The Development of a Novel Therapeutic Strategy to Target Hyaluronan in the Extracellular Matrix of Pancreatic Ductal Adenocarcinoma
Abstract
:1. Introduction
2. Hyaluronan and Its Role in Progression of Pancreatic Ductal Adenocarcinoma
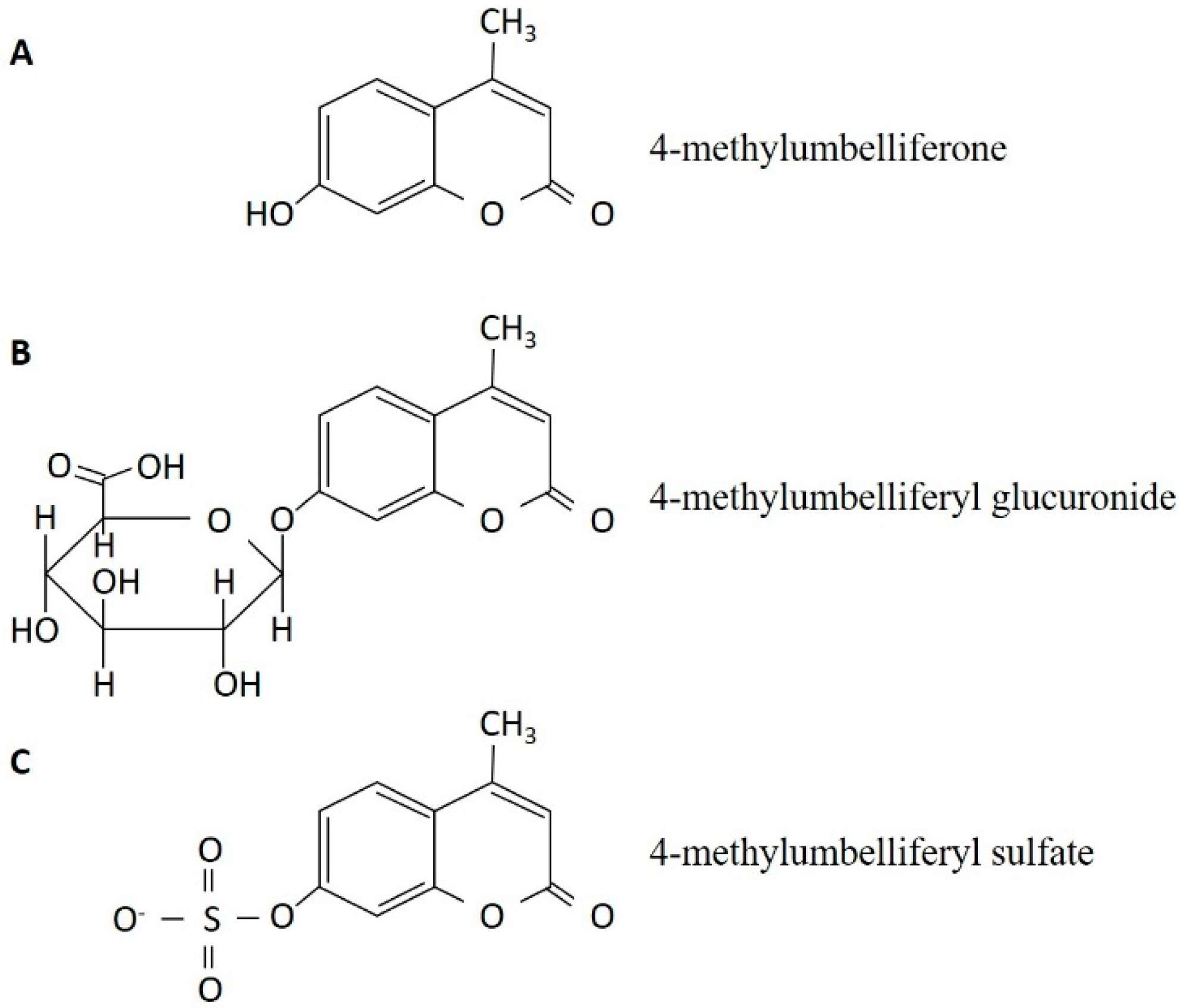
3. The Discovery of 4-Methylumbelliferone as an Inhibitor of Hyaluronan Synthesis and Its Mechanism of Action
4. The Alteration of the Extracellular Matrix in Pancreatic Ductal Adenocarcinoma through Reduction of Hyaluronan
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Stathis, A.; Moore, M.J. Advanced pancreatic carcinoma: Current treatment and future challenges. Nat. Rev. Clin. Oncol. 2010, 7, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, L.; Troiano, M.; Silvestris, N.; Nanni, L.; Latiano, T.P.; Di Maggio, G.; Cinieri, S.; Di Sebastiano, P.; Colucci, G.; Maiello, E. Combined modality treatments in pancreatic cancer. Expert Opin. Ther. Targets 2012, 16, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A., 3rd; Moore, M.J.; Andersen, J.; Green, M.R.; Rothenberg, M.L.; Modiano, M.R.; Cripps, M.C.; Portenoy, R.K.; Storniolo, A.M.; Tarassoff, P.; et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997, 15, 2403–2413. [Google Scholar] [PubMed]
- Moore, M.J.; Goldstein, D.; Hamm, J.; Figer, A.; Hecht, J.R.; Gallinger, S.; Au, H.J.; Murawa, P.; Walde, D.; Wolff, R.A.; et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2007, 20, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ma, Q.; Xu, Q.; Lei, J.; Li, X.; Wang, Z.; Wu, E. Therapeutic potential of perineural invasion, hypoxia and desmoplasia in pancreatic cancer. Curr. Pharm. Des. 2012, 18, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- DuFort, C.C.; DelGiorno, K.E.; Hingorani, S.R. Mounting Pressure in the Microenvironment: Fluids, Solids, and Cells in Pancreatic Ductal Adenocarcinoma. Gastroenterology 2016, 150, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- DuFort, C.C.; DelGiorno, K.E.; Carlson, M.A.; Osgood, R.J.; Zhao, C.; Huang, Z.; Thompson, C.B.; Connor, R.J.; Thanos, C.D.; Scott Brockenbrough, J. Interstitial pressure in pancreatic ductal adenocarcinoma is dominated by a gel-fluid phase. Biophys. J. 2016, 110, 2106–2119. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.; Palmer, J.W. The polysaccharide of the vitreous humor. J. Biol. Chem. 1934, 107, 629–634. [Google Scholar]
- Weissman, B.; Meyer, K. The structure of hyalobiuronic acid and of hyaluronic acid from umbilical cord. J. Am. Chem. Soc. 1954, 76, 1753–1757. [Google Scholar] [CrossRef]
- Itano, N.; Kimata, K. Expression cloning and molecular characterization of HAS protein, a eukaryotic hyaluronan synthase. J. Biol. Chem. 1996, 271, 9875–9878. [Google Scholar] [PubMed]
