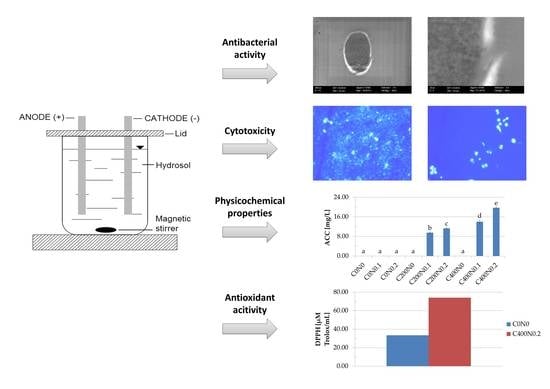
Cytotoxicity, Bactericidal, and Antioxidant Activity of Sodium Alginate Hydrosols Treated with Direct Electric Current
Abstract
:1. Introduction
2. Results
2.1. Physiochemical Properties of Hydrosols
2.2. Antibacterial Properties of Hydrosols
2.3. Effect of Sodium Alginate Hydrosols on Cell Morphology and Intracellular Organization
2.4. In Vitro Assessment of Hydrosols Treated with DC on Cytotoxicity to Mouse RAW 264.7 Macrophages and L929 Fibroblastic Cell Lines
2.5. Antioxidant Activity
3. Materials and Methods
3.1. Apparatus
3.2. Material
3.3. Preparation of the Experimental Material
3.4. Hydrosols Characterization
3.4.1. Physiochemical Properties of Hydrosols
3.4.2. Antibacterial Properties
3.4.3. The Effect of Sodium Alginate Hydrosols on Cell Morphology and Intracellular Organization
3.4.4. In Vitro Assessment of the Tested Hydrosols on Cytotoxicity to RAW 264.7 Macrophages and Mouse L929 Fibroblastic Cell Lines
Cell Culture
Cell Proliferation Assay
Cell Viability
3.5. Statistical Analysis
3.5.1. Antioxidant Activity
Free Radical Scavenging Activity (DPPH)
Ferric Reducing Ion Antioxidant Power (FRAP)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Munoz-Bonilla, A.; Garcia-Fernandez, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Scallan, E.; Griffin, P.M.; Angulo, F.J.; Tauxe, R.V.; Hoekstra, R.M. Foodborne illness acquired in the United States—Unspecified agents. Emerg. Infect. Dis. 2011, 17, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Issa-Zacharia, A.; Kamitani, Y.; Tiisekwa, A.; Morita, K.; Koichi, I. In Vitro inactivation of Escherichia coli, Staphylococcus aureus and Salmonella spp. using slightly acidic electrolyzed water. J. Biosci. Bioeng. 2010, 110, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tay, M.; Palmer, J.; Flint, S. Biofilm formation of Yersinia enterocolitica and its persistence following treatment with different sanitation agents. Food Control 2017, 73, 433–437. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, X.; Zhan, L.; Chen, J.; Zhang, Y.; Zhang, J.; Chen, H.; Zhang, Z.; Zhang, Y.; Lu, Y.; et al. Multilocus sequence type profiles of Bacillus cereus isolates from infant formula in China. Food Microbiol. 2017, 62, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Alboofetileh, M.; Rezaei, M.; Hosseini, H.; Abdollahi, M. Antimicrobial activity of alginate/clay nanocomposite films enriched with essential oils against three common foodborne pathogens. Food Control 2014, 36, 1–7. [Google Scholar] [CrossRef]
- Zhao, A.; Zhu, J.; Ye, X.; Ge, Y.; Li, J. Inhibition of biofilm development and spoilage potential of Shewanella baltica by quorum sensing signal in cell-free supernatant from Pseudomonas fluorescens. Int. J. Food Microbiol. 2016, 239, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuang, H.; Hinton, A.; Bowker, B.; Zhang, J. Photocatalytic disinfection of spoilage bacteria Pseudomonas fluorescens and Micrococcus caseolyticus by nano-TiO2. LWT Food Sci. Technol. 2014, 59, 1009–1017. [Google Scholar] [CrossRef]
