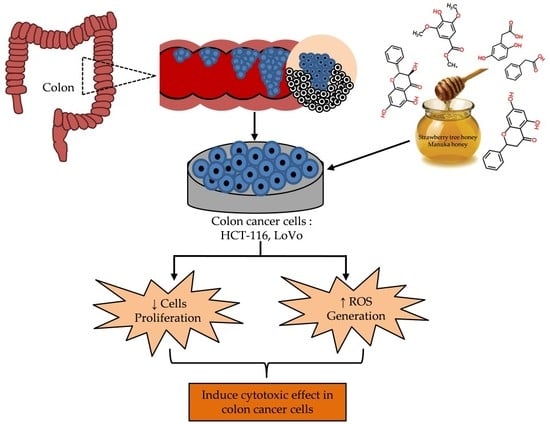
Strawberry-Tree Honey Induces Growth Inhibition of Human Colon Cancer Cells and Increases ROS Generation: A Comparison with Manuka Honey
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Content of STH and MH
2.2. Total Protein and Free Amino Acid Content of STH and MH
2.3. Total Antioxidant Capacity of STH and MH
2.4. Correlations between Biochemical Parameters and Antioxidant Potentials of STH and MH
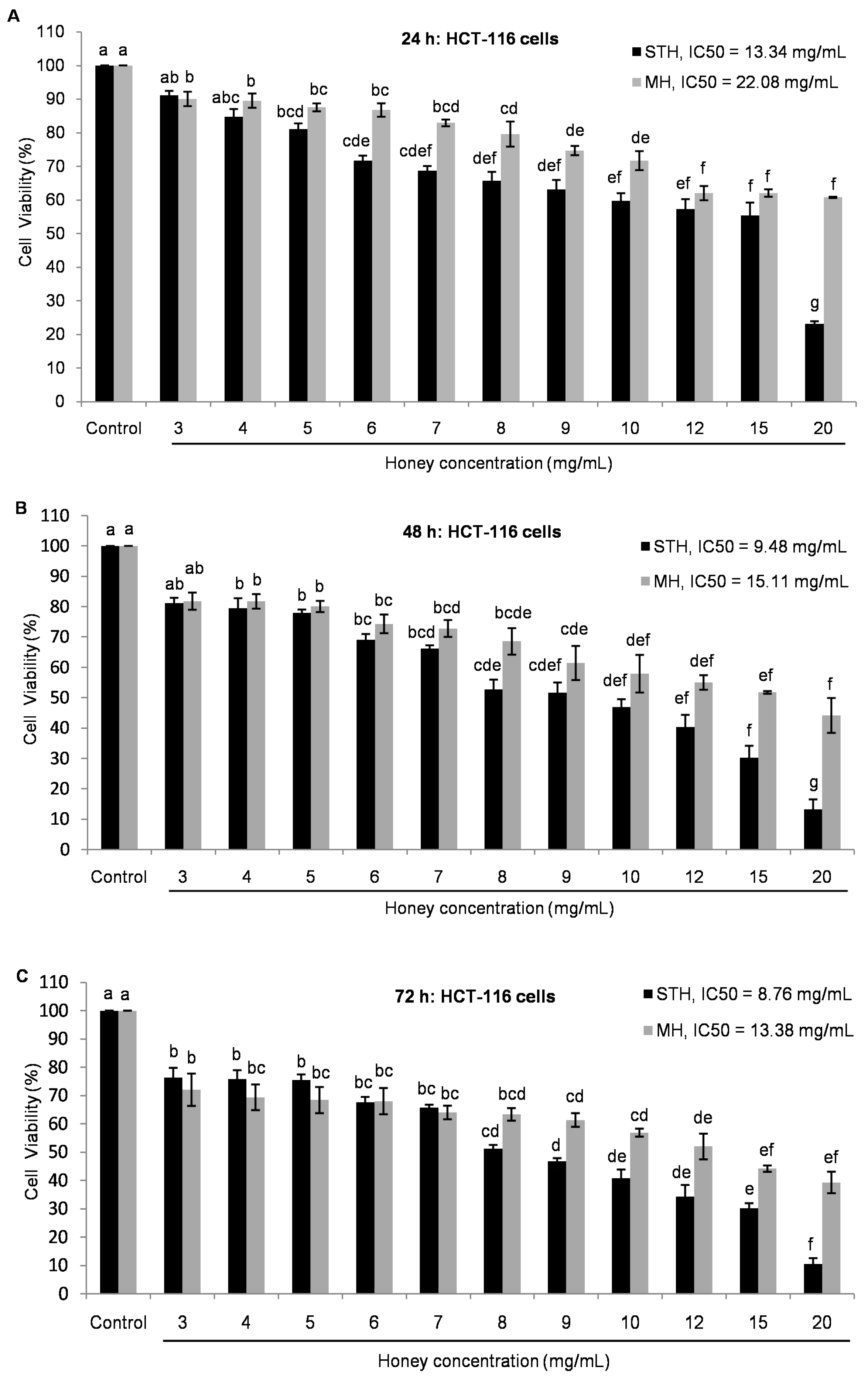
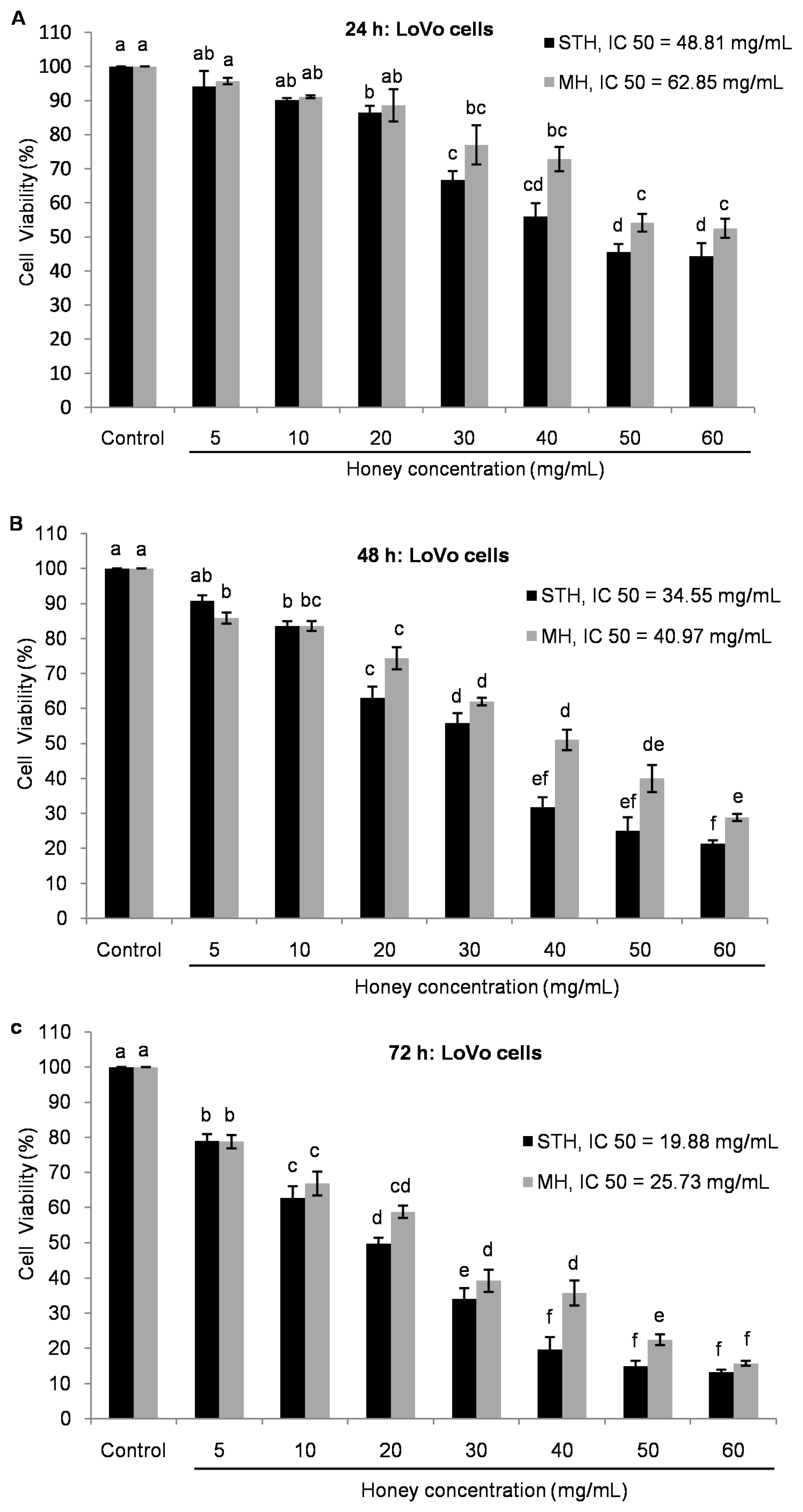
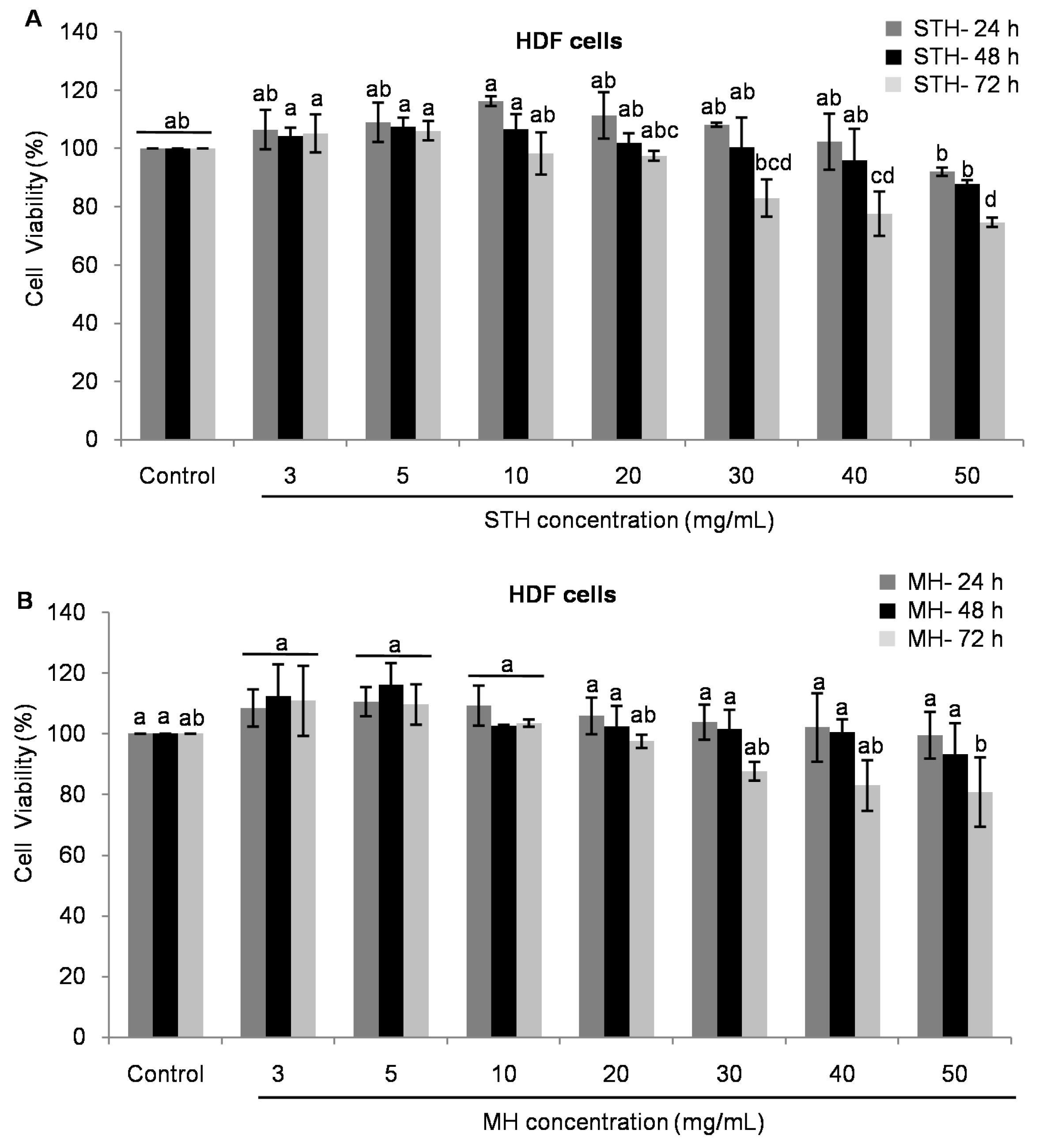
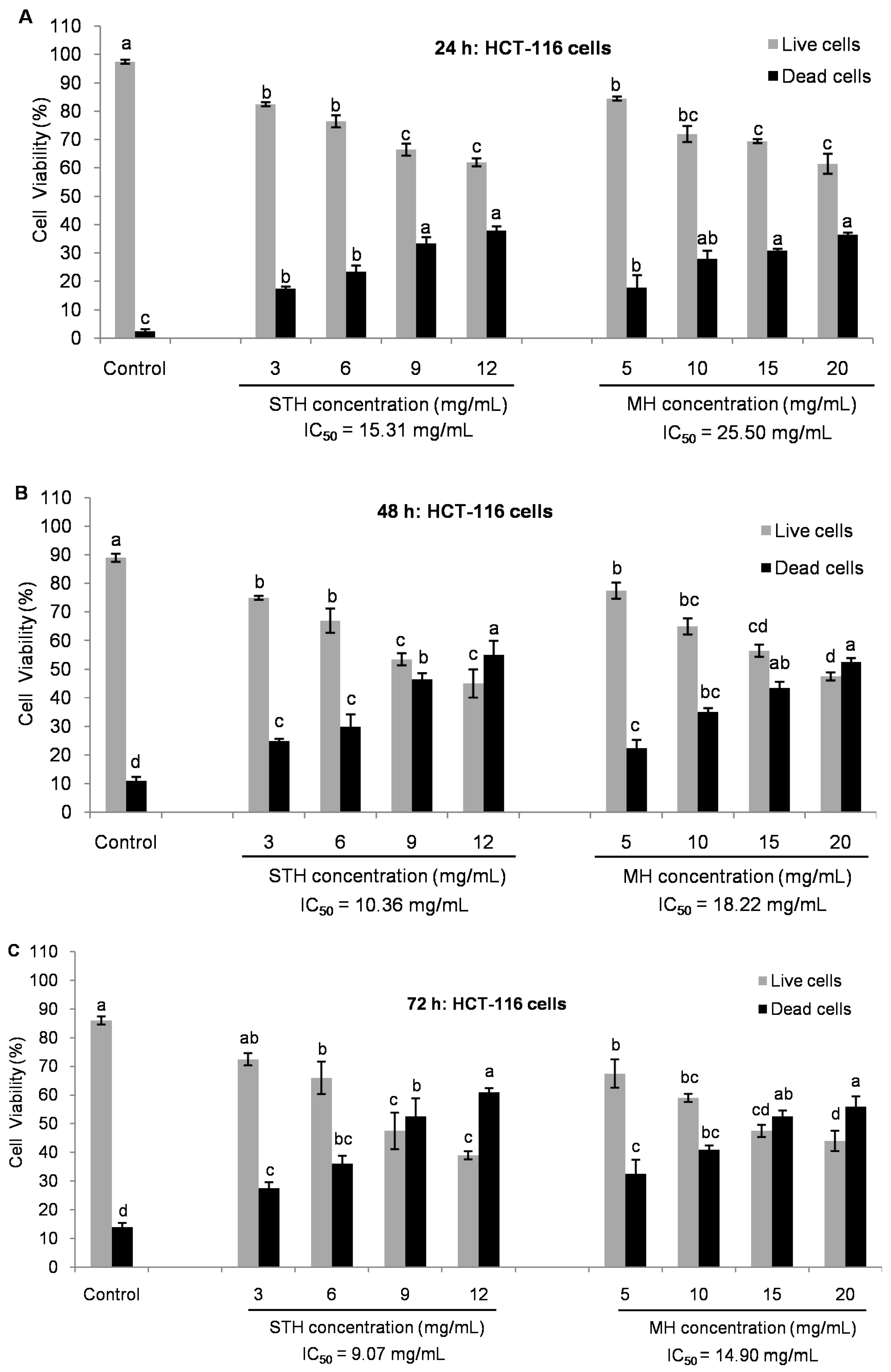
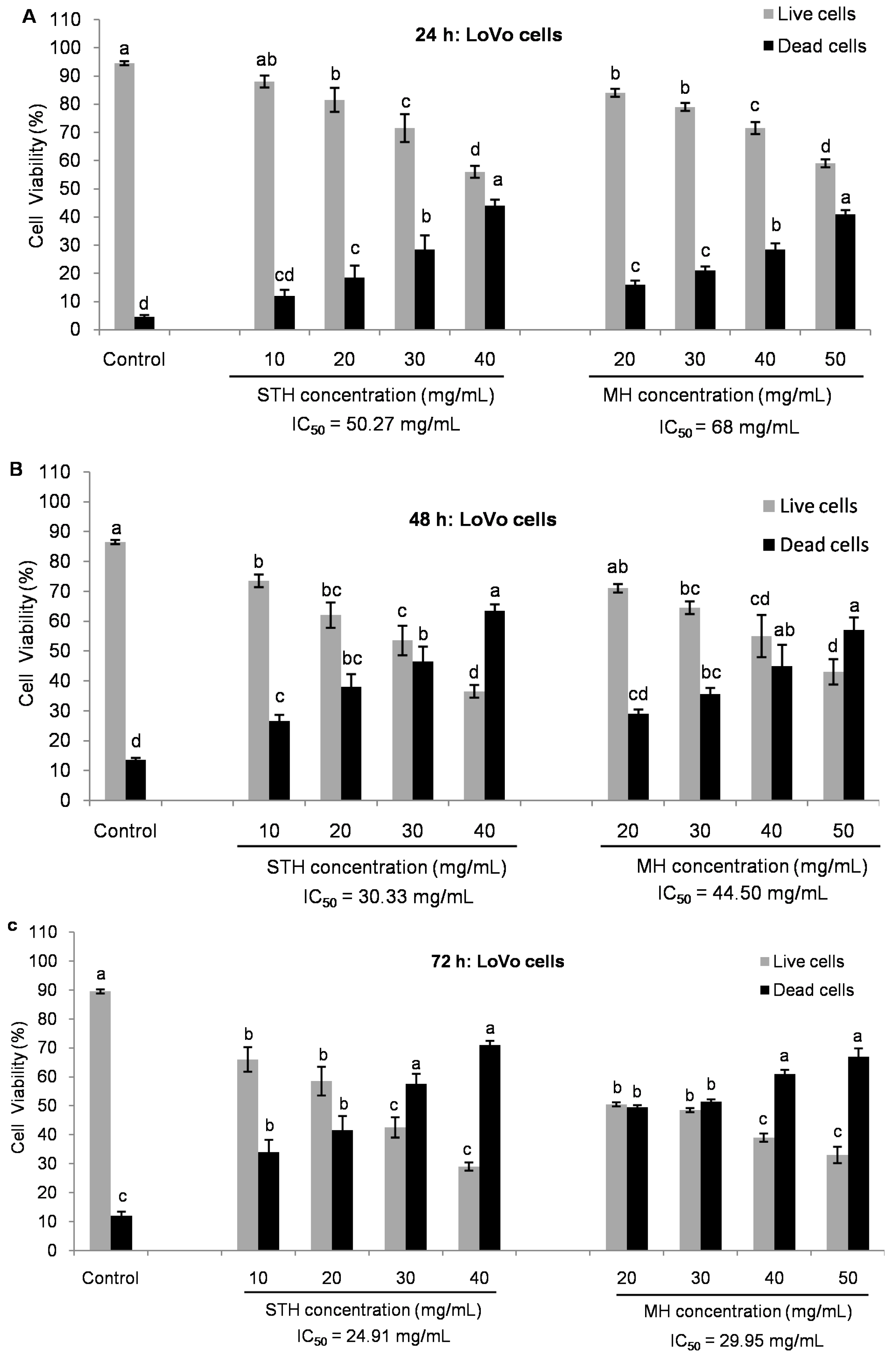
2.5. Cytotoxic Effects MH and STH on HCT-116, Lovo and HDFcells
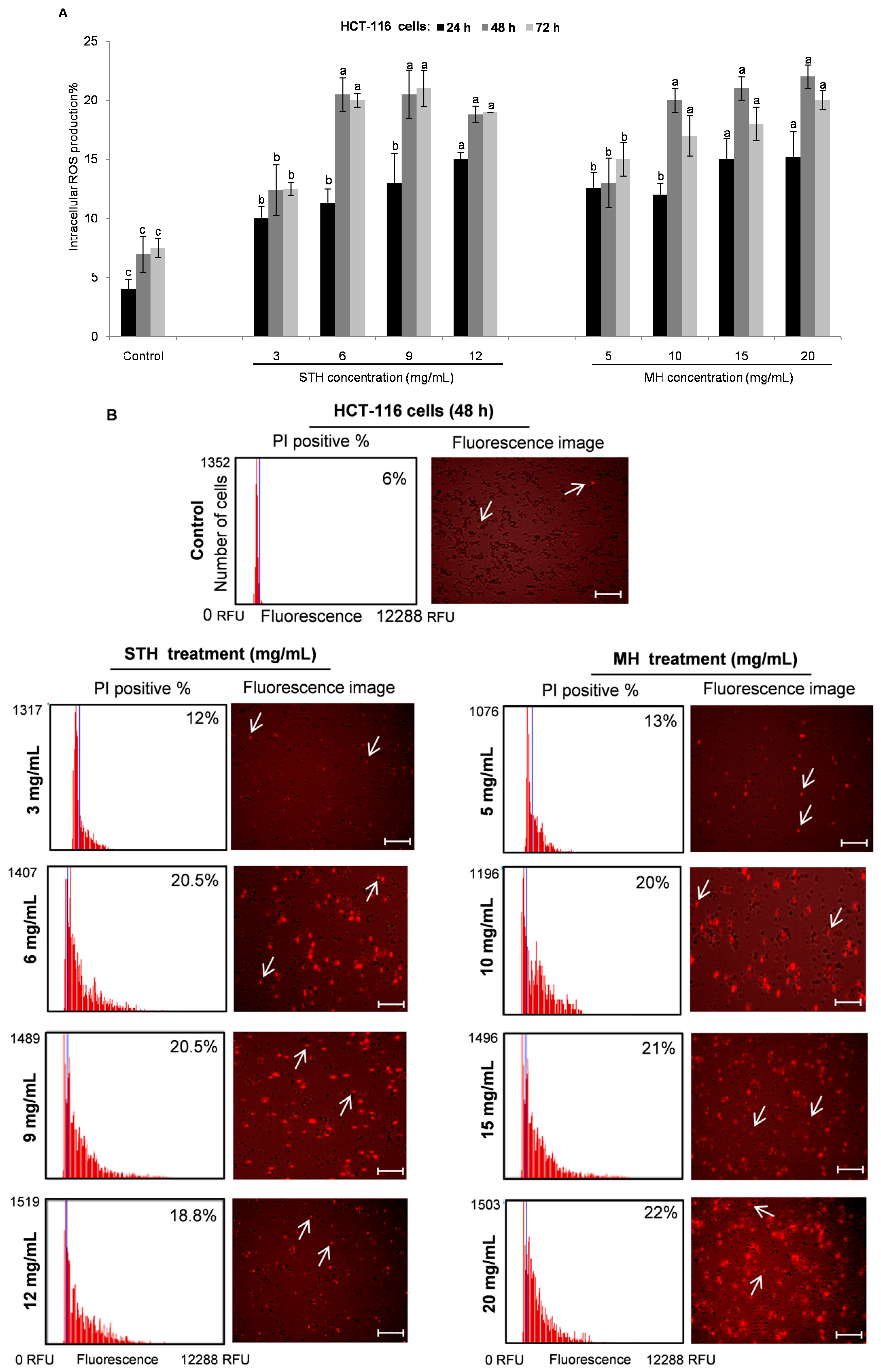
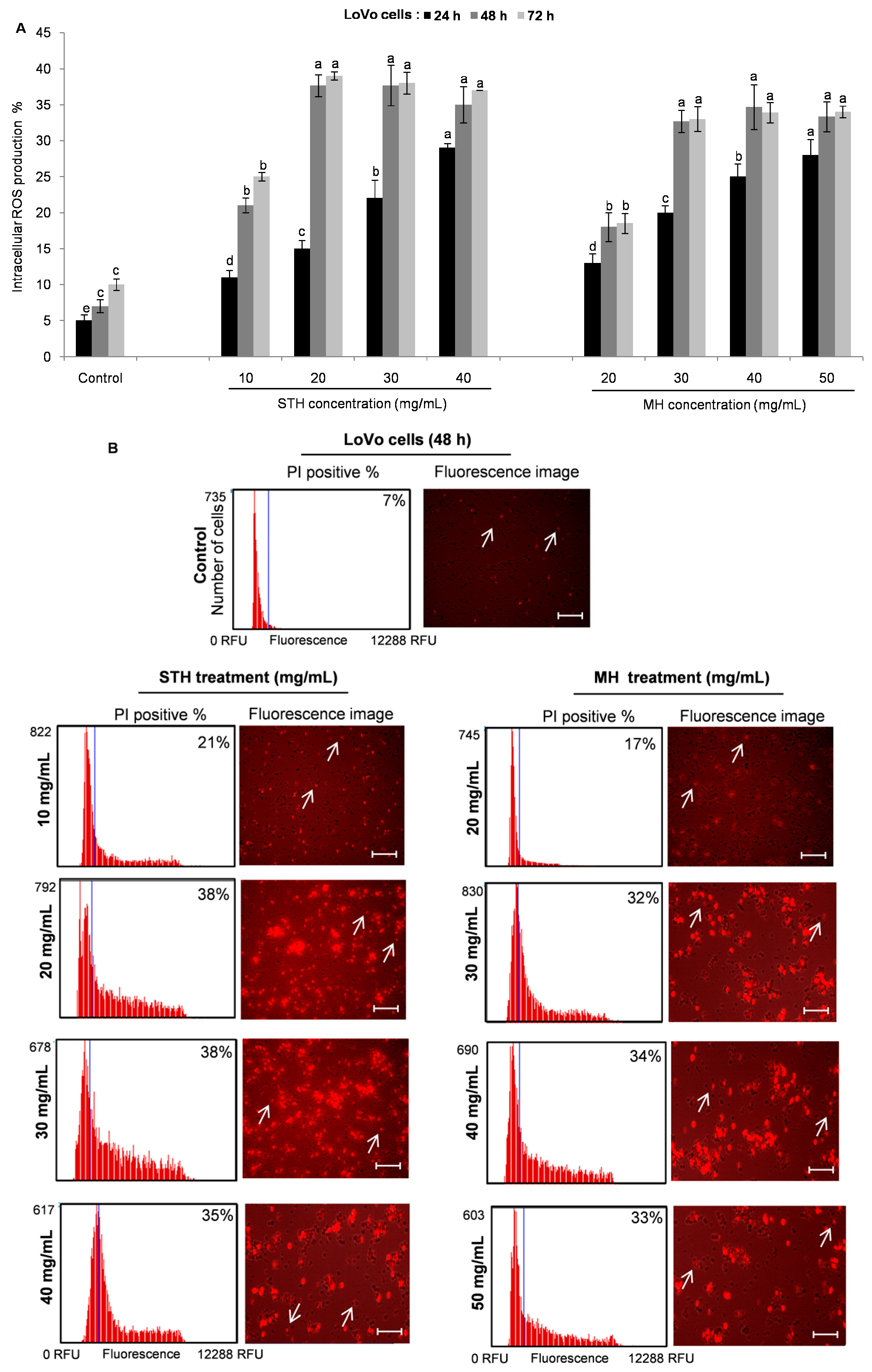
2.6. Intracellular ROS Production by STH and MH on HCT-116 and LoVo Cells
3. Materials and Methods
3.1. Honey Samples
3.2. Chemicals and Reagents
3.3. Determination of TPC and TFC
3.4. Determination of Total Protein and Free Amino acid Content
3.5. Determination of TAC
3.6. Cell Culture
3.7. Determination of Cell Survival Rate by MTT Assay
3.8. Measuring Cell Viability by TALI® Viability Assay
3.9. Determination of Intracellular ROS Generation
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in globocan 2012. Int. J. Cancer 2015, 136, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Tenesa, A.; Dunlop, M.G. New insights into the aetiology of colorectal cancer from genome-wide association studies. Nat. Rev. Genet. 2009, 10, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Dahan, L.; Sadok, A.; Formento, J.-L.; Seitz, J.F.; Kovacic, H. Modulation of cellular redox state underlies antagonism between oxaliplatin and cetuximab in human colorectal cancer cell lines. Br. J. Pharmacol. 2009, 158, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Varela-López, A.; Quiles, J.L.; Mezzetti, B.; Battino, M. Chemopreventive and therapeutic effects of edible berries: A focus on colon cancer prevention and treatment. Molecules 2016, 21, 169. [Google Scholar] [CrossRef]