- Itano, N.; Atsumi, F.; Sawai, T.; Yamada, Y.; Miyaishi, O.; Senga, T.; Hamaguchi, M.; Kimata, K. Abnormal accumulation of hyaluronan matrix diminishes contact inhibition of cell growth and promotes cell migration. Proc. Natl. Acad. Sci. USA 2002, 99, 3609–3614. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.E.; Cummings, C.; Brass, A.; Chen, Y. Secondary and tertiary structures of hyaluronan in aqueous solution, investigated by rotary shadowing-electron microscopy and computer simulation. Hyaluronan is a very efficient network-forming polymer. Biochem. J. 1991, 274, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Salustri, A.; Yanagishita, M.; Underhill, C.B.; Laurent, T.C.; Hascall, V.C. Localization and synthesis of hyaluronic acid in the cumulus cells and mural granulosa cells of the preovulatory follicle. Dev. Biol. 1992, 151, 541–551. [Google Scholar] [CrossRef]
- Tammi, R.; Tammi, M. Correlations between hyaluronan and epidermal proliferation as studied by [3H]glucosamine and [3H]thymidine incorporations and staining of hyaluronan on mitotic keratinocytes. Exp. Cell Res. 1991, 195, 524–547. [Google Scholar] [CrossRef]
- Horton, M.R.; Burdick, M.D.; Strieter, R.M.; Bao, C.; Noble, P.W. Regulation of hyaluronan-induced chemokine gene expression by IL-10 and IFN-γ in mouse macrophages. J. Immunol. 1998, 160, 3023–3030. [Google Scholar] [PubMed]
- D’Agostino, A.; Stellavato, A.; Busico, T.; Papa, A.; Tirino, V.; Papaccio, G.; La Gatta, A.; De Rosa, M.; Schiraldi, C. In vitro analysis of the effects on wound healing of high- and low-molecular weight chains of hyaluronan and their hybrid H-HA/L-HA complexes. BMC Cell Biol. 2015, 11, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, P.; Tammi, R.; Parkkinen, J.; Tammi, M.; Agren, U.; Johansson, R.; Hirvikoski, P.; Eskelinen, M.; Kosma, V.M. Hyaluronan in peritumoral stroma and malignant cells associates with breast cancer spreading and predicts survival. Am. J. Pathol. 2000, 156, 529–536. [Google Scholar] [CrossRef]
- Setälä, L.P.; Tammi, M.I.; Tammi, R.H.; Eskelinen, M.J.; Lipponen, P.K.; Agren, U.M.; Parkkinen, J.; Alhava, E.M.; Kosma, V.M. Hyaluronan expression in gastric cancer cells is associated with local and nodal spread and reduced survival rate. Br. J. Cancer 1999, 79, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Köbel, M.; Weichert, W.; Crüwell, K.; Schmitt, W.D.; Lautenschläger, C.; Hauptmann, S. Epithelial hyaluronic acid and CD44v6 are mutually involved in invasion of colorectal adenocarcinomas and linked to patient prognosis. Virchows Arch. 2004, 445, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Tsara, M.E.; Papageorgacopoulou, N.; Karavias, D.D.; Theocharis, D.A. Pancreatic carcinoma is characterized by elevated content of hyaluronan and chondroitin sulfate with altered disaccharide composition. Biochim. Biophys. Acta 2000, 1502, 201–206. [Google Scholar] [CrossRef]