- Saucier, L. Microbial spoilage, quality and safety within the context of meat sustainability. Meat Sci. 2016, 120, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.R.; Huang, Y.C.; Hsu, S.Y.; Huang, Y.W.; Hwang, D.F. Application of electrolyzed water in the food industry. Food Control 2008, 19, 329–345. [Google Scholar] [CrossRef]
- Król, Ż.; Jarmoluk, A. The effects of using a direct electric current on the chemical properties of gelatine gels and bacterial growth. J. Food Eng. 2015, 170, 1–7. [Google Scholar] [CrossRef]
- Hsu, S.Y. Effects of flow rate, temperature and salt concentration on chemical and physical properties of electrolyzed oxidizing water. J. Food Eng. 2005, 66, 171–176. [Google Scholar] [CrossRef]
- Hao, J.; Wu, T.; Li, H.; Wang, W.; Liu, H. Dual effects of slightly acidic electrolyzed water (SAEW) treatment on the accumulation of γ-aminobutyric acid (GABA) and rutin in germinated buckwheat. Food Chem. 2016, 201, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Król, Ż.; Malik, M.; Marycz, K.; Jarmoluk, A. Physicochemical properties of biopolymer hydrogels treated by direct electric current. Polymers 2016, 8, 248. [Google Scholar] [CrossRef]
- Król, Ż.; Malik, M.; Marycz, K.; Jarmoluk, A. Characteristic of gelatin, carrageenan and sodium alginate hydrosols treated by direct electric current. Polymers 2016, 8, 275. [Google Scholar] [CrossRef]
- Draget, K.I.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Ding, W.; Zhou, J.; Zeng, Y.; Wang, Y.; Shi, B. Preparation of oxidized sodium alginate with different molecular weights and its application for crosslinking collagen fiber. Carbohydr. Polym. 2017, 157, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Manivasagen, P.; Kang, K.H.; Kim, S.K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Liu, H.; Liu, X.; Wang, S.; Wu, J.; Zhang, R.; Min, H.; Huang, M. Development of silver sulfadiazine loaded bacterial cellulose/sodium alginate composite films with enhanced antibacterial property. Carbohydr. Polym. 2015, 132, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Shalumon, K.T.; Anulekha, K.H.; Nair, S.; Nair, S.; Chennazhi, K.P.; Jayakumar, R. Sodium alginate/poly(vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int. J. Biol. Macromol. 2011, 49, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Deka, C.; Deka, D.; Bora, M.M.; Jha, D.K.; Kakati, D.K. Synthesis of peppermint oil-loaded chitosan/alginate polyelectrolyte complexes and study of their antibacterial activity. J. Drug Deliv. Sci. Technol. 2016, 35, 314–322. [Google Scholar] [CrossRef]
