Treatment with 17β-Estradiol Reduced Body Weight and the Risk of Cardiovascular Disease in a High-Fat Diet-Induced Animal Model of Obesity
Abstract
:1. Introduction
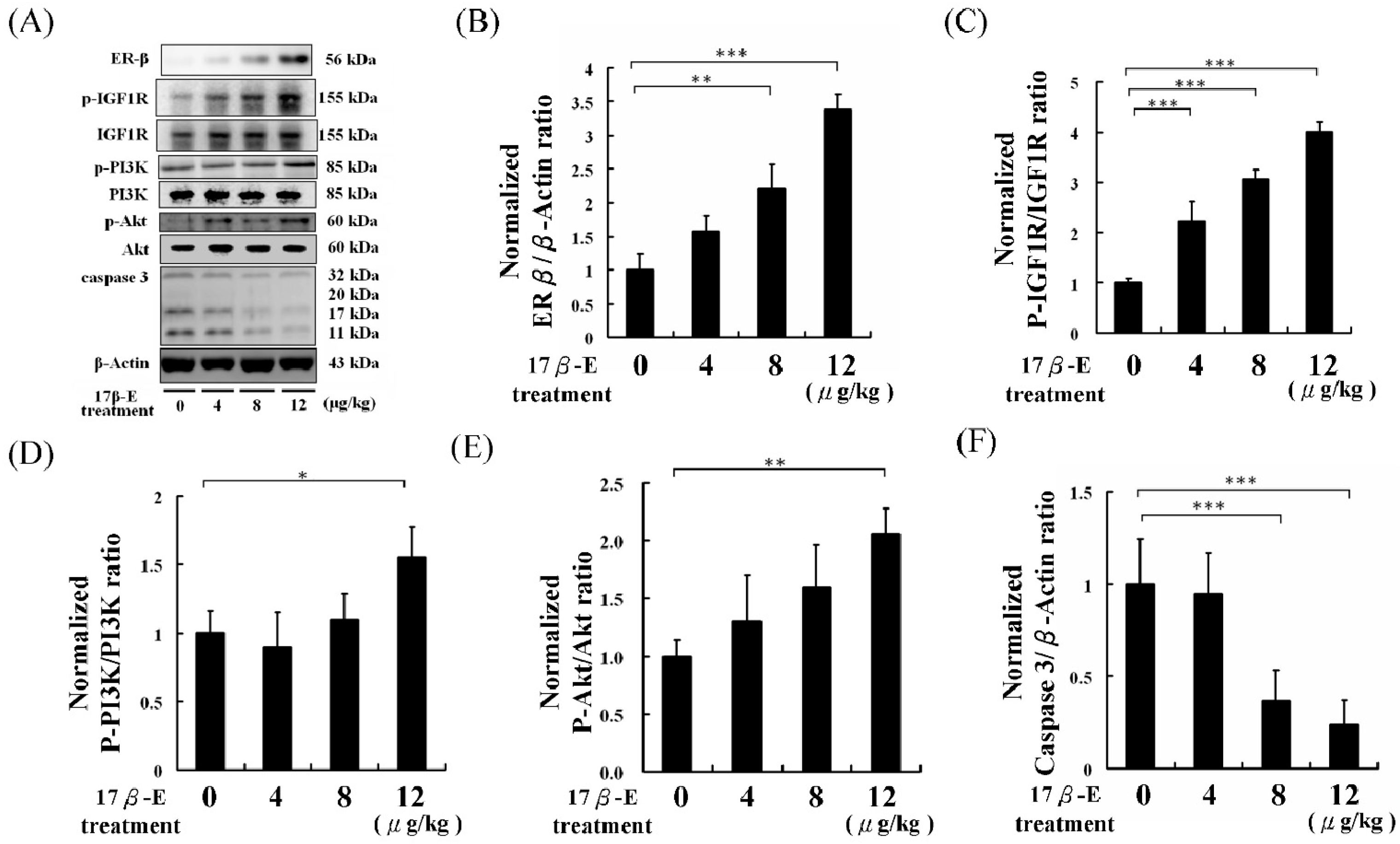
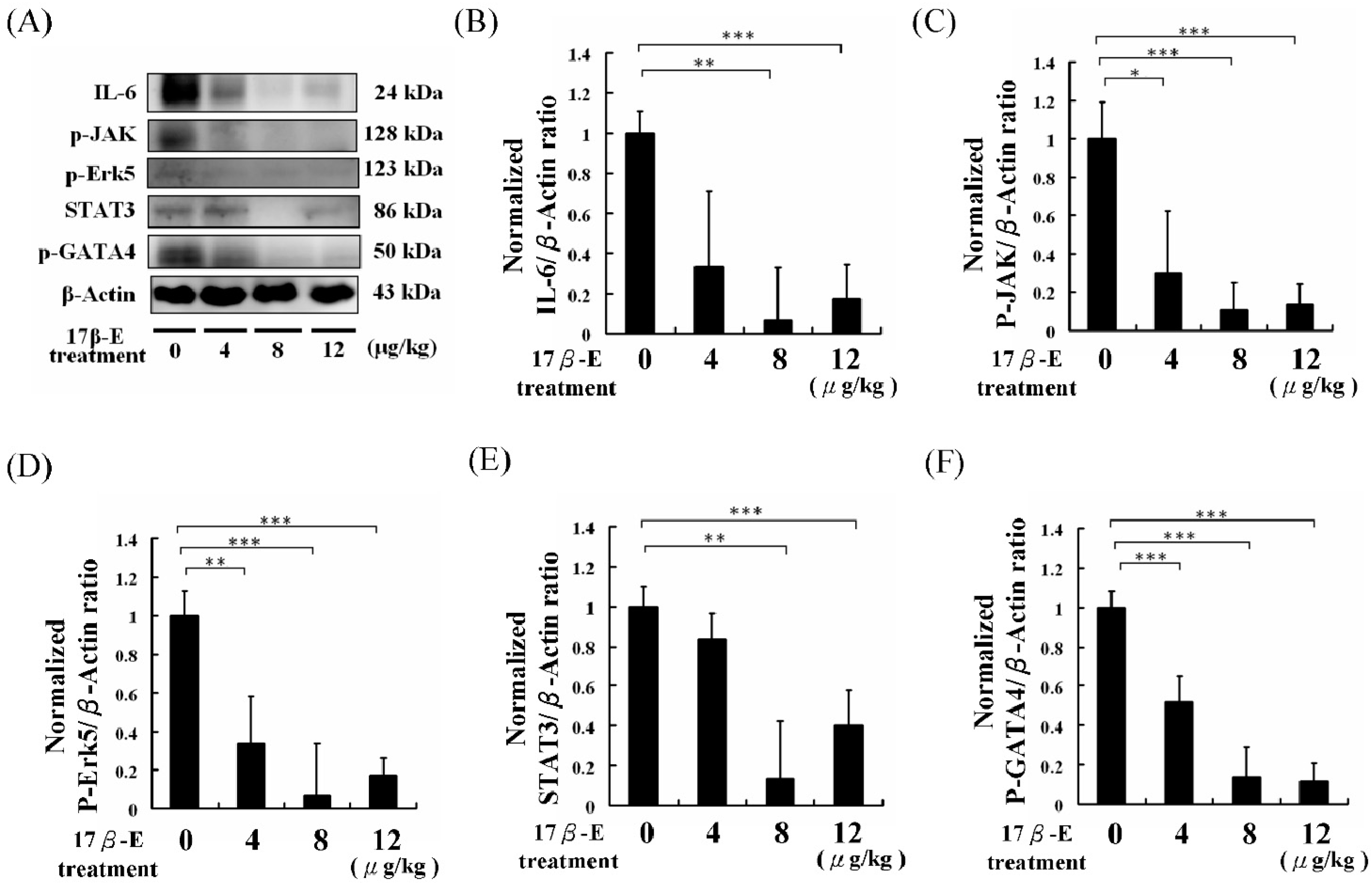
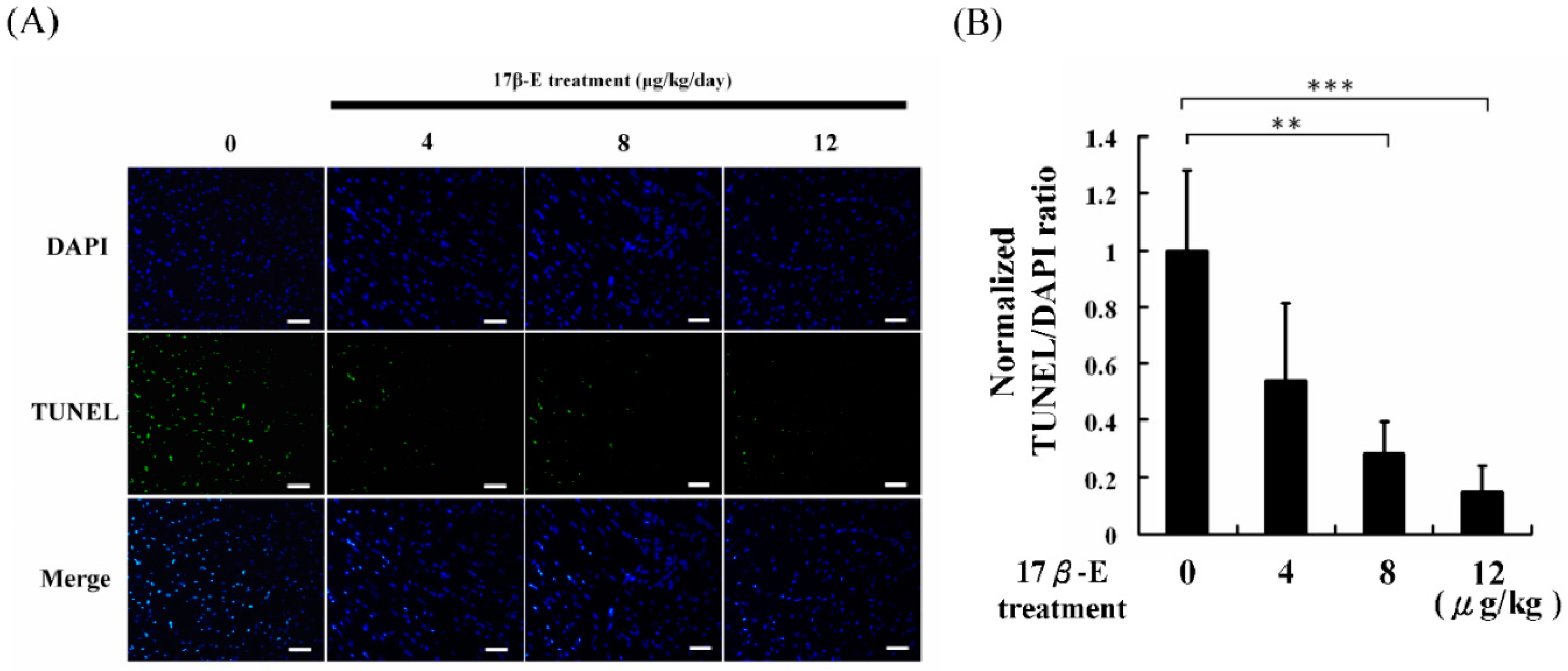
2. Results and Discussion
3. Experimental Section
3.1. Chemicals
3.2. Animals and Experimental Design
3.3. Preparation of Protein Extracts from Heart Tissue
3.4. Western Blot Analysis
3.5. Apoptosis Analysis
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mauvais-Jarvis, F. Estrogen and androgen receptors: Regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol. Metab. 2011, 22, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Meza-Muñoz, D.E.; Fajardo, M.E.; Pérez-Luque, E.L.; Malacara, J.M. Factors associated with estrogen receptors-α (ER-α) and -β (ER-β) and progesterone receptor abundance in obese and non obese pre- and post-menopausal women. Steroids 2006, 71, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Goulart, A.C.; Zee, R.Y.; Rexrode, K.M. Estrogen receptor 1 gene polymorphisms and decreased risk of obesity in women. Metabolism 2009, 58, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Lijnen, H.R. Murine models of obesity and hormonal therapy. Thromb. Res. 2011, 127, S17–S20. [Google Scholar] [CrossRef]
- Abramson, B.L.; Melvin, R.G. Cardiovascular risk in women: Focus on hypertension. Can. J. Cardiol. 2014, 30, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Marco, S.B.; Kim, M.F.; Silvia, G.P.; Peter, C.; Caroline, D.; Ian, G.; Benct, J.; Karin, S.G.; Michal, T. Cardiovascular diseases in women: A statement from the policy conference of the European Society of Cardiology. Eur. Heart J. 2006, 27, 994–1005. [Google Scholar]
