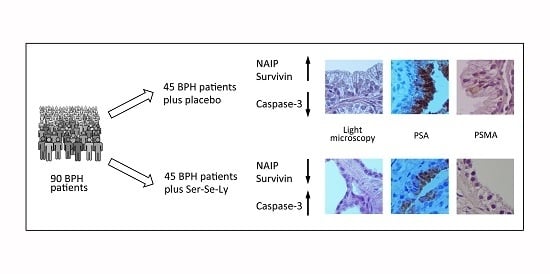
Survivin and NAIP in Human Benign Prostatic Hyperplasia: Protective Role of the Association of Serenoa repens, Lycopene and Selenium from the Randomized Clinical Study
Abstract
:1. Introduction
2. Results
2.1. Clinical Features
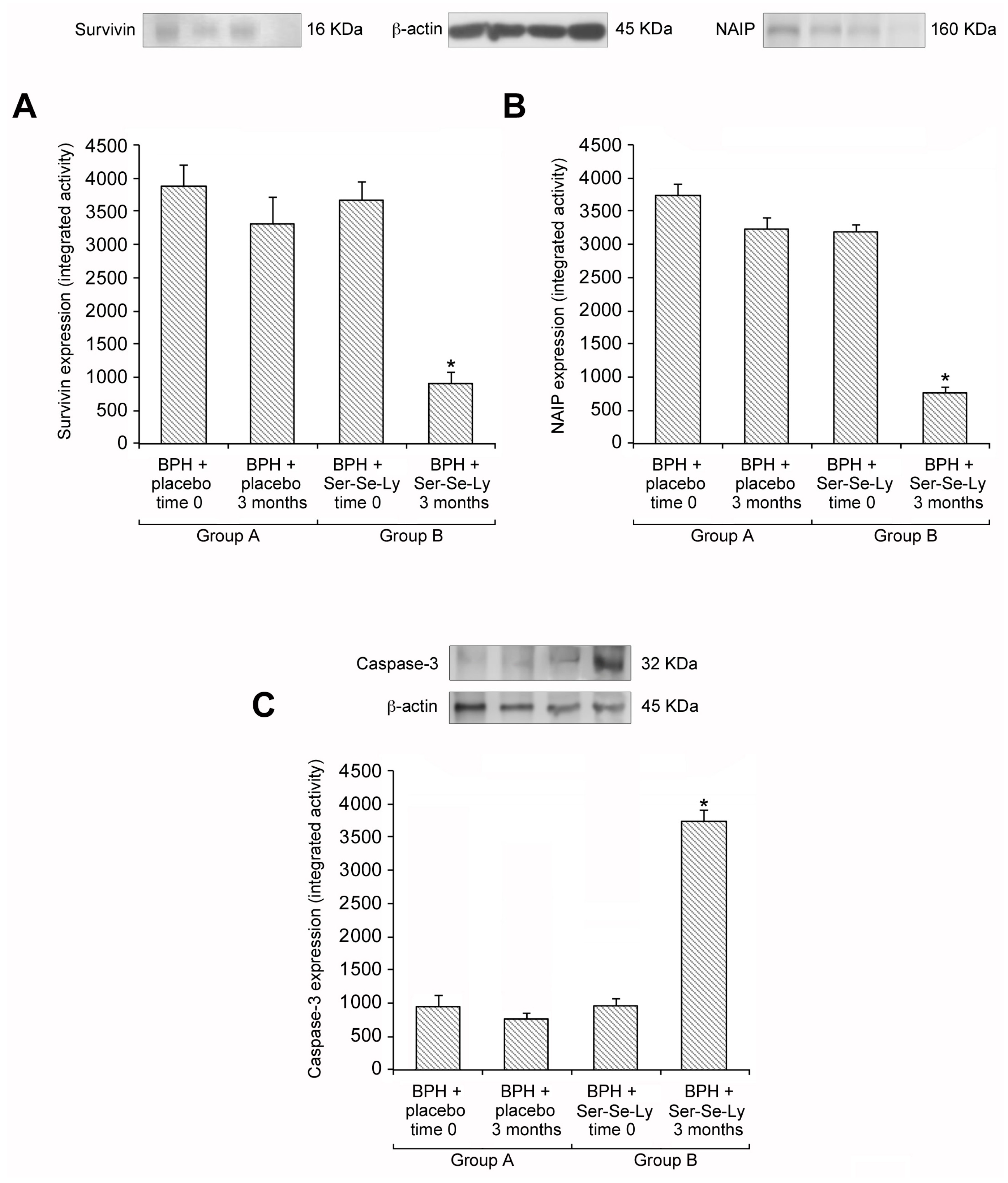
2.2. Ser-Se-Ly Decreases Survivin and NAIP Expression
2.3. Ser-Se-Ly Increases Caspase-3 Expression
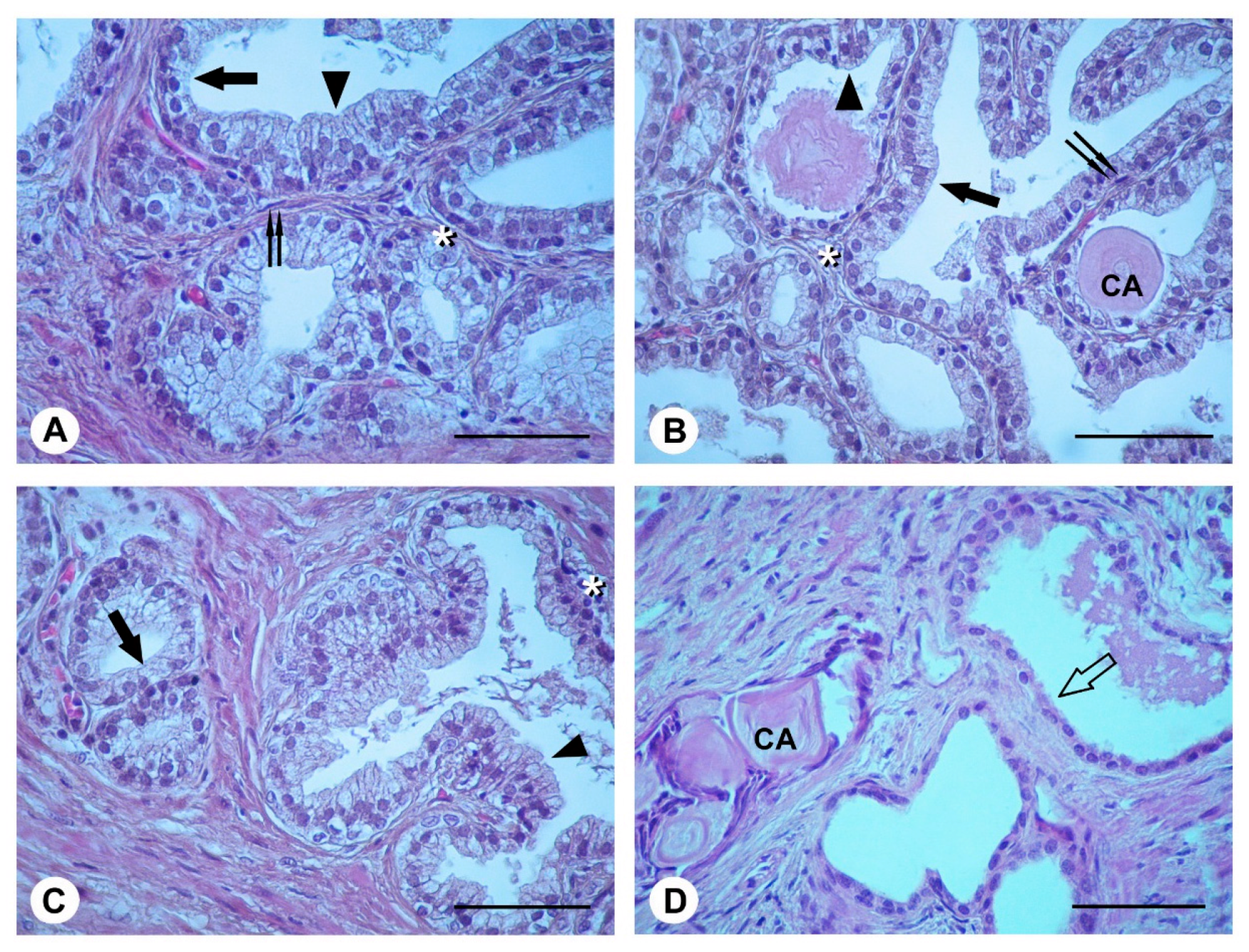
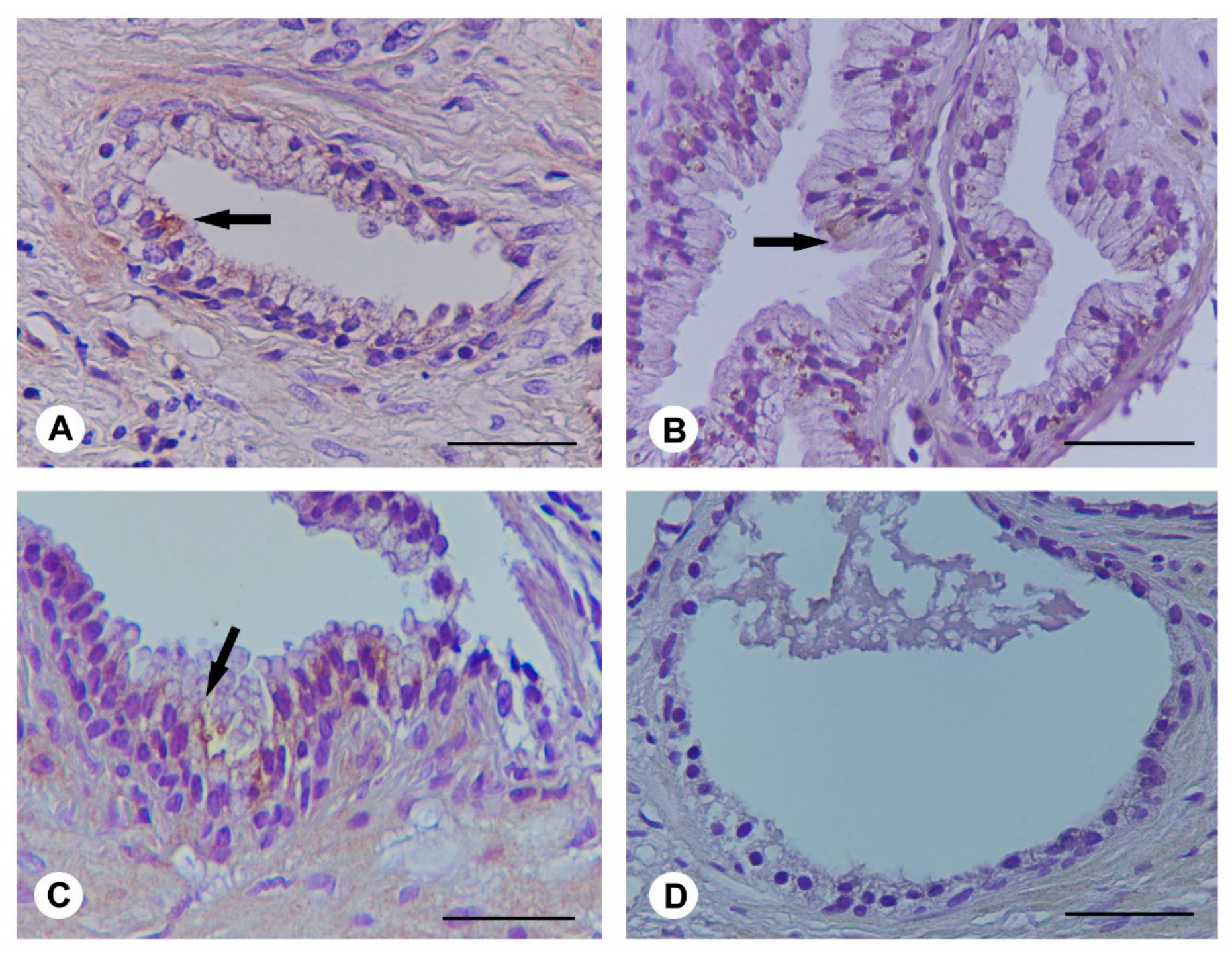
2.4. Histological Evaluation
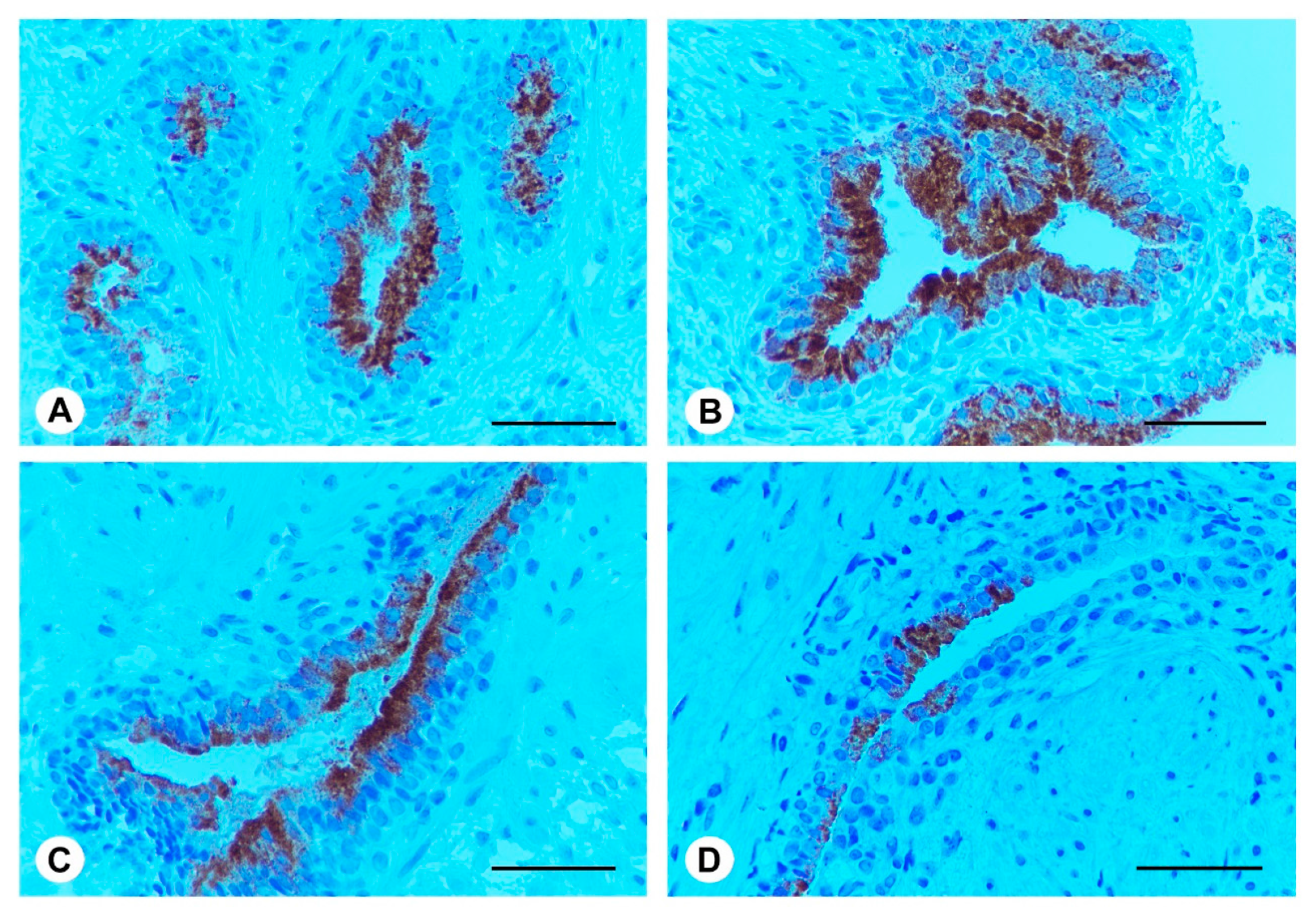
2.5. Ser-Se-Ly Decreases PSA Immunoexpression
2.6. Ser-Se-Ly Decreases in Prostate Specific Membrane Antigen (PSMA) Immunoexpression
3. Discussion
4. Materials and Methods
4.1. Western Blot Analysis
4.2. Light Microscopy (LM)
4.3. Immunohistochemistry for PSA and PSMA
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aaron, L.; Franco, O.E.; Hayward, S.W. Review of prostate anatomy and embryology and the etiology of Benign Prostatic Hyperplasia. Urol. Clin. N. Am. 2016, 43, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Egan, K.B. The epidemiology of Benign Prostatic Hyperplasia associated with Lower Urinary Tract Symptoms: Prevalence and incident rates. Urol. Clin. N. Am. 2016, 43, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Schauer, I.G.; Rowley, D.R. The functional role of reactive stroma in benign prostatic hyperplasia. Differentiation 2011, 82, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Szopinski, T.; Golabek, T.; Borówka, A.; Chłosta, P. Is determination of transition zone volume by transrectal ultrasound in patients with clinically