.; Cutrone, A.; Zurlo, F.; di Castelnuovo, A.; D’Imperio, M.; Giordano, L.; de Curtis, A.; Iacoviello, L.; Rotilio, D.; Cerletti, C.; et al. Both red and blond orange juice intake decreases the procoagulant activity of whole blood in healthy volunteers. Thromb. Res. 2013, 132, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Cancalon, P.F.; King, D. Health benefits of polyphenol-rich orange and grapefruit juices. XII Int. Citrus Congr. Int. Soc. Citric. 2015, 1065, 727–734. [Google Scholar]
- Devaraj, S.; Jialal, I.; Rockwood, J.; Zak, D. Effect of orange juice and beverage with phytosterols on cytokines and PAI-1 activity. Clin. Nutr. 2011, 30, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Simao, T.; Lozovoy, M.; Simao, A.; Oliveira, S.R.; Venturini, D.; Morimoto, H.K.; Miglioranza, L.; Dichi, I. Reduced-energy cranberry juice increases folic acid and adiponectin and reduces homocysteine and oxidative stress in patients with the metabolic syndrome. Br. J. Nutr. 2013, 110, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Bamonti, F.; Novembrino, C.; Ippolito, S.; Soresi, E.; Ciani, A.; Lonati, S.; Scurati-Manzoni, E.; Cighetti, G. Increased free malondialdehyde concentrations in smokers normalise with a mixed fruit and vegetable juice concentrate: A pilot study. Clin. Chem. Lab. Med. 2006, 44, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Bamonti, F.; Pellegatta, M.; Novembrino, C.; Vigna, L.; de Giuseppe, R.; de Liso, F.; Gregori, D.; della Noce, C.; Patrini, L.; Schiraldi, G.; et al. An encapsulated juice powder concentrate improves markers of pulmonary function and cardiovascular risk factors in heavy smokers. J. Am. Coll. Nutr. 2013, 32, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawashima, A.; Madarame, T.; Koike, H.; Komatsu, Y.; Wise, J.A. Four week supplementation with mixed fruit and vegetable juice concentrates increased protective serum antioxidants and folate and decreased plasma homocysteine in Japanese subjects. Asia Pac. J. Clin. Nutr. 2007, 16, 411–421. [Google Scholar] [PubMed]
- Wu, T.; Tang, Q.; Gao, Z.C.; Yu, Z.P.; Song, H.Z.; Zheng, X.D.; Chen, W. Blueberry and mulberry juice prevent obesity development in C57BL/6 mice. PLoS ONE 2013, 8, e77585. [Google Scholar] [CrossRef] [PubMed]
- DeChristopher, L.R.; Uribarri, J.; Tucker, K.L. Intake of high-fructose corn syrup sweetened soft drinks, fruit drinks and apple juice is associated with prevalent arthritis in US adults, aged 20–30 years. Nutr. Diabetes 2016, 6, e199. [Google Scholar] [CrossRef] [PubMed]
- Shefferly, A.; Scharf, R.J.; DeBoer, M.D. Longitudinal evaluation of 100% fruit juice consumption on BMI status in 2–5-year-old children. Pediatr. Obes. 2016, 11, 221–227. [Google Scholar] [CrossRef] [PubMed]
| Juices | Effective Components | Subjects | Study Types | Results | Reference |
|---|---|---|---|---|---|
| Fruit juices | |||||
| Sweetie fruit juice | Naringin | Stage I hypertension | Cross-over | Decrease in systolic blood pressure (SBP) dose-dependent decrease in diastolic blood pressure (DBP) | [44] |
| Pomegranate juice | Ellagitannins and anthocyanins | Hypertensive | Randomized controlled | Decrease in SBP and DBP | [45] |
| Grapefruit juice | Flavanones | Healthy postmenopausal women | Randomized, controlled, crossover | No effect | [53] |
| Orange juice | - | Persons with hypercholesterolemia and hypertriglyceridemia | Randomized controlled | No effect | [54] |
| Clear and cloudy apple juices | Polyphenols | Healthy persons | Randomized crossover | No effect | [55] |
| Guava fruit juice | - | Healthy volunteers | Randomized, controlled | Decrease in SBP and DBP | [56] |
| Cherry juice | Anthocyanin | Healthy volunteers | Crossover | Decrease in SBP and DBP | [57] |
| Polyphenol-rich juices 1 | Polyphenols | Healthy individuals | Randomized, controlled | Decrease in SBP and DBP | [58] |