- Chu, C.H.; Lo, J.F.; Hu, W.S.; Lu, R.B.; Chang, M.H.; Tsai, F.J.; Tsai, C.H.; Weng, Y.S.; Tzang, B.S.; Huang, C.Y. Histone acetylation is essential for ANG-II-induced IGF-IIR gene expression in H9c2 cardiomyoblast cells and pathologically hypertensive rat heart. J. Cell. Physiol. 2012, 227, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.H.; Kuo, W.W.; Li, P.C.; Lin, D.Y.; Lee, S.D.; Tsai, F.J.; Jong, G.P.; Lin, Y.M.; Huang, C.Y.; Wu, W.J. Down regulation of IGF-I and IGF-IR gene expression in right atria tissue of ventricular septal defect infants with right atria hypoxemia. Clin. Chim. Acta 2007, 379, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Lin, W.D.; Bau, D.T.; Chou, I.C.; Tsai, C.H.; Tsai, F.J. Appearance of acanthosis nigricans may precede obesity: An involvement of the insulin/IGF receptor signaling pathway. BioMedicine 2013, 3, 82–87. [Google Scholar] [CrossRef]
- Duran, J.; Oyarce, C.; Pavez, M.; Valladares, D.; Basualto-Alarcon, C.; Lagos, D.; Barrientos, G.; Troncoso, M.F.; Ibarra, C.; Estrada, M. GSK-3β/NFAT Signaling Is Involved in Testosterone-Induced Cardiac Myocyte Hypertrophy. PLoS ONE 2016, 11, e0168255. [Google Scholar] [CrossRef] [PubMed]
- Hirota, H.; Chen, J.; Betz, U.A.; Rajewsky, K.; Gu, Y.; Ross, J., Jr.; Müller, W.; Chien, K.R. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell 1999, 97, 189–198. [Google Scholar] [CrossRef]