- Chen, T.Y.; Kuo, S.H.; Chen, S.T.; Hwang, D.F. Differential proteomics to explore the inhibitory effects of acidic, slightly acidic electrolysed water and sodium hypochlorite solution on Vibrio parahaemolytucus. Food Chem. 2016, 194, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Forghani, F.; Park, J.H.; Oh, D.H. Effect of water hardness on the production and microbial efficacy of slightly acidic electrolyzed water. Food Microbiol. 2015, 48, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Brychcy, E.; Malik, M.; Drożdżewski, E.; Król, Ż.; Jarmoluk, A. Antibacterial and physicochemical properties of carrageenan and gelatine hydrosols and hydrogels incorporated with acidic electrolysed water. Polymers 2015, 7, 2638–2649. [Google Scholar] [CrossRef]
- Cui, X.; Shang, Y.; Shi, Z.; Xin, H.; Cao, W. Physicochemical properties and bactericidal efficiency of neutral and acidic electrolyzed water under different storage conditions. J. Food Eng. 2009, 91, 582–586. [Google Scholar] [CrossRef]
- Park, H.; Hung, Y.-C.; Chung, D. Effects of chlorine and pH on efficacy of electrolyzed water for inactivating Escherichia coli O157:H7 and Listeria monocytogenes. Int. J. Food Microbiol. 2004, 91, 13–18. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Vorobjeva, L.I.; Khodjaev, E.Y. The bactericidal effects of electrolyzed oxidizing water on bacterial strains involved in hospital infections. J. Artif. Organs 2003, 28, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Venkitanarayanan, K.S.; Ezeike, G.O.; Hung, Y.-C.; Doyle, M.P. Efficacy of electrolyzed oxidizing water for inactivating Escherichia coli O157:H7, Salmonella enteritidis and Listeria monocytogenes. Appl. Environ. Microbiol. 1999, 65, 4276–4279. [Google Scholar] [PubMed]
- Zeng, X.; Tang, W.; Ye, W.; Ouyang, T.; Tian, L.; Ni, Y.; Li, P. Studies on disinfection mechanism of electrolyzed oxidizing water on E. coli and Staphylococcus aureus. J. Food. Sci. Technol. 2010, 75, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Tango, C.N.; Miskeen, S.; Lee, B.H.; Oh, D.H. Hurdle technology: A novel approach for enhanced food quality and safety—A review. Food Control 2016, 73, 1426–1444. [Google Scholar] [CrossRef]
- Yamaner, C.; Ayvaz, M.; Konak, R.; Tan, N.; Kosoglu, I.; Dimoglo, A. Efficacy of neutralised electrolysed water and mild heat against foodborne pathogens isolated from Ficus carica. Ital. J. Food Sci. 2016, 28, 1–13. [Google Scholar]
- Ding, T.; Ge, Z.; Shi, J.; Xu, Y.T.; Jones, C.L.; Liu, D.H. Impact of slightly acidic electrolyzed water (SAEW) and ultrasound on microbial loads and quality of fresh fruits. LWT Food Sci. Technol. 2015, 60, 1195–1199. [Google Scholar] [CrossRef]
- Kim, C.; Huang, Y.-C.; Brackett, R.E. Roles of oxidation-reduction potential in electrolyzed oxidizing and chemically modified water for the inactivation of food-related pathogens. J. Food Prot. 2000, 63, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Al-Haq, M.I.; Sugiyama, J.; Isobe, S. Application of electrolyzed water in agriculture & food industries. Food Sci. Technol. Res. 2005, 11, 135–150. [Google Scholar]
