The Carotid Intima-Media Thickness and Arterial Stiffness of Pediatric Mucopolysaccharidosis Patients Are Increased Compared to Both Pediatric and Adult Controls
Abstract
:1. Introduction
2. Results
2.1. Study Cohort Descriptions
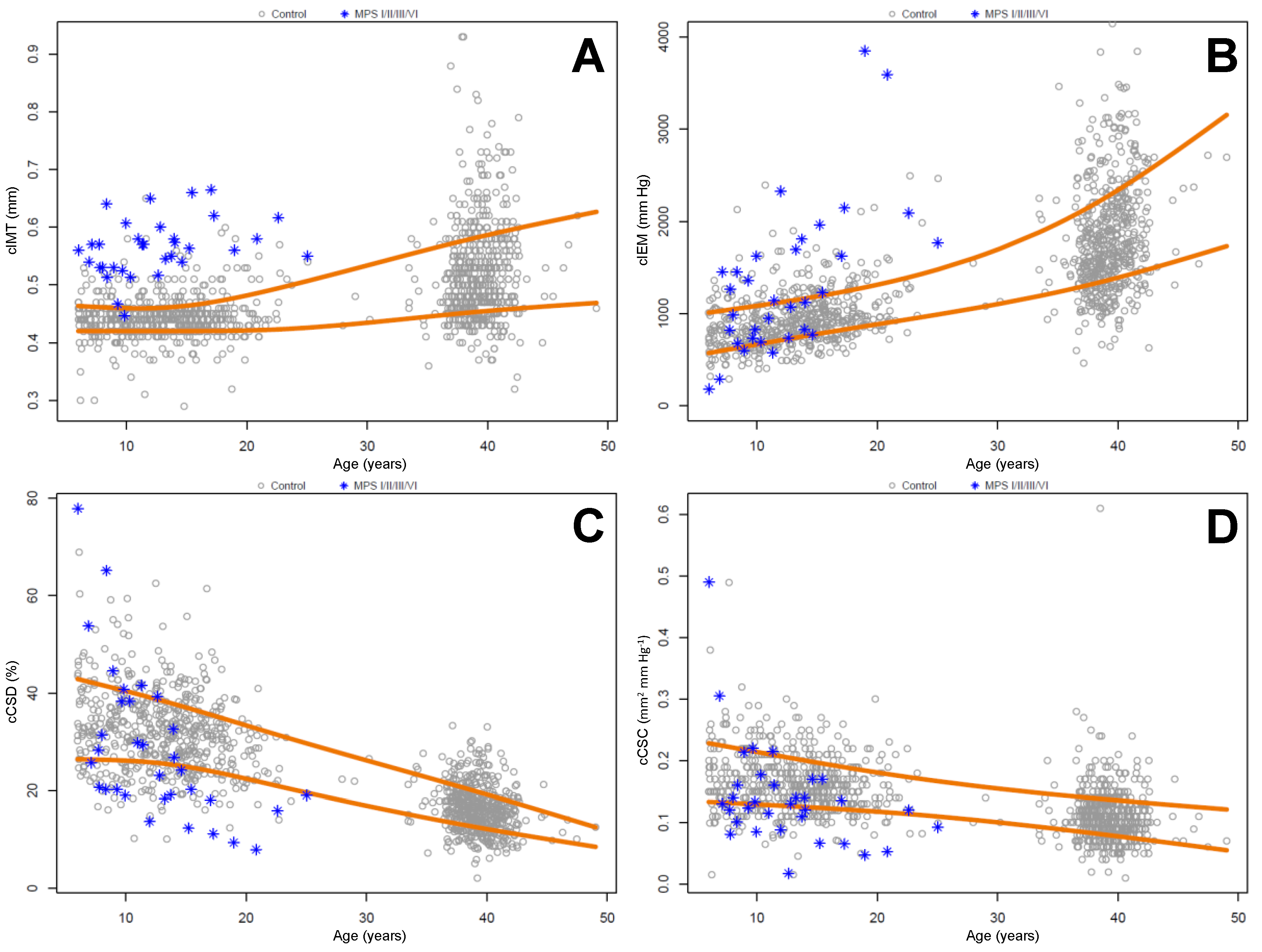
2.2. Carotid Intima Media Thickness and Stiffness Measurements
3. Discussion
4. Materials and Methods
4.1. Human Subjects
4.2. Study Design
4.3. Carotid Artery Imaging and Analysis
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Neufeld, E.F.; Muenzer, J. The Mucopolysaccharidoses. In The Metabolic and Molecular Basis of Inherited Disease; Scriver, C., Beaudet, A.L., Sly, W., Valle, D., Eds.; McGraw-Hill: New York, NY, USA, 2001; pp. 3421–3452. [Google Scholar]
- Braunlin, E.A.; Stauffer, N.R.; Peters, C.H.; Berry, J.M.; Bass, J.L.; Hopwood, J.J.; Krivit, W. Usefulness of bone marrow transplantation in the Hurler syndrome. Am. J. Cardiol. 2003, 93, 882–886. [Google Scholar] [CrossRef]
- Lin, H.Y.; Lin, S.P.; Chuang, C.K.; Chen, M.R.; Chen, B.F.; Wraith, J.E. Mucopolysaccharidosis I under enzyme replacement therapy with laronidase—A mortality case with autopsy report. J. Inherit. Metab. Dis. 2005, 28, 1146–1148. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Moseley, K.; Pavlova, Z. Postmortem studies on a patient with mucopolysaccharidosis type I: Histopathological findings after one year of enzyme replacement therapy. J. Inherit. Metab. Dis. 2009, 32, S53–S57. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, L.; Backx, A.P.; Coolen, H.; Wijburg, F.A.; Wevers, R.; Morava, E.; Neeleman, C. Fatal coronary artery disease in an infant with severe mucopolysaccharidosis type I. Pediatrics 2011, 127, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; O’Meara, A.; Wynn, R.; McDermott, M. Fatal and unanticipated cardiorespiratory disease in a two-year-old child with Hurler syndrome following successful stem cell transplant. JIMD Rep. 2013, 10, 1–5. [Google Scholar] [PubMed]
- Aldenhoven, M.; Wynn, R.F.; Orchard, P.J.; O’Meara, A.; Veys, P.; Fischer, A.; Valayannopoulos, V.; Neven, B.; Rovelli, A.; Prasad, VK.; et al. Long-term outcome of Hurler syndrome patients after hematopoietic cell transplantation: An international multicenter study. Blood 2015, 125, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Chuang, C.K.; Huang, Y.H.; Tu, R.Y.; Lin, F.J.; Lin, S.J.; Chiu, P.C.; Niu, D.M.; Tsai, F.J.; Hwu, W.L.; et al. Causes of death and clinical characteristics of 34 patients with Mucopolysaccharidosis II in Taiwan from 1995–2012. Orphanet. J. Rare Dis. 2016, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Braunlin, E.; Orchard, P.J.; Whitley, C.B.; Schroeder, L.; Reed, R.C.; Manivel, J.C. Unexpected coronary artery findings in mucopolysaccharidosis. Report of four cases and literature review. Cardiovasc. Pathol. 