- Skandalis, S.S.; Kletsas, D.; Kyriakopoulou, D.; Stavropoulos, M.; Theocharis, D.A. The greatly increased amounts of accumulated versican and decorin with specific post-translational modifications may be closely associated with the malignant phenotype of pancreatic cancer. Biochim. Biophys. Acta 2006, 1760, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.B.; Sato, N.; Kohi, S.; Yamaguchi, K. Prognostic impact of hyaluronan and its regulators in pancreatic ductal adenocarcinoma. PLoS ONE 2013, 8, e80765. [Google Scholar] [CrossRef] [PubMed]
- Abetamann, V.; Kern, HF.; Elsässer, H.P. Differential expression of the hyaluronan receptors CD44 and RHAMM in human pancreatic cancer cells. Clin. Cancer Res. 1996, 2, 1607–1618. [Google Scholar] [PubMed]
- Sugahara, K.N.; Hirata, T.; Hayasaka, H.; Stern, R.; Murai, T.; Miyasaka, M. Tumor cells enhance their own CD44 cleavage and motility by generating hyaluronan fragments. J. Biol. Chem. 2006, 281, 5861–5868. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, S.; Ikeshita, S.; Miyatake, Y.; Kasahara, M. Pancreatic cancer cells express CD44 variant 9 and multidrug resistance protein 1 during mitosis. Exp. Mol. Pathol. 2015, 98, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Wang, S.C.; Sun, C.; Tao, Y.; Piao, H.L.; Wang, X.Q.; Du, M.; Da-Jin, Li. Hyaluronan-CD44 interaction promotes growth of decidual stromal cells in human first-trimester pregnancy. PLoS ONE 2013, 8, e74812. [Google Scholar] [CrossRef] [PubMed]
- Lokeshwar, V.B.; Mirza, S.; Jordan, A. Targeting hyaluronic acid family for cancer chemoprevention and therapy. Adv. Cancer Res. 2014, 123, 35–65. [Google Scholar] [PubMed]
- Zhang, Y.; Wei, J.; Wang, H.; Xue, X.; An, Y.; Tang, D.; Yuan, Z.; Wang, F.; Wu, J.; Zhang, J.; et al. Epithelial mesenchymal transition correlates with CD24+CD44+ and CD133+ cells in pancreatic cancer. Oncol. Rep. 2012, 27, 1599–1605. [Google Scholar] [PubMed]
- Wei, X.; Senanayake, T.H.; Warren, G.; Vinogradov, S.V. Hyaluronic acid-based nanogel-drug conjugates with enhanced anticancer activity designed for the targeting of CD44-positive and drug-resistant tumors. Bioconjug. Chem. 2013, 24, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Cuevas, C.; Chang, A.E.; Goel, V.K.; Von Hoff, D.D.; Hingorani, S.R. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012, 21, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Spicer, A.P.; McDonald, J.A. Characterization and molecular evolution of a vertebrate hyaluronan synthase gene family. J. Biol. Chem. 1998, 273, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Csóka, A.B.; Scherer, S.W.; Stern, R. Expression analysis of six paralogous human hyaluronidase genes clustered on chromosomes 3p21 and 7q31. Genomics 1999, 60, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.B.; Kohi, S.; Koga, A.; Hirata, K.; Sato, N. Hyaluronan stimulates pancreatic cancer cell motility. Oncotarget 2016, 26, 4829–4840. [Google Scholar] [CrossRef]
- Saarni, H.; Hopsu-Havu, V.K. The decrease of hyaluronate synthesis by anti-inflammatory steroids in vitro. Br. J. Dermatol. 1978, 98, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.L.; Toole, B.P. Monensin inhibition of hyaluronate synthesis in rat fibrosarcoma cells. J. Biol. Chem. 1983, 258, 7041–7046. [Google Scholar] [PubMed]