- Ruiz, R.B.; Hernández, P.S. Cancer chemoprevention by dietary phytochemicals: Epidemiological evidence. Maturitas 2016, 94, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.A. Effects of honey and its mechanisms of action on the development and progression of cancer. Molecules 2014, 19, 2497–2522. [Google Scholar] [CrossRef] [PubMed]
- Kumar Jaganathan, S.; Balaji, A.; Vignesh Vellayappan, M.; Kumar Asokan, M.; Priyadharshni Subramanian, A.; Aruna John, A.; Supriyanto, E.; Izwan Abd Razak, S.; Marvibaigi, M. A review on antiproliferative and apoptotic activities of natural honey. Anticancer Agents Med. Chem. 2015, 15, 48–56. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Giampieri, F. The composition and biological activity of honey: A focus on Manuka honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Candiracci, M.; Piatti, E.; Dominguez-Barragán, M.; García-Antrás, D.; Morgado, B.; Ruano, D.; Gutiérrez, J.F.; Parrado, J.; Castaño, A.L. Anti-inflammatory activity of a honey flavonoid extract on lipopolysaccharide-activated N13 microglial cells. J. Agric. Food Chem. 2012, 60, 12304–12311. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; Damiani, E.; Astolfi, P.; Fattorini, D.; Regoli, F.; Quiles, J.L.; Battino, M. Radical-scavenging activity, protective effect against lipid peroxidation and mineral contents of monofloral Cuban honeys. Plant Foods Hum. Nutr. 2012, 67, 31–38. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Tulipani, S.; Díaz, D.; Estevez, Y.; Romandini, S.; Giampieri, F.; Damiani, E.; Astolfi, P.; Bompadre, S.; Battino, M. Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food Chem. Toxicol. 2010, 48, 2490–2499. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; Battino, M. Honey as a source of dietary antioxidants: Structures, bioavailability and evidence of protective effects against human chronic diseases. Curr. Med. Chem. 2013, 20, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Tuberoso, C.I.G.; Atzeri, A.; Melis, M.P.; Bifulco, E.; Dessì, M.A. Antioxidant profile of strawberry tree honey and its marker homogentisic acid in several models of oxidative stress. Food Chem. 2011, 129, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, P.A.; Maia, M.; Brigas, A.F. Physicochemical parameters and bioactive compounds of strawberry tree (Arbutus unedo L.) honey. J. Chem. 2015, 2015, 602792. [Google Scholar] [CrossRef]
- Ciulu, M.; Spano, N.; Pilo, M.I.; Sanna, G. Recent advances in the analysis of phenolic compounds in unifloral honeys. Molecules 2016, 21, 451. [Google Scholar] [CrossRef] [PubMed]
- Spano, N.; Ciulu, M.; Floris, I.; Panzanelli, A.; Pilo, M.I.; Piu, P.C.; Salis, S.; Sanna, G. A direct RP-HPLC method for the determination of furanic aldehydes and acids in honey. Talanta 2009, 78, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Spano, N.; Piras, I.; Ciulu, M.; Floris, I.; Panzanelli, A.; Pilo, M.I.; Piu, P.C.; Sanna, G. Reversed-phase liquid chromatographic profile of free amino acids in strawberry-tree (Arbutus unedo L.) honey. J. AOAC Int. 2009, 92, S1145–S1156. [Google Scholar]
- Tuberoso, C.I.G.; Boban, M.; Bifulco, E.; Budimir, D.; Pirisi, F.M. Antioxidant capacity and vasodilatory properties of Mediterranean food: The case of Cannonau wine, myrtle berries liqueur and strawberry-tree honey. Food Chem. 2013, 140, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Cherchi, A.; Spanedda, L.; Tuberoso, C.; Cabras, P. Solid-phase extraction and high-performance liquid chromatographic determination of organic acids in honey. J. Chromatogr. A 1994, 669, 59–64. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A.; Tuberoso, C.; Floris, I.; Reniero, F.; Guillou, C.; Ghelli, S. Homogentisic acid: A phenolic acid as a marker of strawberry-tree (Arbutus unedo) honey. J. Agric. Food Chem. 1999, 47, 4064–4067. [Google Scholar] [CrossRef] [PubMed]