benign prostatic enlargement sufficiently reliable in the clinical setting? Wideochir. Inne Tech. Maloinwazyjne 2014, 9, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Nickel, J.C.; Roehrborn, C.G.; Castro-Santamaria, R.; Freedland, S.J.; Moreira, D.M. Chronic prostate inflammation is associated with severity and progression of Benign Prostatic Hyperplasia, Lower Urinary Tract Symptoms and risk of Acute Urinary Retention. J. Urol. 2016, 196, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Briganti, A.; Gontero, P.; Mondaini, N.; Novara, G.; Salonia, A.; Sciarra, A.; Montorsi, F. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int. 2013, 112, 432–441. [Google Scholar] [CrossRef] [PubMed]
- La Vignera, S.; Condorelli, R.A.; Russo, G.I.; Morgia, G.; Calogero, A.E. Endocrine control of benign prostatic hyperplasia. Andrology 2016, 4, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Norström, M.M.; Rådestad, E.; Sundberg, B.; Mattsson, J.; Henningsohn, L.; Levitsky, V.; Uhlin, M. Progression of benign prostatic hyperplasia is associated with pro-inflammatory mediators and chronic activation of prostate-infiltrating lymphocytes. Oncotarget 2016, 7, 23581–23593. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.R.; Ricke, W.A. A historical perspective on the role of stroma in the pathogenesis of benign prostatic hyperplasia. Differentiation 2011, 82, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.S.; An, H.J.; Cheon, S.Y.; Kwon, K.R.; Lee, K.H. Bee venom suppresses testosterone-induced benign prostatic hyperplasia by regulating the inflammatory response and apoptosis. Exp. Biol. Med. 2015, 240, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Kiraz, Y.; Adan, A.; Kartal Yandim, M.; Baran, Y. Major apoptotic mechanisms and genes involved in apoptosis. Tumour Biol. 2016, 37, 8471–8486. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; López-Soto, A.; Kumar, S.; Kroemer, G. Caspases connect cell-death signaling to organismal homeostasis. Immunity 2016, 44, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Kocab, A.J.; Duckett, C.S. Inhibitor of apoptosis proteins as intracellular signaling intermediates. FEBS J. 2016, 283, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Silke, J.; Meier, P. Inhibitor of apoptosis (IAP) proteins-modulators of cell death and inflammation. Cold Spring Harb. Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Berriguete, G.; Fraile, B.; de Bethencourt, F.R.; Prieto-Folgado, A.; Bartolome, N.; Nuñez, C.; Prati, B.; Martínez-Onsurbe, P.; Olmedilla, G.; Paniagua, R.; et al. Role of IAPs in prostate cancer progression: Immunohistochemical study in normal and pathological (benign hyperplastic, prostatic intraepithelial neoplasia and cancer) human prostate. BMC Cancer 2010, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Minutoli, L.; Altavilla, D.; Marini, H.; Rinaldi, M.; Irrera, N.; Pizzino, G.; Bitto, A.; Arena, S.; Cimino, S.; Squadrito, F.; et al. Inhibitors of apoptosis proteins in experimental benign prostatic hyperplasia: Effects of serenoa repens, selenium and lycopene. J. Biomed. Sci. 2014, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Allkanjari, O.; Vitalone, A. What do we know about phytotherapy of benign prostatic hyperplasia? Life Sci. 2015, 126, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Morgia, G.; Cimino, S.; Favilla, V.; Russo, G.I.; Squadrito, F.; Mucciardi, G.; Masieri, L.; Minutoli, L.; Grosso, G.; Castelli, T. Effects of Serenoa repens, selenium and lycopene (Profluss®) on chronic inflammation associated with benign prostatic hyperplasia: Results of “FLOG” (Flogosis and Profluss in Prostatic and Genital Disease), a multicentre Italian study. Int. Braz. J. Urol. 2013, 39, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Altavilla, D.; Bitto, A.; Polito, F.; Irrera, N.; Marini, H.; Arena, S.; Favilla, V.; Squadrito, F.; Morgia, G.; Minutoli, L. The combination of Serenoa repens, selenium and lycopene is more effective than serenoa repens alone to prevent hormone dependent prostatic growth. J. Urol. 2011, 186, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Morgia, G.; Russo, G.I.; Voce, S.; Palmieri, F.; Gentile, M.; Giannantoni, A.; Blefari, F.; Carini, M.; Minervini, A.; Ginepri, A.; et al. Serenoa repens, lycopene and selenium versus tamsulosin for the treatment of LUTS/BPH. An Italian multicenter double-blinded randomized study between single or combination therapy (PROCOMB trial). Prostate 2014, 74, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.W.; Lim, S.W.; Kim, J.H.; Ahn, S.H.; Choi, J.D. Comparison of tamsulosin plus serenoa repens with tamsulosin in the treatment of benign prostatic hyperplasia in Korean men: 1-year randomized open label study. Urol. Int. 2015, 94, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.I.; Vanella, L.; Castelli, T.; Cimino, S.; Reale, G.; Urzì, D.; Li Volti, G.; Gacci, M.; Carini, M.; Motta, F.; et