- Mason, R.M.; Lineham, J.D.; Phillipson, M.A.; Black, C.M. Selective inhibition of proteoglycan and hyaluronate synthesis in chondrocyte cultures by cyclofenil diphenol, a non-steroidal weak oestrogen. Biochem. J. 1984, 223, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Prehm, P. Inhibition of hyaluronate synthesis. Biochem. J. 1985, 225, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Gressner, A.M.; Haarmann, R. Effect of n-butyrate on the synthesis of sulfated glycosaminoglycans and hyaluronate by rat liver fat-storing cells (Ito cells). Biochem. Pharmacol. 1988, 37, 3771–3776. [Google Scholar] [CrossRef]
- Smith, T.J. Glucocorticoid regulation of glycosaminoglycan synthesis in cultured human skin fibroblasts: Evidence for a receptor-mediated mechanism involving effects on specific de novo protein synthesis. Metabolism 1988, 37, 179–184. [Google Scholar] [CrossRef]
- Smith, T.J. Retinoic acid inhibition of hyaluronate synthesis in cultured human skin fibroblasts. J. Clin. Endocrinol. Metab. 1990, 70, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Gressner, A.M. Proliferation and transformation of cultured liver fat-storing cells (perisinusoidal lipocytes) under conditions of β-d-xyloside-induced abrogation of proteoglycan synthesis. Exp. Mol. Pathol. 1991, 55, 143–169. [Google Scholar] [CrossRef]
- Honda, A.; Noguchi, N.; Takehara, H.; Ohashi, Y.; Asuwa, N.; Mori, Y. Cooperative enhancement of hyaluronic acid synthesis by combined use of IGF-I and EGF, and inhibition by tyrosine kinase inhibitor genistein, in cultured mesothelial cells from rabbit pericardial cavity. J. Cell Sci. 1991, 98, 91–98. [Google Scholar] [PubMed]
- August, E.M.; Duncan, K.L.; Malinowski, N.M.; Cysyk, R.L. Inhibition of fibroblast hyaluronic acid production by suramin. Oncol. Res. 1993, 5, 415–422. [Google Scholar] [PubMed]
- Zaharevitz, D.W.; Chisena, C.A.; Duncan, K.L.; August, E.M.; Cysyk, R.L. Vanadate inhibition of hyaluronic acid synthesis in Swiss 3T3 fibroblasts. Biochem. Mol. Biol. Int. 1993, 31, 627–633. [Google Scholar] [PubMed]
- Nakamura, T.; Takagaki, K.; Shibata, S.; Tanaka, K.; Higuchi, T.; Endo, M. Hyaluronic-acid-deficient extracellular matrix induced by addition of 4-methylumbelliferone to the medium of cultured human skin fibroblasts. Biochem. Biophys. Res. Commun. 1995, 208, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Yaron, I.; Shirazi, I.; Judovich, R.; Levartovsky, D.; Caspi, D.; Yaron, M. Fluoxetine and amitriptyline inhibit nitric oxide, prostaglandin E2, and hyaluronic acid production in human synovial cells and synovial tissue cultures. Arthritis Rheum. 1999, 42, 2561–2568. [Google Scholar] [CrossRef]