- Hati, S.; Mandal, S.; Minz, P.S.; Vij, S.; Khetra, Y.; Singh, B.P.; Yadav, D. Electrolyzed Oxidized Water (EOW): Non-Thermal approach for decontamination of foodborne microorganisms in food industry. Food Nutr. Sci. 2012, 3, 760–768. [Google Scholar] [CrossRef]
- Koseki, S.; Yshida, K.; Isobe, S.; Itoh, K. Decontamination of lettuce using acidic electrolyzed water. J. Food Prot. 2001, 64, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Gomi, K.; Makini, T.; Suzuki, S.; Hasegawa, M.; Maeda, N.; Arai, T. Microbicidal and cytotoxic effects of functional water in vitro. Quintessence Int. 2010, 41, 166–172. [Google Scholar]
- Kubota, A.; Nose, K.; Yonekura, T.; Kosumi, T.; Yamauchi, K.; Oyanagi, H. Effect of electrolyzed strong acid water on peritoneal irrigation of experimental perforated peritonitis. Surg. Today 2009, 29, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Morita, C.; Nishida, T.; Ito, K. Biological toxicity of acid electrolyzed functional water: Effect of oral administration on mouse digestive tract and changes in body weight. Arch. Oral. Biol. 2011, 56, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Urakami, H.; Tamura, A. Cytotoxicity and microbicidal activity of electrolyzed strong acid water and acidic hypochlorite solution under isotonic conditions. Kansenshogaku Zasshi 1999, 73, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Mokudai, T.; Kanno, T.; Niwano, Y. Involvement of reactive oxygen species in the cytotoxic effect of acid-electrolyzed water. J. Toxicol. Sci. 2015, 40, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H. Electrolyzed water as a disinfectant for fresh-cut vegetables. J. Food Sci. 1999, 64, 536–539. [Google Scholar] [CrossRef]
- Trash, J.C.; Coates, J.D. Review: Direct and indirect electrical stimulation of microbial metabolism. Environ. Sci. Technol. 2008, 42, 3921–3931. [Google Scholar] [CrossRef]
- Diao, M.; Li, X.Y.; Gu, J.D.; Shi, H.C.; Xie, Z.M. Electron microscopic investigation of the bactericidal action of electrochemical disinfection in comparison with chlorination, ozonation and Fenton reaction. Process Biochem. 2004, 39, 1421–1426. [Google Scholar] [CrossRef]
- She, P.; Song, B.; Xing, X.-H.; Loosdrecht, M.; Liu, Z. Electrolytic stimulation of bacteria Enterobacter dissolvens by a direct current. Biochem. Eng. J. 2006, 28, 23–29. [Google Scholar] [CrossRef]
- Nakanashi, K.; Tokuda, H.; Soga, T.; Yoshinaga, T.; Takeda, M. Effect of electric current on growth and alcohol production by yeast cells. J. Ferment. Bioeng. 1998, 85, 250–253. [Google Scholar] [CrossRef]
- Kulig, D.; Zimoch-Korzycka, A.; Król, Ż.; Oziembłowski, M.; Jarmoluk, A. Effect of film-forming alginate chitosan polyelectrolyte complex on the storage quality of pork. Molecules 2017, 22, 98. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Yasuda, M.; Ota, T.; Suzuki, T. Antioxidative activity of a cathodic solution by the electrolysis of a dilute NaCl solution. Biosci. Biotechnol. Biochem. 1999, 63, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Miks-Krajnik, M.; Feng, L.X.J.; Bang, W.S.; Yuk, H.-G. Inactivation of Listeria monocytogenes and natural microbiota on raw salmon fillets using acidic electrolyzed water, ultraviolet light or/and ultrasound. Food Control 2017, 74, 54–60. [Google Scholar] [CrossRef]