2014, 23, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Wendelhag, I.; Gustavsson, T.; Suurküla, M.; Berglund, G.; Wikstrand, J. Ultrasound measurement of wall thickness in the carotid artery: Fundamental principles and description of a computerized analysing system. Clin. Physiol. 1991, 11, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Y.; Covault, K.K.; Halcrow, E.M.; Gardner, A.J.; Cao, X.; Newcomb, R.L.; Dauben, R.D.; Chang, A.C. Carotid intima-media thickness is increased in patients with mucopolysaccharidoses. Mol. Genet. Metab. 2011, 104, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Y.; Braunlin, E.A.; Rudser, K.D.; Dengel, D.R.; Metzig, A.M.; Covault, K.K.; Polgreen, L.E.; Shapiro, E.; Steinberger, J.; Kelly, A.S. Carotid intima-media thickness is increased in patients with treated mucopolysaccharidosis types I and II, and correlates with arterial stiffness. Mol. Genet. Metab. 2014, 111, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.; Berger, K.I.; Borgo, A.; Braunlin, E.A.; Burton, B.K.; Ghotme, K.A.; Kircher, S.G.; Molter, D.; Orchard, P.J.; Palmer, J.; et al. Unique medical issues in adult patients with mucopolysaccharidoses. Eur. J. Intern. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Braunlin, E.; Wang, R. Cardiac issues in adults with the mucopolysaccharidoses: Current knowledge and emerging needs. Heart 2016. [Google Scholar] [CrossRef] [PubMed]
- Pignoli, P.; Tremoli, E.; Poli, A.; Oreste, P.; Paoletti, R. Intimal plus medial thickness of the arterial wall: A direct measurement with ultrasound imaging. Circulation 1986, 74, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of clinical cardiovascular events with carotid intima-media thickness. Circulation 2007, 115, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Ylä-Herttuala, S.; Solakivi, T.; Hirvonen, J.; Laaksonen, H.; Möttönen, M.; Pesonen, E.; Raekallio, J.; Akerblom, H.K.; Nikkari, T. Glycosaminoglycans and apolipoproteins B and A-I in human aortas. Chemical and immunological analysis of lesion-free aortas from children and adults. Arteriosclerosis 1987, 7, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Funderburgh, J.L.; Funderburgh, M.L.; Mann, M.M.; Conrad, G.W. Arterial lumican. Properties of a corneal-type keratan sulfate proteoglycan from bovine aorta. J. Biol. Chem. 1991, 266, 24773–24777. [Google Scholar] [PubMed]