- Scanu, R.; Spano, N.; Panzanelli, A.; Pilo, M.I.; Piu, P.C.; Sanna, G.; Tapparo, A. Direct chromatographic methods for the rapid determination of homogentisic acid in strawberry tree (Arbutus unedo L.) honey. J. Chromatogr. A 2005, 1090, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Spano, N.; Casula, L.; Panzanelli, A.; Pilo, M.I.; Piu, P.C.; Scanu, R.; Tapparo, A.; Sanna, G. An RP-HPLC determination of 5-hydroxymethylfurfural in honey: The case of strawberry tree honey. Talanta 2006, 68, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.A.; Blair, S.E.; Cokcetin, N.N.; Bouzo, D.; Brooks, P.; Schothauer, R.; Harry, E.J. Therapeutic Manuka honey: No longer so alternative. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Giampieri, F.; Cordero, M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Afrin, S.; Beltrán-Ayala, P.; González-Paramás, A.M.; Santos-Buelga, C. Activation of AMPK/Nrf2 signalling by Manuka honey protects human dermal fibroblasts against oxidative damage by improving antioxidant response and mitochondrial function promoting wound healing. J. Funct. Foods 2016, 25, 38–49. [Google Scholar] [CrossRef]
- Bischofberger, A.S.; Dart, C.M.; Horadagoda, N.; Perkins, N.R.; Jeffcott, L.B.; Little, C.B.; Dart, A.J. Effect of Manuka honey gel on the transforming growth factor β1 and β3 concentrations, bacterial counts and histomorphology of contaminated full-thickness skin wounds in equine distal limbs. Aust. Vet. J. 2016, 94, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.W.; Deadman, B.J.; Manley-Harris, M.; Wilkins, A.L.; Alber, D.G.; Harry, E. Analysis of the flavonoid component of bioactive New Zealand Mänuka (Leptospermum scoparium) honey and the isolation, characterisation and synthesis of an unusual pyrrole. Food Chem. 2013, 141, 1772–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cabezudo, M.J.; El-Kharrag, R.; Torab, F.; Bashir, G.; George, J.A.; El-Taji, H.; Al-Ramadi, B.K. Intravenous administration of Manuka honey inhibits tumor growth and improves host survival when used in combination with chemotherapy in a melanoma mouse model. PLoS ONE 2013, 8, e55993. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Mandal, M. Honey constituents and its apoptotic effect in colon cancer cells. J. Apiproduct Apimedical Sci. 2009, 1, 29–36. [Google Scholar] [CrossRef]