al. Heme oxygenase levels and metaflammation in benign prostatic hyperplasia patients. World J. Urol. 2016, 34, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, V.; Rossanese, M.; Zazzara, M.; Giannarini, G.; Abbinante, M.; Bartoletti, R.; Mirone, V.; Scaglione, F. The role of inflammation in lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH) and its potential impact on medical therapy. Curr. Urol. Rep. 2014, 15, 463. [Google Scholar] [CrossRef] [PubMed]
- Bonvissuto, G.; Minutoli, L.; Morgia, G.; Bitto, A.; Polito, F.; Irrera, N.; Marini, H.; Squadrito, F.; Altavilla, D. Effect of Serenoa repens, lycopene, and selenium on proinflammatory phenotype activation: An in vitro and in vivo comparison study. Urology 2011, 77. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahsan, H.; Mukhtar, H.; Ahmad, N. Combination of vitamin E and selenium causes an induction of apoptosis of human prostate cancer cells by enhancing Bax/Bcl-2 ratio. Prostate 2008, 68, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Qadir, M.I.; Perveen, N.; Ahmad, B.; Saleem, U.; Irshad, T.; Ahmad, B. Inhibitors of apoptotic proteins: New targets for anticancer therapy. Chem. Biol. Drug Des. 2013, 82, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.K.; Lahoua, Z.; Gendron, N.H.; Fetni, R.; Johnston, A.; Davoodi, J.; Rasper, D.; Roy, S.; Slack, R.S.; Nicholson, D.W.; et al. The neuronal apoptosis inhibitory protein is a direct inhibitor of caspases 3 and 7. J. Neurosci. 2002, 22, 2035–2043. [Google Scholar] [PubMed]