| Purple grape juice | Anthocyanin | Smokers | Pilot | Decrease in DBP | [59] |
| Vegetable juices | |||||
| Commercial vegetable juice 2 | Minerals and vitamins | Healthy persons | Randomized, controlled, parallel-arm | Decrease in BP | [34] |
| Beetroot juice | Nitrate | Healthy persons | Randomized crossover, Randomized, controlled | Decrease in BP | [47,48,49,50] |
| Persons with type 2 diabetes | Randomized, crossover | No effect | [60] | ||
| Hypertensive subjects | Randomized, crossover | Decrease in BP | [61] | ||
| Hypertensive individuals | Randomized controlled | No effects | [62] | ||
| Carrot juice | Fiber, potassium, nitrates, and vitamin C | Persons with elevated Plasma cholesterol and Triglyceride levels | Pilot | Decrease in SBP | [51] |
| Commercial vegetable juice 2 | Vitamin C and potassium | Persons with metabolic syndrome | Randomized, controlled, parallel-arm | No effects | [63] |
| Yam bean root juice | nitrate | Healthy volunteers | Randomized, controlled | Decrease in DBP | [56] |
| Mixture juice | |||||
| Fruit and vegetable powder juice 3 | - | Healthy persons | Pilot | Decrease in SBP and DBP | [52] |
| Juices | Effective Components | Subjects | Study Types | Results | Reference |
|---|---|---|---|---|---|
| Fruit juices | |||||
| Pomegranate juice | Ellagitannins and anthocyanins | Hypertensive | Randomized controlled | No effect | [45] |
| Orange juice | Vitamin C, folate, and potassium | Persons with hypercholesterolemia and hypertriglyceridemia | Randomized controlled | No effect | [54] |
| Persons with normal and moderately high cholesterol blood levels | Cross-sectional | TC, LDL-C, apo B and LDL/HDL ratio were all significantly lowered 1 | [64] | ||
| Apple juice | Polyphenols | Healthy persons | Randomized crossover | Lower serum LDL-C for cloudy juice, no effect for clear juice | [55] |
| Vitamin C | Healthy persons | Randomized crossover | Decreased trend in total cholesterol | [66] | |
| Acai berry juice | Polyphenols | Junior hurdlers | Pilot | Improvement in lipid profile | [65] |
| Cranberry juice | Polyphenols | Healthy persons | Cross-sectional, association | A tendency of lower levels of cholesterol | [67] |
| Chokeberry juice | - | Healthy volunteers | Pilot | No significant effect | [71] |
| Vegetable juices | |||||
| Carrot juice | Carotenoid, anthocyanin | Healthy persons | Pilot | No effect | [51] |
| Tomato juice | Lycopene, minerals | Healthy persons | Randomized controlled | Decrease in serum cholestol, increase in adiponectin and triglyceride | [68] |
| Tree tomato juice | - | Persons with hypercholesterolemia | Randomized, controlled | Decrease in TC and LDL-C | [72] |
| Mixture juices | |||||
| Mixture of vegetables and fruits juice 2 | Minerals, vitamins, and polyphenols | Prehypertensive and hypertensive | Pilot | Decrease in HDL-C and apo A | [52] |
| Fruit and komatsuna juice 3 | Minerals, vitamins. and polyphenols | Healthy persons | Randomized controlled | Decrease in TC and LDL-C | [69] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Zhou, Y.; Li, S.; Zhang, P.; Zhou, T.; Xu, D.-P.; Li, H.-B. Effects and Mechanisms of Fruit and Vegetable Juices on Cardiovascular Diseases. Int. J. Mol. Sci. 2017, 18, 555. https://doi.org/10.3390/ijms18030555
Zheng J, Zhou Y, Li S, Zhang P, Zhou T, Xu D-P, Li H-B. Effects and Mechanisms of Fruit and Vegetable Juices on Cardiovascular Diseases. International Journal of Molecular Sciences. 2017; 18(3):555. https://doi.org/10.3390/ijms18030555
Chicago/Turabian StyleZheng, Jie, Yue Zhou, Sha Li, Pei Zhang, Tong Zhou, Dong-Ping Xu, and Hua-Bin Li. 2017. "Effects and Mechanisms of Fruit and Vegetable Juices on Cardiovascular Diseases" International Journal of Molecular Sciences 18, no. 3: 555. https://doi.org/10.3390/ijms18030555