- Hakim, L.; Alias, E.; Makpol, S.; Ngah, W.Z.; Morad, N.A.; Yusof, Y.A. Gelam honey and ginger potentiate the anti cancer effect of 5-FU against HCT116 colorectal cancer cells. Asian Pac. J. Cancer Prev. 2013, 15, 4651–4657. [Google Scholar] [CrossRef]
- Tahir, A.A.; Sani, N.F.A.; Murad, N.A.; Makpol, S.; Ngah, W.Z.W.; Yusof, Y.A.M. Combined ginger extract & gelam honey modulate Ras/ERK and PI3K/AKT pathway genes in colon cancer HT29 cells. Nutr. J. 2015, 14, 1–10. [Google Scholar]
- Wee, L.H.; Morad, N.A.; Aan, G.J.; Makpol, S.; Ngah, W.Z.W.; Yusof, Y.A.M. Mechanism of chemoprevention against colon cancer cells using combined gelam honey and ginger extract via mTOR and Wnt/β-catenin pathways. Asian Pac. J. Cancer Prev. 2015, 16, 6549–6556. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.T.P.; Hussein, S.Z.; Abdullah, S.; Karim, N.A.; Makpol, S.; Yusof, Y.A.M. Gelam and nenas honeys inhibit proliferation of HT29 colon cancer cells by inducing DNA damage and apoptosis while suppressing inflammation. Asian Pac. J. Cancer Prev. 2012, 13, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, H.A.; Alsabehi, R.; Boukraâ, L.; Abdellah, F.; Bellik, Y.; Bakhotmah, B.A. Antibacterial and antioxidant potency of floral honeys from different botanical and geographical origins. Molecules 2012, 17, 10540–10549. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Sirajudeen, K.N.S.; Swamy, M.; Yaacob, M.; Sulaiman, S. Studies on the antioxidant properties of Tualang honey of Malaysia. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 59–63. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Khalil, M.I.; Moniruzzaman, M.; Boukraâ, L.D.; Benhanifia, M.; Islam, M.A.; Islam, M.N.; Sulaiman, S.A.; Gan, S.H. Physicochemical and antioxidant properties of Algerian honey. Molecules 2012, 17, 11199–11215. [Google Scholar] [CrossRef] [PubMed]