- Ueki, N.; Taguchi, T.; Takahashi, M.; Adachi, M.; Ohkawa, T.; Amuro, Y.; Hada, T.; Higashino, K. Inhibition of hyaluronan synthesis by vesnarinone in cultured human myofibroblasts. Biochim. Biophys. Acta 2000, 1495, 160–167. [Google Scholar] [CrossRef]
- Jokela, T.A.; Jauhiainen, M.; Auriola, S.; Kauhanen, M.; Tiihonen, R.; Tammi, M.I.; Tammi, R.H. Mannose inhibits hyaluronan synthesis by down-regulation of the cellular pool of UDP-N-acetylhexosamines. J. Biol. Chem. 2008, 283, 7666–7673. [Google Scholar] [CrossRef] [PubMed]
- Kultti, A.; Kärnä, R.; Rilla, K.; Nurminen, P.; Koli, E.; Makkonen, K.M.; Si, J.; Tammi, M.I.; Tammi, R.H. Methyl-β-cyclodextrin suppresses hyaluronan synthesis by down-regulation of hyaluronan synthase 2 through inhibition of Akt. J. Biol. Chem. 2010, 285, 22901–22910. [Google Scholar] [CrossRef] [PubMed]
- Freudenberger, T.; Röck, K.; Dai, G.; Dorn, S.; Mayer, P.; Heim, H.K.; Fischer, J.W. Estradiol inhibits hyaluronic acid synthase 1 expression in human vascular smooth muscle cells. Basic Res. Cardiol. 2011, 106, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Röck, K.; Grandoch, M.; Majora, M.; Krutmann, J.; Fischer, J.W. Collagen fragments inhibit hyaluronan synthesis in skin fibroblasts in response to ultraviolet B (UVB): New insights into mechanisms of matrix remodeling. J. Biol. Chem. 2011, 286, 18268–18276. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.R.; Relling, M.V. Automated high-performance liquid chromatographic assay for the determination of 7-ethoxycoumarin and umbelliferone. J. Chromatogr. 1992, 578, 141–145. [Google Scholar] [CrossRef]
- Kudo, D.; Kon, A.; Yoshihara, S.; Kakizaki, I.; Sasaki, M.; Endo, M.; Takagaki, K. Effect of a hyaluronan synthase suppressor, 4-methylumbelliferone, on B16F-10 melanoma cell adhesion and locomotion. Biochem. Biophys. Res. Commun. 2004, 321, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, S.; Kon, A.; Kudo, D.; Nakazawa, H.; Kakizaki, I.; Sasaki, M.; Endo, M.; Takagaki, K. A hyaluronan synthase suppressor, 4-methylumbelliferone, inhibits liver metastasis of melanoma cells. FEBS Lett. 2005, 579, 2722–2726. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, H.; Yoshihara, S.; Kudo, D.; Morohashi, H.; Kakizaki, I.; Kon, A.; Takagaki, K.; Sasaki, M. 4-methylumbelliferone, a hyaluronan synthase suppressor, enhances the anticancer activity of gemcitabine in human pancreatic cancer cells. Cancer Chemother. Pharmacol. 2006, 57, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Lokeshwar, V.B.; Lopez, L.E.; Munoz, D.; Chi, A.; Shirodkar, S.P.; Lokeshwar, S.D.; Escudero, D.O.; Dhir, N.; Altman, N. Antitumor activity of hyaluronic acid synthesis inhibitor 4-methylumbelliferone in prostate cancer cells. Cancer Res. 2010, 70, 2613–2623. [Google Scholar] [CrossRef] [PubMed]