- Schiattarella, G.G.; Cerulo, G.; de Pasquale, V.; Cocchiaro, P.; Paciello, O.; Avallone, L.; Belfiore, M.P.; Iacobellis, F.; di Napoli, D.; Magliulo, F.; et al. The murine model of mucopolysaccharidosis IIIB develops cardiopathies over time leading to heart failure. PLoS ONE 2015, 10, e0131662. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, E.; Fushimi, K.; Suzuki, Y.; Shimizu, K.; Takami, T.; Zustin, J.; Patel, P.; Ruhnke, K.; Shimada, T.; Boyce, B.; et al. Pathogenesis of Morquio A syndrome: An autopsied case reveals systemic storage disorder. Mol. Genet. Metab. 2013, 109, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.A.; Linders, B.; Wu, S.; Bigg, P.; O’Donnell, P.; Sleeper, M.M.; Whyte, M.P.; Haskins, M.; Ponder, K.P. Upregulation of elastase activity in aorta in mucopolysaccharidosis I and VII dogs may be due to increased cytokine expression. Mol. Genet. Metab. 2010, 99, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.A.; Dickson, P.I.; Wall, J.S.; Passage, M.B.; Ellinwood, N.M.; Kakkis, E.D.; McEntee, M.F. Arterial pathology in canine mucopolysaccharidosis-I and response to therapy. Lab. Investig. 2011, 91, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Khalid, O.; Vera, M.U.; Gordts, P.L.; Ellinwood, N.M.; Schwartz, P.H.; Dickson, P.I.; Esko, J.D.; Wang, R.Y. Immune-mediated inflammation may contribute to the pathogenesis of cardiovascular disease in mucopolysaccharidosis type I. PLoS ONE 2016, 11, e0150850. [Google Scholar] [CrossRef] [PubMed]
- Simonaro, C.M.; Tomatsu, S.; Sikora, T.; Kubaski, F.; Frohbergh, M.; Guevara, J.M.; Wang, R.Y.; Vera, M.; Kang, J.L.; Smith, L.J.; et al. Pentosan polysulfate: Oral versus subcutaneous injection in mucopolysaccharidosis type I dogs. PLoS ONE 2016, 11, e0153136. [Google Scholar] [CrossRef] [PubMed]
- Syeda, B.; Gottsauner-Wolf, M.; Denk, S.; Pichler, P.; Khorsand, A.; Glogar, D. Arterial compliance: A diagnostic marker for atherosclerotic plaque burden? Am. J. Hypertens. 2003, 16, 356–362. [Google Scholar] [CrossRef]
- Kiotsekoglou, A.; Moggridge, J.C.; Kapetanakis, V.; Newey, V.R.; Kourliouros, A.; Mullen, M.J.; Kaski, J.C.; Nassiri, D.K.; Camm, J.; Sutherland, G.R.; et al. Assessment of carotid compliance using real time vascular ultrasound image analysis in Marfan syndrome. Echocardiography 2009, 26, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Hinderer, C.; Bell, P.; Gurda, B.L.; Wang, Q.; Louboutin, J.P.; Zhu, Y.; Bagel, J.; O’Donnell, P.; Sikora, T.; Ruane, T.; et al. Liver-directed gene therapy corrects cardiovascular lesions in feline mucopolysaccharidosis type I. Proc. Natl. Acad. Sci. USA. 2014, 111, 14894–14899. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, L.; Hennig, A.K.; Kovacs, A.; Fu, A.; Chung, S.; Lee, D.; Wang, B.; Herati, R.S.; Mosinger Ogilvie, J.; et al. Liver-directed neonatal gene therapy prevents cardiac, bone, ear, and eye disease in mucopolysaccharidosis I mice. Mol. Ther. 2005, 11, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Marlatt, K.L.; Kelly, A.S.; Steinberger, J.; Dengel, D.R. The influence of gender on carotid artery compliance and distensibility in children and adults. J. Clin. Ultrasound 2013, 41, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Steffen, L.M.; Sinaiko, A.R.; Zhou, X.; Moran, A.; Jacobs, D.R., Jr.; Korenfeld, Y.; Dengel, D.R.; Chow, L.S.; Steinberger, J. Relation of adiposity, television and screen time in offspring to their parents. BMC Pediatr. 2013, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, T.; Moran, A.; Jacobs, D.R., Jr.; Steffen, L.M.; Sinaiko, A.R.; Zhou, X.; Steinberger, J. Relation of cardiometabolic risk factors between parents and children. J. Pediatr. 2015, 167, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Ryder, J.R.; Dengel, D.R.; Jacobs, D.R., Jr.; Sinaiko, A.R.; Kelly, A.S.; Steinberger, J. Relations among adiposity and insulin resistance with flow-mediated dilation, carotid intima-media thickness, and arterial stiffness in children. J. Pediatr. 2016, 168, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Neocleous, T.; Portnoy, S. Partially linear censored quantile regression. Lifetime Data Anal. 2009, 15, 357–378. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
| Covariate | Control: Pediatric | Control: Adult | MPS |
|---|---|---|---|
| (n = 560) | (n = 554) | (n = 33) | |
| Female | 259 (46.2%) | 285 (51.4%) 1 | 10 (30.3%) |
| Age at visit (years) | 13.14 (4.01) | 39.16 (2.24) 1 | 12.47 (4.66) |
| Height (cm) | 155 (18.30) | 171 (12.80) 110 | 132 (16.59) |
| Weight (kg) | 53.80 (23.54) | 86.39 (23.33) 108 | 36.66 (16.74) |
| BMI (kg/m2) | 21.44 (5.84) | 29.46 (7.47) 114 | 20.03 (4.50) |
| BMI percentile | 63.44 (27.33) | n.a. | 65.89 (26.29) |
| SBP (mm Hg) | 106 (10.42) 4 | 125 (15.59) 3 | 106 (10.98) |
| DBP (mm Hg) | 58.00 (7.78) 4 | 71.97 (10.39) 3 | 55.00 (13.73) |
| HR (min−1) | 79.39 (72.38) 5 | n.m. | 87.70 (14.54) |
| Race | |||
| White | 379 (67.7%) | 371 (67.0%) | 27 (81.8%) |
| Black | 124 (22.1%) | 138 (24.9%) | 2 (6.1%) |
| Native American | 14 (2.5%) | 16 (2.9%) | 0 (0.0%) |
| Asian | 16 (2.9%) | 18 (3.2%) | 0 (0.0%) |
| Hispanic | 3 (0.5%) | 1 (0.2%) | 0 (0.0%) |
| Other | 24 (4.3%) | 1 (0.2%) | 3 (9.1%) |
| Missing race | 0 (0.0%) | 9 (1.6%) | 1 (3.0%) |