- Aazza, S.; Lyoussi, B.; Antunes, D.; Miguel, M.G.A. Physicochemical characterization and antioxidant activity of commercial Portuguese honeys. J. Food Sci. 2013, 78, C1159–C1165. [Google Scholar] [CrossRef] [PubMed]
- Kishore, R.K.; Halim, A.S.; Syazana, M.S.N.; Sirajudeen, K.N.S. Tualang honey has higher phenolic content and greater radical scavenging activity compared with other honey sources. Nutr. Res. 2011, 31, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Sulaiman, S.A.; Khalil, M.I.; Gan, S.H. Evaluation of physicochemical and antioxidant properties of sourwood and other Malaysian honeys: A comparison with Manuka honey. Chem. Cent. J. 2013, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M.; Tulipani, S.; Romandini, S.; Bertoli, E.; Battino, M. Contribution of honey in nutrition and human health: A review. Med. J. Nutr. Metab. 2010, 3, 15–23. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Islam, A.; Khalil, I.; Islam, N.; Moniruzzaman, M.; Mottalib, A.; Sulaiman, S.A.; Gan, S.H. Physicochemical and antioxidant properties of Bangladeshi honeys stored for more than one year. BMC Complement. Alter. Med. 2012, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.; Nigam, P.S.; Owusu-Apenten, R.K. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food Chem. 2015, 174, 119–123. [Google Scholar]
- Saxena, S.; Gautam, S.; Sharma, A. Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem. 2010, 118, 391–397. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mandal, M. Antiproliferative effects of honey and of its polyphenols: A review. BioMed Res. Int. 2009, 2009, 830616. [Google Scholar] [CrossRef] [PubMed]
- Hornsveld, M.; Dansen, T.B. The hallmarks of cancer from a redox perspective. Antioxid. Redox Signal. 2016, 25, 300–325. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Mandal, M. Involvement of non-protein thiols, mitochondrial dysfunction, reactive oxygen species and p53 in honey-induced apoptosis. Investig. New Drugs 2010, 28, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Kim, N.-H.; Paul, S.; Jakhar, R.; Han, J.; Kang, S.C. 5-Hydroxy-7-methoxyflavone triggers mitochondrial-associated cell death via reactive oxygen species signaling in human colon carcinoma cells. PLoS ONE 2016, 11, e0154525. [Google Scholar] [CrossRef] [PubMed]