- Eslami, M.; Khamechian, T.; Mazoochi, T.; Ehteram, H.; Sehat, M.; Alizargar, J. Evaluation of survivin expression in prostate specimens of patients with prostate adenocarcinoma and benign prostate hyperplasia underwent transurethral resection of the prostate or prostatectomy. Springerplus 2016, 5, 621. [Google Scholar] [CrossRef] [PubMed]
- Altieri, D.C. Survivin—The inconvenient IAP. Semin. Cell Dev. Biol. 2015, 39, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Ren, L.; Wang, X.; Qu, C.; Zhang, Y. Expression of livin, survivin and caspase-3 in prostatic cancer and their clinical significance. Int. J. Clin. Exp. Pathol. 2015, 8, 14034–14039. [Google Scholar] [PubMed]
- Estornes, Y.; Bertrand, M.J. IAPs, regulators of innate immunity and inflammation. Semin. Cell Dev. Biol. 2015, 39, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Ben Jemaa, A.; Bouraoui, Y.; Sallami, S.; Banasr, A.; Nouira, Y.; Oueslati, R. PSA-PSMA profiles and their impact on sera PSA levels and angiogenic activity in hyperplasia and human prostate cancer. Pathol. Biol. 2014, 62, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Gorges, T.M.; Riethdorf, S.; von Ahsen, O.; Nastał, Y.P.; Röck, K.; Boede, M.; Peine, S.; Kuske, A.; Schmid, E.; Kneip, C.; et al. Heterogeneous PSMA expression on circulating tumor cells—A potential basis for stratification and monitoring of PSMA-directed therapies in prostate cancer. Oncotarget 2016, 7, 34930–34941. [Google Scholar] [CrossRef] [PubMed]
- Ieni, A.; Barresi, V.; Grosso, M.; Rosa, M.A.; Tuccari, G. Immunohistochemical evidence of lactoferrin in human embryo-fetal bone and cartilage tissues. Cell Biol. Int. 2010, 34, 845–849. [Google Scholar]
| Parameters | Group A (n = 45) | Group B (n = 45) |