| Covariate | MPS | MPS I | MPS II | MPS IIIA | MPS VI |
|---|---|---|---|---|---|
| (n = 33) | (n = 17) | (n = 9) | (n = 4) | (n = 3) | |
| Male | 23 (69.7%) | 10 (58.8%) | 9 (100.0%) | 3 (75.0%) | 1 (33.3%) |
| Female | 10 (30.3%) | 7 (41.2%) | 0 (0.0%) | 1 (25.0%) | 2 (66.7%) |
| Treatment: HSCT only | 9 (27.3%) | 9 (52.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Treatment: HSCT, now ERT | 3 (9.1%) | 2 (11.8%) | 0 (0.0%) | 0 (0.0%) | 1 (33.3%) |
| Treatment: ERT only | 17 (51.5%) | 6 (35.3%) | 9 (100.0%) | 0 (0.0%) | 2 (66.7%) |
| Treatment: None | 4 (12.1%) | 0 (0.0%) | 0 (0.0%) | 4 (100.0%) | 0 (0.0%) |
| Age at visit (years) | 12.47 (4.66) | 12.18 (4.72) | 10.80 (3.17) | 16.97 (5.48) | 13.13 (5.50) |
| SBP (mm Hg) | 106 (10.98) | 106 (10.99) | 103 (11.96) | 118 (5.91) | 103 (2.65) |
| DBP (mm Hg) | 55.00 (13.73) | 50.18 (13.74) | 54.11 (6.94) | 73.00 (15.08) | 61.00 (8.19) |
| HR (min−1) | 87.70 (14.54) | 87.06 (16.05) | 92.00 (12.30) | 81.75 (17.63) | 86.33 (9.61) |
| Height (cm) | 132 (16.59) | 133 (15.59) | 134 (11.48) | 136 (26.78) | 115 (19.04) |
| Weight (kg) | 36.66 (16.74) | 37.02 (18.61) | 38.05 (12.65) | 40.77 (22.30) | 24.97 (8.82) |
| BMI (kg/m2) | 20.03 (4.50) | 19.82 (5.46) | 20.54 (3.41) | 20.93 (4.37) | 18.43 (2.06) |
| BMI percentile | 65.89 (26.29) 4 | 60.28 (24.62) 3 | 81.63 (14.21) | 65.67 (39.58) 1 | 45.07 (37.07) |
| Race | |||||
| White | 27 (81.8%) | 15 (88.2%) | 6 (66.7%) | 4 (100.0%) | 2 (66.7%) |
| Black | 2 (6.1%) | 0 (0.0%) | 2 (22.2%) | 0 (0.0%) | 0 (0.0%) |
| Native American | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Asian | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Hispanic | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Other | 3 (9.1%) | 2 (11.8%) | 1 (11.1%) | 0 (0.0%) | 0 (0.0%) |
| Missing race | 1 (3.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (33.3%) |
| Vascular Measure | Control (Pediatric) | Control (Adult) | MPS | MPS I | MPS II | MPS IIIA | MPS VI |
|---|---|---|---|---|---|---|---|
| (n = 560) | (n = 554) | (n = 33) | (n = 17) | (n = 9) | (n = 4) | (n = 3) | |
| cIMT (mm) | 0.44 (0.04) | 0.52 (0.09) | 0.56 (0.05) | 0.57 (0.04) | 0.55 (0.07) | 0.59 (0.05) | 0.57 (0.04) |
| cCSD (%) | 32.03 (8.35) | 16.33 (4.73) 1 | 28.36 (15.70) | 28.47 (14.30) | 32.01 (19.66) | 21.59 (2.39) | 25.76 (24.28) |
| cCSC (mm2·mm Hg−1) | 0.16 (0.05) | 0.11 (0.04) 2 | 0.14 (0.08) | 0.12 (0.06) | 0.18 (0.12) | 0.14 (0.04) | 0.15 (0.14) |
| cIEM (mm Hg) | 951 (379.84) | 1856 (755.3) 2 | 1341 (817.06) | 1422 (973.19) | 1203 (632.77) | 1210 (416.98) | 1467 (1021.21) |