- Kato, Y.; Kravchenko, V.V.; Tapping, R.I.; Han, J.; Ulevitch, R.J.; Lee, J.D. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 1997, 16, 7054–7066. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Marinissen, M.J.; Chiariello, M.; Gutkind, J.S. Signaling from G protein-coupled receptors to ERK5/Big MAPK 1 involves Gαq and Gα12/13 families of heterotrimeric G proteins. Evidence for the existence of a novel Ras and Rho-independent pathway. J. Biol. Chem. 2000, 275, 21730–21736. [Google Scholar] [CrossRef] [PubMed]
- Rebekka, L.N.; Norbert, F.; Gray, P.; Melanie, C.; James, R.; Eric, N.O. Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. EMBO J. 2001, 20, 2757–2767. [Google Scholar]
- Logan, S.M.; Tessier, S.N.; Tye, J.; Storey, K.B. Response of the JAK-STAT pathway to mammalian hibernation in 13-lined ground squirrel striated muscle. Mol. Cell. Biochem. 2016, 414, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Ihle, J.N. Cytokine receptor signalling. Nature 1995, 377, 591–594. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Gadina, M.; Schreiber, R.D. Cytokine signaling in 2002: New surprises in the Jak/Stat pathway. Cell 2002, 109, S121–S131. [Google Scholar] [CrossRef]
- Doppler, S.A.; Deutsch, M.A.; Lange, R.; Krane, M. Direct Reprogramming-The Future of Cardiac Regeneration? Int. J. Mol. Sci. 2015, 16, 17368–17393. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Krane, M.; Deutsch, M.A.; Wang, L.; Rav-Acha, M.; Gregoire, S.; Engels, M.C.; Rajarajan, K.; Karra, R.; Abel, E.D.; et al. Inefficient reprogramming of fibroblasts into cardiomyocytes using Gata4, Mef2c, and Tbx5. Circ. Res. 2012, 111, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Barry, S.P.; Townsend, P.A. What causes a broken heart—Molecular insights into heart failure. Int. Rev. Cell Mol. Biol. 2010, 284, 113–179. [Google Scholar] [PubMed]