|---|---|---|
| Age, median (IQR) | 65 (55–75) | 65 (56–76) |
| PSA (ng/mL) | 6.0 (4.2–7.8) | 6.2 (4.3–8.0) |
| Prostate volume (cc), median (IQR) | 43 (25–60) | 45 (25–65) |
| Qmax (mL/s), median (IQR) | 12 (5.4–15.0) | 12.0 (6.0–14.5) |
| PVR (cc), median (IQR) | 30 (10.0–80.0) | 32 (10.0–75.0) |
| IPSS, median (IQR) | 18 (12–28) | 20.0 (12.0–29.0) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgia, G.; Micali, A.; Rinaldi, M.; Irrera, N.; Marini, H.; Puzzolo, D.; Pisani, A.; Privitera, S.; Russo, G.I.; Cimino, S.; et al. Survivin and NAIP in Human Benign Prostatic Hyperplasia: Protective Role of the Association of Serenoa repens, Lycopene and Selenium from the Randomized Clinical Study. Int. J. Mol. Sci. 2017, 18, 680. https://doi.org/10.3390/ijms18030680
Morgia G, Micali A, Rinaldi M, Irrera N, Marini H, Puzzolo D, Pisani A, Privitera S, Russo GI, Cimino S, et al. Survivin and NAIP in Human Benign Prostatic Hyperplasia: Protective Role of the Association of Serenoa repens, Lycopene and Selenium from the Randomized Clinical Study. International Journal of Molecular Sciences. 2017; 18(3):680. https://doi.org/10.3390/ijms18030680
Chicago/Turabian StyleMorgia, Giuseppe, Antonio Micali, Mariagrazia Rinaldi, Natasha Irrera, Herbert Marini, Domenico Puzzolo, Antonina Pisani, Salvatore Privitera, Giorgio I. Russo, Sebastiano Cimino, and et al. 2017. "Survivin and NAIP in Human Benign Prostatic Hyperplasia: Protective Role of the Association of Serenoa repens, Lycopene and Selenium from the Randomized Clinical Study" International Journal of Molecular Sciences 18, no. 3: 680. https://doi.org/10.3390/ijms18030680