- Sankpal, U.T.; Nagaraju, G.P.; Gottipolu, S.R.; Hurtado, M.; Jordan, C.G.; Simecka, J.W.; Shoji, M.; El-Rayes, B.; Basha, R. Combination of tolfenamic acid and curcumin induces colon cancer cell growth inhibition through modulating specific transcription factors and reactive oxygen species. Oncotarget 2016, 7, 3186. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Doi, E.; Shibata, D.; Matoba, T. Modified colorimetric ninhydrin methods for peptidase assay. Anal. Biochem. 1981, 118, 173–184. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Remple, K.; Stone, L. Assessment of GFP expression and viability using the Tali image-based cytometer. J. Vis. Exp. 2011, 57, 3659. [Google Scholar] [CrossRef] [PubMed]
| Type of Honey | Total Polyphenols | Total Flavonoids |
|---|---|---|
| (g GAE/Kg) | (mg CAE/kg) | |
| Strawberry Tree Honey | ||
| Monti | 0.86 ± 0.01 b | 92.68 ± 14.17 a,b |
| Luras | 0.77 ± 0.02 b,c | 69.96 ± 3.62 b,c |
| Sadali | 0.76 ± 0.02 b,c | 65.74 ± 2.50 c |
| Olbia | 0.69 ± 0.01 c | 66.18 ± 0.61 c |
| Berchidda | 1.00 ± 0.02 a | 108.20 ± 2.69 a |
| Manuka Honey | 0.89 ± 0.01 a,b | 71.90 ± 0.03 b,c |
| Type of Honey | Total Protein | Total Free Amino Acids |
|---|---|---|
| (g BSA/100 g) | (mg LE/100 g) | |
| Strawberry Tree Honey | ||
| Monti | 0.04 ± 0.00 b,c | 14.56 ± 0.93 b |
| Luras | 0.03 ± 0.00 c | 12.86 ± 0.05 b |
| Sadali | 0.03 ± 0.00 c | 10.28 ± 0.86 b |
| Olbia | 0.04 ± 0.01 b,c | 13.18 ± 1.35 b |
| Berchidda | 0.07 ± 0.00 a | 51.67 ± 9.64 a |
| Manuka Honey | 0.05 ± 0.00 a,b | 14.34 ±0.13 b |
| Type of Honey | FRAP Values | TEAC Values | DPPH Values | |
|---|---|---|---|---|
| mmol TE/100 g | mmol Fe(II)/100 g | mmol TE/100 g | mmol TE/100 g | |
| Strawberry Tree Honey | ||||