- Twarock, S.; Tammi, M.I.; Savani, R.C.; Fischer, J.W. Hyaluronan stabilizes focal adhesions, filopodia, and the proliferative phenotype in esophageal squamous carcinoma cells. J. Biol. Chem. 2010, 285, 23276–23284. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.P.; Pan, Y.R.; Fu, C.Y.; Chang, H.Y. Down-regulation of UDP-glucose dehydrogenase affects glycosaminoglycans synthesis and motility in HCT-8 colorectal carcinoma cells. Exp. Cell Res. 2010, 316, 2893–2902. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, H.; Nishida, Y.; Wasa, J.; Arai, E.; Zhuo, L.; Kimata, K.; Kozawa, E.; Futamura, N.; Ishiguro, N. Inhibition of hyaluronan synthesis in breast cancer cells by 4-methylumbelliferone suppresses tumorigenicity in vitro and metastatic lesions of bone in vivo. Int. J. Cancer 2012, 130, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, F.; Malvicini, M.; Garcia, M.G.; Rodriguez, A.; Atorrasagasti, C.; Kippes, N.; Piedra Buena, I.T.; Rizzo, M.M.; Bayo, J.; Aquino, J.; et al. Antitumor effects of hyaluronic acid inhibitor 4-methylumbelliferone in an orthotopic hepatocellular carcinoma model in mice. Glycobiology 2012, 22, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Arai, E.; Nishida, Y.; Wasa, J.; Urakawa, H.; Zhuo, L.; Kimata, K.; Kozawa, E.; Futamura, N.; Ishiguro, N. Inhibition of hyaluronan retention by 4-methylumbelliferone suppresses osteosarcoma cells in vitro and lung metastasis in vivo. Br. J. Cancer 2011, 105, 1839–1849. [Google Scholar] [CrossRef] [PubMed]
- Lompardía, S.L.; Papademetrio, D.L.; Mascaró, M.; Álvarez, E.M.; Hajos, S.E. Human leukemic cell lines synthesize hyaluronan to avoid senescence and resist chemotherapy. Glycobiology 2013, 23, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, I.; Kojima, K.; Takagaki, K.; Endo, M.; Kannagi, R.; Ito, M.; Maruo, Y.; Sato, H.; Yasuda, T.; Mita, S.; et al. A novel mechanism for the inhibition of hyaluronan biosynthesis by 4-methylumbelliferone. J. Biol. Chem. 2004, 279, 33281–33289. [Google Scholar] [CrossRef] [PubMed]
- Kultti, A.; Pasonen-Seppänen, S.; Jauhiainen, M.; Rilla, K.J.; Kärnä, R.; Pyöriä, E.; Tammi, R.H.; Tammi, M.I. 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and downregulation of hyaluronan synthase 2 and 3. Exp. Cell Res. 2009, 315, 1914–1923. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Kudo, D.; Suto, A.; Yoshida, E.; Suto, S.; Negishi, M.; Kakizaki, I.; Hakamada, K. 4-Methylumbelliferone suppresses hyaluronan synthesis and tumor progression in scid mice intra-abdominally inoculated with pancreatic cancer cells. Pancreas 2016, 46, 190. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Aburada, M. The choleretic mechanism of coumarin compounds and phenolic compounds. J. Pharmacobiodyn. 1981, 4, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.; Dimartino, V.; Spina, P.; Costa, P.L.; Lombardo, C.; Santini, A.; Del Piano, M.; Alimonti, P. Hymecromone in the treatment of motor disorders of the bile ducts: A multicenter, double-blind, placebo-controlled clinical study. Drugs Exp. Clin. Res. 2001, 27, 223–231. [Google Scholar] [PubMed]