| Vascular Measure | Covariate | Adjusted Difference (95% CI) | p-Value |
|---|---|---|---|
| cIMT (mm) | Control (adult) vs. MPS | −0.10 (−0.14, −0.06) | <0.001 |
| Control (pediatric) vs. MPS | −0.12 (−0.14, −0.10) | <0.001 | |
| Female | −0.01 (−0.02, −0.01) | <0.001 | |
| Age (per 10 years) | +0.02 (0.01, 0.03) | <0.001 | |
| cCSD (%) | Control (adult) vs. MPS | +4.37 (0.31, 8.42) | 0.035 |
| Control (pediatric) vs. MPS | +4.08 (1.64, 6.51) | 0.001 | |
| Female | +0.02 (−0.79, 0.82) | 0.963 | |
| Age (per 10 years) | −6.15 (−7.36, −4.93) | <0.001 | |
| cCSC (mm2·mm Hg−1) | Control (adult) vs. MPS | +0.04 (0.01, 0.07) | 0.003 |
| Control (pediatric) vs. MPS | +0.03 (0.01, 0.04) | <0.001 | |
| Female | −0.01 (−0.02, −0.01) | <0.001 | |
| Age (per 10 years) | −0.03 (−0.03, −0.02) | <0.001 | |
| cIEM (mm Hg) | Control (adult) vs. MPS | −501.93 (−848.54, −155.32) | 0.005 |
| Control (pediatric) vs. MPS | −410.83 (−618.72, −202.93) | <0.001 | |
| Female | −26.26 (−95.05, 42.52) | 0.454 | |
| Age (per 10 years) | +383.35 (279.51, 487.19) | <0.001 |
| Vascular Measure | Number (%) > Pediatric Cohort 80th Percentile | Number (%) > Adult Cohort 50th Percentile |
| cIMT (mm) | 32 (97%) | 31 (94%) |
| cIEM (mm Hg) | 16 (48%) | 8 (24%) |
| Vascular Measure | Number (%) < Pediatric Cohort 20th percentile | Number (%) < Adult Cohort 50th percentile |
| cCSD (%) | 17 (52%) | 6 (18%) |
| cCSC (mm2·mm Hg−1) | 16 (48%) | 11 (33%) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.Y.; Rudser, K.D.; Dengel, D.R.; Braunlin, E.A.; Steinberger, J.; Jacobs, D.R.; Sinaiko, A.R.; Kelly, A.S. The Carotid Intima-Media Thickness and Arterial Stiffness of Pediatric Mucopolysaccharidosis Patients Are Increased Compared to Both Pediatric and Adult Controls. Int. J. Mol. Sci. 2017, 18, 637. https://doi.org/10.3390/ijms18030637
Wang RY, Rudser KD, Dengel DR, Braunlin EA, Steinberger J, Jacobs DR, Sinaiko AR, Kelly AS. The Carotid Intima-Media Thickness and Arterial Stiffness of Pediatric Mucopolysaccharidosis Patients Are Increased Compared to Both Pediatric and Adult Controls. International Journal of Molecular Sciences. 2017; 18(3):637. https://doi.org/10.3390/ijms18030637
Chicago/Turabian StyleWang, Raymond Y., Kyle D. Rudser, Donald R. Dengel, Elizabeth A. Braunlin, Julia Steinberger, David R. Jacobs, Alan R. Sinaiko, and Aaron S. Kelly. 2017. "The Carotid Intima-Media Thickness and Arterial Stiffness of Pediatric Mucopolysaccharidosis Patients Are Increased Compared to Both Pediatric and Adult Controls" International Journal of Molecular Sciences 18, no. 3: 637. https://doi.org/10.3390/ijms18030637