| Monti | 0.39 ± 0.00 b | 0.81 ± 0.00 b | 0.10 ± 0.00 c | 0.09 ± 0.00 b |
| Luras | 0.30 ± 0.00 c | 0.68 ± 0.00 c | 0.11 ± 0.00 c | 0.09 ± 0.00 b |
| Sadali | 0.24 ± 0.00 d | 0.63 ± 0.00 d | 0.11 ± 0.00 c | 0.09 ± 0.00 b |
| Olbia | 0.21 ± 0.00 e | 0.51 ± 0.00 e | 0.11 ± 0.00 c | 0.09 ± 0.00 b |
| Berchidda | 0.54 ± 0.00 a | 0.92 ± 0.02 a | 0.39 ± 0.01 a | 0.20 ± 0.01 a |
| Manuka Honey | 0.14 ± 0.00 f | 0.29 ± 0.00 f | 0.22 ± 0.00 b | 0.06 ± 0.00 c |
| Variable | TPC | TFC | FRAP | TEAC | DPPH | Protein |
|---|---|---|---|---|---|---|
| TFC | 0.856 * | |||||
| FRAP | 0.663 * | 0.878 ** | ||||
| TEAC | 0.870 ** | 0.678 * | 0.586 ns | |||
| DPPH | 0.678 * | 0.796 ** | 0.807 ** | 0.704 ** | ||
| Protein | 0.863 ** | 0.817 * | 0.660 * | 0.923 ** | 0.772 ** | |
| Free AA | 0.728 * | 0.740 * | 0.694 * | 0.899 * | 0.922 ** | 0.947 ** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afrin, S.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Bompadre, S.; Quiles, J.L.; Sanna, G.; Spano, N.; Giampieri, F.; Battino, M. Strawberry-Tree Honey Induces Growth Inhibition of Human Colon Cancer Cells and Increases ROS Generation: A Comparison with Manuka Honey. Int. J. Mol. Sci. 2017, 18, 613. https://doi.org/10.3390/ijms18030613
Afrin S, Forbes-Hernandez TY, Gasparrini M, Bompadre S, Quiles JL, Sanna G, Spano N, Giampieri F, Battino M. Strawberry-Tree Honey Induces Growth Inhibition of Human Colon Cancer Cells and Increases ROS Generation: A Comparison with Manuka Honey. International Journal of Molecular Sciences. 2017; 18(3):613. https://doi.org/10.3390/ijms18030613
Chicago/Turabian StyleAfrin, Sadia, Tamara Y. Forbes-Hernandez, Massimiliano Gasparrini, Stefano Bompadre, José L. Quiles, Gavino Sanna, Nadia Spano, Francesca Giampieri, and Maurizio Battino. 2017. "Strawberry-Tree Honey Induces Growth Inhibition of Human Colon Cancer Cells and Increases ROS Generation: A Comparison with Manuka Honey" International Journal of Molecular Sciences 18, no. 3: 613. https://doi.org/10.3390/ijms18030613