- Xuan, X.-T.; Ding, T.; Li, J.; Ahn, J.-H.; Zhao, Y.; Chen, S.-G.; Ye, X.-Q.; Liu, D.-H. Estimation of growth parameters of Listeria monocytogenes after sublethal heat and slightly acidic electrolyzed water (SAEW) treatment. Food Control 2017, 71, 17–25. [Google Scholar] [CrossRef]
- Luo, K.; Kim, S.Y.; Wang, J.; Oh, D.-H. A combined hurdle approach of slightly acidic electrolyzed water simultaneous with ultrasound to inactivate Bacillus cereus on potato. LWT Food Sci. Technol. 2016, 73, 615–621. [Google Scholar] [CrossRef]
- Tirawat, D.; Phongpaichit, S.; Benjakul, S.; Sumpacapol, P. Microbial load reduction of sweet basil using acidic electrolyzed water and lactic acid in combination with mild heat. Food Control 2016, 64, 29–36. [Google Scholar] [CrossRef]
- Navarro-Rico, J.; Artes-Hernandez, F.; Gomez, P.A.; Nunez-Sanchez, M.A.; Artes, F.; Martinez-Hernandez, G.B. Neutral and acidic electrolyzed water kept microbial quality and healt promoting compounds of fresh-cut broccoli throughout shelf life. Innov. Food Sci. Emerg. 2014, 21, 74–81. [Google Scholar] [CrossRef]
- The American Public Health Assoc (APHA). Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association Inc.: Washington, DC, USA, 1998. [Google Scholar]
- Kaliński, K.; Marycz, K.; Czogała, J.; Serwa, E.; Janeczek, W. An application of scanning electron microscopy combined with roentgen microanalysis (SEM-EDS) in canine urolithiasis. J. Electron. Microsc. 2011, 61, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Beatty, W.; Weil, G.; Fisher, P. High pressure freezing/freeze substitution fixation improves the ultrastructural assessment of Wolbachia endosymbiont—Filarial nematode host interaction. PLoS ONE 2014, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Grzesiak, J.; Marycz, K.; Wrzeszcz, K. Isolation and morphological characterization of ovine adipose-derived mesenchymal stem cells in culture. Int. J. Stem Cells 2011, 4, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.; Marycz, K.; Poniewierska-Baran, A. Very small embryonic-like stem cells as a novel development concept and the hierarchy of the stem cell compartment. Adv. Med. Sci. 2014, 59, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Yeh, J.Y.; Chen, P.C.; Hsu, C.K. Phenolic content and DPPH radical scavenging activity of yam-containing surimi gels influenced by salt and heating. Asian J. Health Inf. Sci. 2007, 2, 1–11. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]






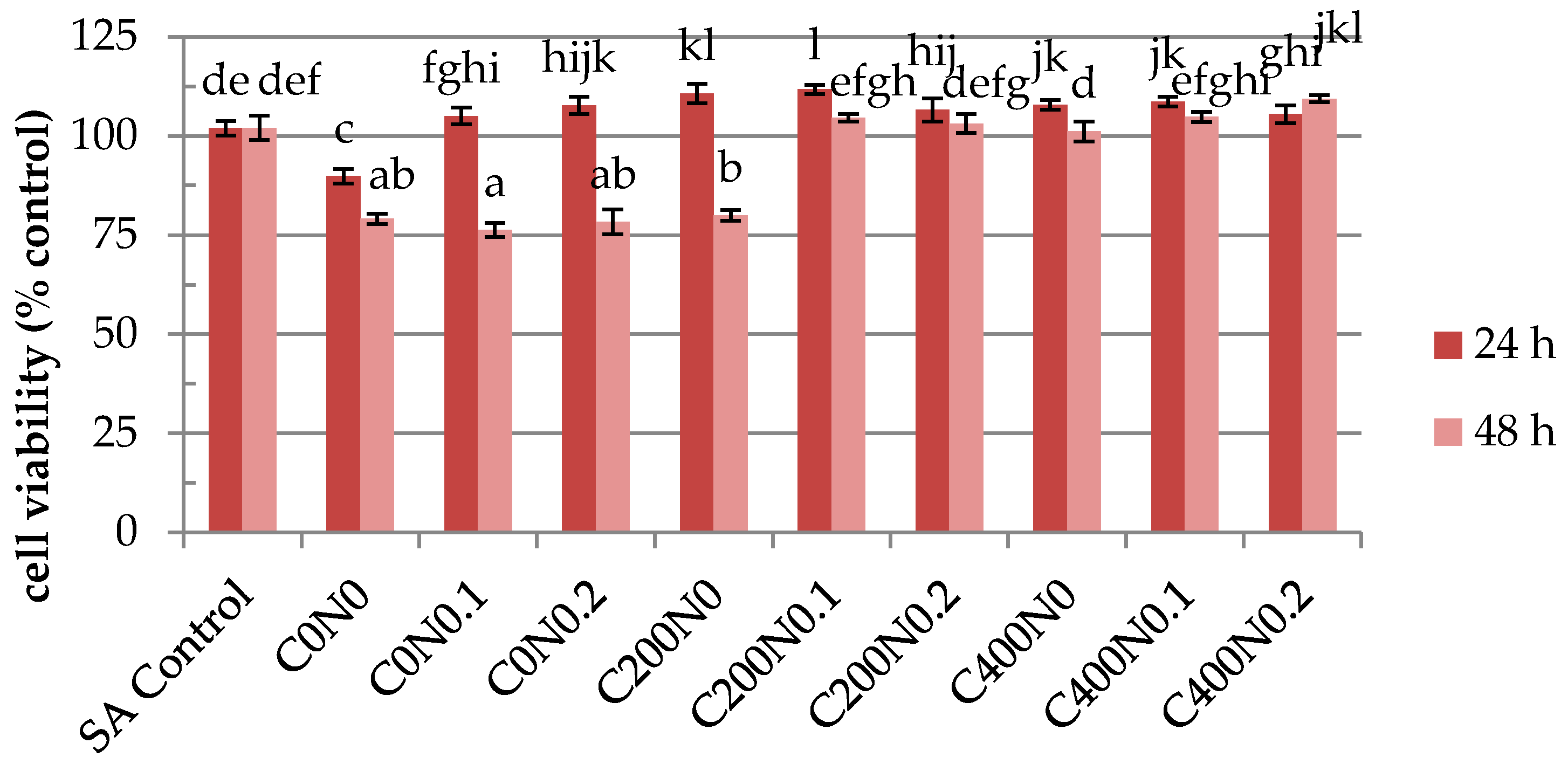


| Variants | S. aureus | L. monocytogenes | B. cereus | M. luteus | E. coli | S. enteritidis | Y. enterocolitica | P. fluorescens |
|---|---|---|---|---|---|---|---|---|