- Hingorani, S.R.; Harris, W.P.; Beck, J.T.; Berdov, B.A.; Wagner, S.A.; Pshevlotsky, E.M.; Tjulandin, S.A.; Gladkov, O.A.; Holcombe, R.F.; Korn, R.; et al. Phase Ib study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin. Cancer Res. 2016, 22, 2848–2854. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.B.; Shepard, H.M.; O’Connor, P.M.; Kadhim, S.; Jiang, P.; Osgood, R.J.; Bookbinder, L.H.; Li, X.; Sugarman, B.J.; Connor, R.J.; et al. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol. Cancer Ther. 2010, 9, 3052–3064. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, E.; Kudo, D.; Nagase, H.; Shimoda, H.; Suto, S.; Negishi, M.; Kakizaki, I.; Endo, M.; Hakamada, K. Antitumor effects of the hyaluronan inhibitor 4-methylumbelliferone on pancreatic cancer. Oncol. Lett. 2016, 12, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Katsuda, M.; Maguchi, H.; Katanuma, A.; Ishii, H.; Ozaka, M.; Yamao, K.; Imaoka, H.; Kawai, M.; Hirono, S.; et al. Phase II clinical trial using novel peptide cocktail vaccine as a postoperative adjuvant treatment for surgically resected pancreatic cancer patients. Int. J. Cancer 2016. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Wu, Z.; Jiang, Z.; Li, J.; Zong, L.; Chen, X.; Duan, W.; Xu, Q.; Zhang, L.; Han, L.; et al. Pancreatic carcinoma-specific immunotherapy using novel tumor specific cytotoxic T cells. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Mace, T.A.; Shakya, R.; Pitarresi, J.R.; Swanson, B.; McQuinn, C.W.; Loftus, S.; Nordquist, E.; Cruz-Monserrate, Z.; Yu, L.; Young, G.; et al. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut 2016. [Google Scholar] [CrossRef] [PubMed]
- Tewari, N.; Zaitoun, A.M.; Arora, A.; Madhusudan, S.; Ilyas, M.; Lobo, D.N. The presence of tumour-associated lymphocytes confers a good prognosis in pancreatic ductal adenocarcinoma: An immunohistochemical study of tissue microarrays. BMC Cancer 2013, 13, 436. [Google Scholar] [CrossRef] [PubMed]
- Homma, Y.; Taniguchi, K.; Murakami, T.; Nakagawa, K.; Nakazawa, M.; Matsuyama, R.; Mori, R.; Takeda, K.; Ueda, M.; Ichikawa, Y.; et al. Immunological impact of neoadjuvant chemoradiotherapy in patients with borderline resectable pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 2014, 21, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Nagano, S.; Ichise, H.; Kataoka, K.; Yamada, D.; Ogawa, S.; Koseki, H.; Kitawaki, T.; Kadowaki, N.; Takaori-Kondo, A.; et al. Regeneration of CD8αβ T Cells from T-cell-derived iPSC imparts potent tumor antigen-specific cytotoxicity. Cancer Res. 2016, 76, 6839–6850. [Google Scholar] [CrossRef] [PubMed]