- Von Kaisenberg, C.S.; Huggon, I.; Hyett, J.A.; Farzaneh, F.; Nicolaides, K.H. Cardiac gene expression of GATA-4 transcription factor in human trisomy 21 fetuses with increased nuchal translucency. Prenat. Diagn. 1998, 18, 267–271. [Google Scholar] [CrossRef]
- Weng, Y.S.; Wang, H.F.; Pai, P.Y.; Jong, G.P.; Lai, C.H.; Chung, L.C.; Hsieh, D.J.; HsuanDay, C.; Kuo, W.W.; Huang, C.Y. Tanshinone IIA Prevents Leu27IGF-II-Induced Cardiomyocyte Hypertrophy Mediated by Estrogen Receptor and Subsequent Akt Activation. Am. J. Chin. Med. 2015, 43, 1567–1591. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.L.; Chen, Y.S.; Lin, J.Y.; Tien, Y.C.; Peng, W.H.; Kuo, C.H.; Tzang, B.S.; Wang, H.L.; Tsai, F.J.; Chou, M.C.; et al. Neuron Regeneration and Proliferation Effects of Danshen and Tanshinone IIA. Evid. Based Complement. Altern. Med. 2011, 2011, 378907. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; O’Malley, B.W.; DeMayo, F.J. An estrogen receptor α activity indicator model in mice. Genesis 2009, 47, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Charn, T.H.; Liu, E.T.; Chang, E.C.; Lee, Y.K.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Genome-wide dynamics of chromatin binding of estrogen receptors α and β: Mutual restriction and competitive site selection. Mol. Endocrinol. 2010, 24, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Newbold, R.R.; Padilla-Banks, E.; Jefferson, W.N. Environmental estrogens and obesity. Mol. Cell. Endocrinol. 2009, 304, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Tabata, M.; Kadomatsu, T.; Fukuhara, S.; Miyata, K.; Ito, Y.; Endo, M.; Urano, T.; Zhu, H.J.; Tsukano, H.; Tazume, H.; et al. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 2009, 10, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Puurunen, V.P.; Lepojärvi, E.S.; Piira, O.P.; Hedberg, P.; Junttila, M.J.; Ukkola, O.; Huikuri, H.V. High plasma leptin levels are associated with impaired diastolic function in patients with coronary artery disease. Peptides 2016, 84, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Manrique, C.; Lastra, G.; Habibi, J.; Mugerfeld, I.; Garro, M.; Sowers, J.R. Loss of Estrogen Receptor α Signaling Leads to Insulin Resistance and Obesity in Young and Adult Female Mice. Cardiorenal. Med. 2012, 2, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Yasrebi, A.; Rivera, J.A.; Krumm, E.A.; Yang, J.A.; Roepke, T.A. Activation of Estrogen Response Element-independent ERα signaling protects female mice from diet-induced obesity. Endocrinology 2016. [Google Scholar] [CrossRef] [PubMed]
- Grépin, C.; Dagnino, L.; Robitaille, L.; Haberstroh, L.; Antakly, T.; Nemer, M. A hormone-encoding gene identifies a pathway for cardiac but not skeletal muscle gene transcription. Mol. Cell. Biol. 1994, 14, 3115–3129. [Google Scholar] [CrossRef] [PubMed]
- Arias-Loza, P.A.; Hu, K.; Dienesch, C.; Mehlich, A.M.; König, S.; Jazbutyte, V.; Neyses, L.; Hegele-Hartung, C.; Heinrich Fritzemeier, K.; Pelzer, T. Both estrogen receptor subtypes, alpha and beta, attenuate cardiovascular remodeling in aldosterone salt-treated rats. Hypertension 2007, 50, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.R.; Cheng, W.C.; Su, Y.M.; Chiu, C.H.; Liou, Y.M. Molecular targets for anti-oxidative protection of green tea polyphenols against myocardial ischemic injury. BioMedicine 2014, 4, 7–16. [Google Scholar] [CrossRef] [PubMed]