| Control | 7.82 ± 0.08 a | 7.49 ± 0.05 a | 7.21 ± 0.03 d | 7.69 ± 0.01 a | 7.24 ± 0.04 a | 7.79 ± 0.03 a,b | 7.46 ± 0.01 a | 7.77 ± 0.06 a |
| SA Control | 7.06 ± 0.04 b | 7.57 ± 0.03 a | 7.51 ± 0.01 a | 7.67 ± 0.01 a | 6.95 ± 0.00 b | 7.75 ± 0.01 a,b | 7.44 ± 0.01 a | 6.92 ± 0.01 c,d |
| C0N0 | 6.78 ± 0.00 d | 7.50 ± 0.02 a | 7.23 ± 0.04 d | 7.62 ± 0.02 a | 6.06 ± 0.04 c | 7.84 ± 0.03 a | 7.41 ± 0.01 a | 6.86 ± 0.03 d |
| C0N0.1 | 6.79 ± 0.01 d | 7.58 ± 0.07 a | 7.29 ±0.02 c | 7.65 ± 0.02 a | 5.95 ± 0.10 c | 7.69 ± 0.01 b | 7.41 ± 0.02 a | 6.96 ± 0.05 c |
| C0N0.2 | 6.90 ± 0.08 c | 7.52 ± 0.04 a | 7.41 ± 0.01 b | 7.59 ± 0.03 a | 6.01 ± 0.09 c | 7.69 ± 0.11 b | 7.44 ± 0.01 a | 7.03 ± 0.05 b |
| C200N0 | 6.63 ± 0.06 f | 6.75 ± 0.05 b | 6.21 ± 0.06 e | 6.72 ± 0.02 b | 5.37 ± 0.02 e | 6.63 ± 0.00 d | 7.33 ± 0.01 b | 6.95 ± 0.03 c |
| C200N0.1 | 4.59 ± 0.00 g | 1.80 ± 0.28 c | 4.21 ± 0.01 g | 2.33 ± 0.17 d | 5.35 ± 0.10 e | 4.59 ± 0.09 e | 1.65 ± 0.02 d | ND f |
| C200N0.2 | ND h | ND d | ND h | ND e | 3.38 ± 0.01 f | 3.49 ± 0.01 g | 1.01 ± 0.05 e | ND f |
| C400N0 | 6.68 ± 0.05 e | 6.59 ± 0.21 b | 5.26 ± 0.02 f | 5.70 ± 0.12 c | 5.74 ± 0.03 d | 6.54 ± 0.06 c | 7.22 ± 0.05 c | 6.63 ± 0.01 e |
| C400N0.1 | ND h | ND d | ND h | ND e | 3.35 ± 0.17 f | 3.88 ± 0.03 f | ND f | ND f |
| C400N0.2 | ND h | ND d | ND h | ND e | ND g | ND h | ND f | ND f |
| Variants | DPPH (μM Trolox/mL) | FRAP (μM Fe(II)/mL) |
|---|---|---|
| C0N0 | 124.17 ± 3.04 e | 1.00 ± 0.03 a |
| C0N0.1 | 75.67 ± 6.81 c | 0.99 ± 0.04 a |
| C0N0.2 | 74.00 ± 5.35 b,c | 1.01 ± 0.04 a |
| C200N0 | 97.08 ± 15.36 d | 0.98 ± 0.01 a |
| C200N0.1 | 62.25 ± 8.82 b | 0.97 ± 0.03 a |
| C200N0.2 | 43.33 ± 3.19 a | 1.00 ± 0.03 a |
| C400N0 | 75.42 ± 11.66 c | 0.99 ± 0.02 a |
| C400N0.1 | 36.67 ± 6.16 a | 1.00 ± 0.01 a |
| C400N0.2 | 33.33 ± 2.89 a | 1.00 ± 0.01 a |
| Run Code Letters | Current (C) (mA) | NaCl (N) (%) |
|---|---|---|
| C0N0 | 0 | 0.0 |
| C0N0.1 | 0.1 | |
| C0N0.2 | 0.2 | |
| C200N0 | 200 | 0.0 |
| C200N0.1 | 0.1 | |
| C200N0.2 | 0.2 | |
| C400N0 | 400 | 0.0 |
| C400N0.1 | 0.1 | |
| C400N0.2 | 0.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Król, Ż.; Marycz, K.; Kulig, D.; Marędziak, M.; Jarmoluk, A. Cytotoxicity, Bactericidal, and Antioxidant Activity of Sodium Alginate Hydrosols Treated with Direct Electric Current. Int. J. Mol. Sci. 2017, 18, 678. https://doi.org/10.3390/ijms18030678
Król Ż, Marycz K, Kulig D, Marędziak M, Jarmoluk A. Cytotoxicity, Bactericidal, and Antioxidant Activity of Sodium Alginate Hydrosols Treated with Direct Electric Current. International Journal of Molecular Sciences. 2017; 18(3):678. https://doi.org/10.3390/ijms18030678
Chicago/Turabian StyleKról, Żaneta, Krzysztof Marycz, Dominika Kulig, Monika Marędziak, and Andrzej Jarmoluk. 2017. "Cytotoxicity, Bactericidal, and Antioxidant Activity of Sodium Alginate Hydrosols Treated with Direct Electric Current" International Journal of Molecular Sciences 18, no. 3: 678. https://doi.org/10.3390/ijms18030678