- McKallip, R.J.; Ban, H.; Uchakina, O.N. Treatment with the hyaluronic Acid synthesis inhibitor 4-methylumbelliferone suppresses LPS-induced lung inflammation. Inflammation 2015, 38, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Colombaro, V.; Declèves, A.E.; Jadot, I.; Voisin, V.; Giordano, L.; Habsch, I.; Nonclercq, D.; Flamion, B.; Caron, N. Inhibition of hyaluronan is protective against renal ischaemia-reperfusion injury. Nephrol. Dial. Transplant. 2013, 28, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Kozawa, E.; Urakawa, H.; Arai, E.; Futamura, N.; Zhuo, L.; Kimata, K.; Ishiguro, N.; Nishida, Y. Suppression of hyaluronan synthesis alleviates inflammatory responses in murine arthritis and in human rheumatoid synovial fibroblasts. Arthritis Rheum. 2013, 65, 1160–1170. [Google Scholar] [CrossRef] [PubMed]

| No. | Substance | Cells | Author (year) | Ref. |
|---|---|---|---|---|
| 1. | anti-inflammatory steroids | Human skin fibroblasts | Saarni, H. et al. (1978) | [37] |
| 2. | Monensin | Rat fibrosarcoma cells | Goldberg, R.L. and Toole, B.P. (1983) | [38] |
| 3. | Cyclofenil diphemol | Rat chondrocytes | Mason, R.M. et al. (1984) | [39] |
| 4. | Periodae-oxidized UDP-GlcNAc | Human fibrosarcoma cells | Prehm P. (1985) | [40] |
| 5. | n-Butylate | Rat liver fat-storing cells | Gressner, A.M. and Haarmann, R. (1988) | [41] |
| 6. | Dexamethasone | Human skin fibroblasts | Smith, T.J. (1988) | [42] |
| 7. | All-trans retinoic acid | Human skin fibroblasts | Smith, T.J. (1990) | [43] |
| 8. | p-Nitrophenol-β-d-xyloside | Rat liver fat-storing cells | Gressner, A.M. (1991) | [44] |
| 9. | Genistein | Rabbit mesothelial cells | Honda, A. et al. (1991) | [45] |
| 10. | Suramin | Mouse skin fibroblasts | August, E.M. et al. (1993) | [46] |
| 11. | Vanadate | Mouse skin fibroblasts | Zaharevitz, D.W. et al. (1993) | [47] |
| 12. | 4-methylumbelliferone | Human skin fibroblasts | Nakamura, T. et al. (1995) | [48] |
| 13. | Fluoxetine, amitriptyline | Human synovial cells | Yaron, I. et al. (1999) | [49] |
| 14. | Vesnarinone | Human myofibroblasts | Ueki, N. et al. (2000) | [50] |
| 15. | Mannose | Human myofibroblasts | Jokela, T.A. et al. (2008) | [51] |
| 16. | Methyl-β-cyclodextrin | Human breast cancer cells | Kultti, A. et al. (2010) | [52] |
| 17. | Estradiol | Human vascular smooth muscle cells | Freudenberger, T. et al (2011) | [53] |
| 18. | Collagen fragments | Human skin fibroblasts | Röck, K. et al. (2011) | [54] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudo, D.; Suto, A.; Hakamada, K. The Development of a Novel Therapeutic Strategy to Target Hyaluronan in the Extracellular Matrix of Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2017, 18, 600. https://doi.org/10.3390/ijms18030600
Kudo D, Suto A, Hakamada K. The Development of a Novel Therapeutic Strategy to Target Hyaluronan in the Extracellular Matrix of Pancreatic Ductal Adenocarcinoma. International Journal of Molecular Sciences. 2017; 18(3):600. https://doi.org/10.3390/ijms18030600
Chicago/Turabian StyleKudo, Daisuke, Akiko Suto, and Kenichi Hakamada. 2017. "The Development of a Novel Therapeutic Strategy to Target Hyaluronan in the Extracellular Matrix of Pancreatic Ductal Adenocarcinoma" International Journal of Molecular Sciences 18, no. 3: 600. https://doi.org/10.3390/ijms18030600