| 17β-E (μg/kg/day) | 0 | 4 | 8 | 12 |
|---|---|---|---|---|
| HW (g) | 0.22 ± 0.03 | 0.19 ± 0.04 | 0.16 ± 0.01 | 0.16 ± 0.03 |
| TL (mm) | 22.80 ± 0.13 | 22.73 ± 0.14 | 22.59 ± 0.15 | 22.87 ± 0.12 |
| HW/TL ratio (×103) | 9.65 ± 1.31 | 8.36 ± 1.76 | 7.08 ± 0.44 ** | 7.00 ± 1.31 * |
| GAW (g) | 0.25 ± 0.07 | 0.21 ± 0.03 | 0.18 ± 0.02 | 0.17 ± 0.06 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ting, W.-J.; Huang, C.-Y.; Jiang, C.-H.; Lin, Y.-M.; Chung, L.-C.; Shen, C.-Y.; Pai, P.; Lin, K.-H.; Viswanadha, V.P.; Liao, S.-C. Treatment with 17β-Estradiol Reduced Body Weight and the Risk of Cardiovascular Disease in a High-Fat Diet-Induced Animal Model of Obesity. Int. J. Mol. Sci. 2017, 18, 629. https://doi.org/10.3390/ijms18030629
Ting W-J, Huang C-Y, Jiang C-H, Lin Y-M, Chung L-C, Shen C-Y, Pai P, Lin K-H, Viswanadha VP, Liao S-C. Treatment with 17β-Estradiol Reduced Body Weight and the Risk of Cardiovascular Disease in a High-Fat Diet-Induced Animal Model of Obesity. International Journal of Molecular Sciences. 2017; 18(3):629. https://doi.org/10.3390/ijms18030629
Chicago/Turabian StyleTing, Wei-Jen, Chih-Yang Huang, Chong-He Jiang, Yueh-Min Lin, Li-Chin Chung, Chia-Yao Shen, Peiying Pai, Kuan-Ho Lin, Vijaya Padma Viswanadha, and Shih-Chieh Liao. 2017. "Treatment with 17β-Estradiol Reduced Body Weight and the Risk of Cardiovascular Disease in a High-Fat Diet-Induced Animal Model of Obesity" International Journal of Molecular Sciences 18, no. 3: 629. https://doi.org/10.3390/ijms18030629