Expression of the Antioxidative Enzyme Peroxiredoxin 2 in Multiple Sclerosis Lesions in Relation to Inflammation
Abstract
:1. Introduction
2. Results
2.1. Tissue and Lesion Classification
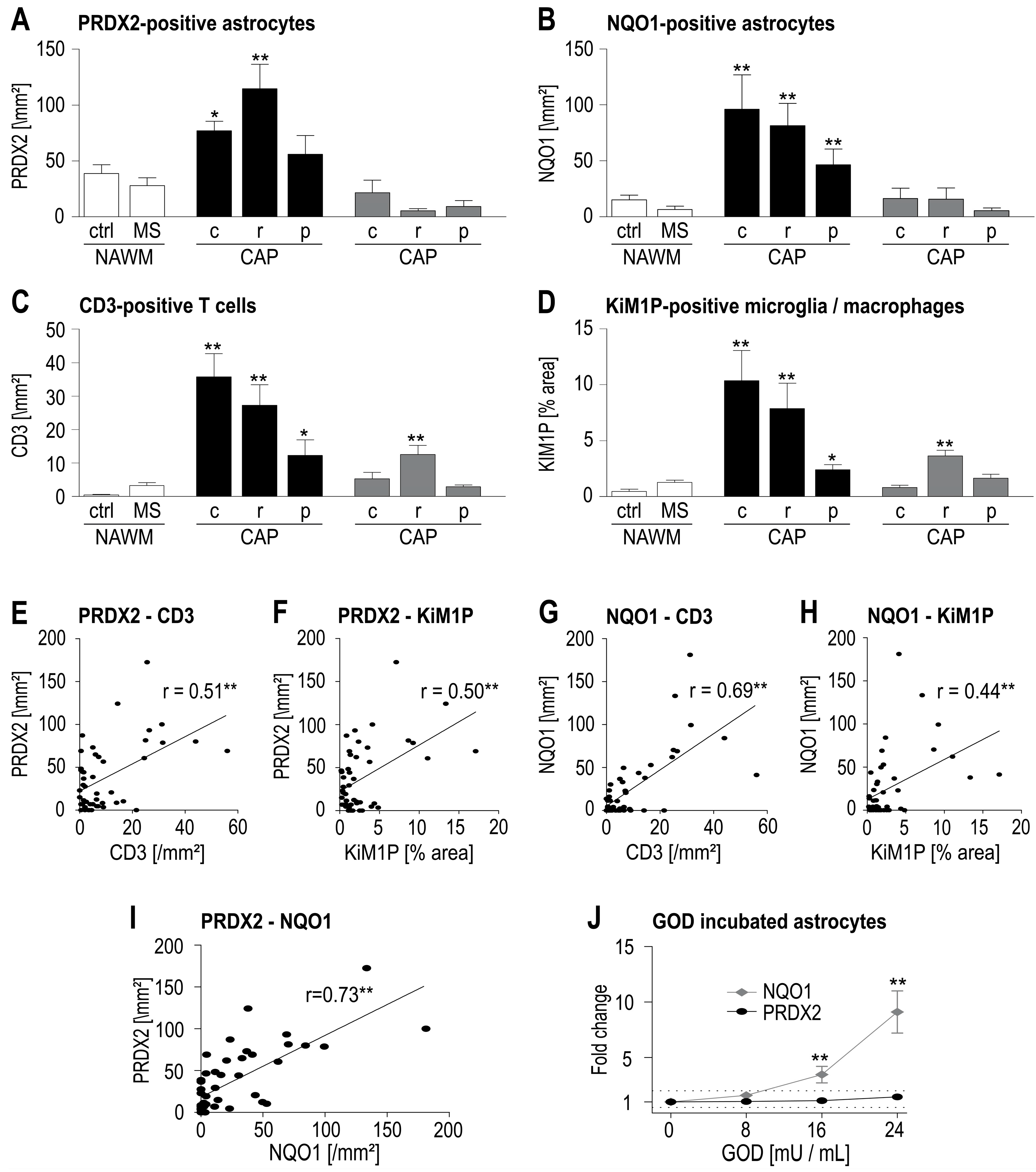
2.2. Astrocytes Show Enhanced Expression of PRDX2 in WML
2.3. PRDX2 Expression Correlates with Inflammation and Oxidative Stress in WML
3. Discussion
4. Material and Methods
4.1. Human Brain Tissue
4.2. Histology and Immunohistochemistry
4.3. Morphometric Analysis and Data Acquisition
4.4. Cell Culture Experiments with Primary Murine Astrocytes
4.5. RNA Extraction and cDNA Synthesis
4.6. Quantitative PCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jack, C.; Antel, J.; Brück, W.; Kuhlmann, T. Contrasting potential of nitric oxide and peroxynitrite to mediate oligodendrocyte injury in multiple sclerosis. Glia 2007, 55, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Haider, L.; Fischer, M.T.; Frischer, J.M.; Bauer, J.; Hoftberger, R.; Botond, G.; Esterbauer, H.; Binder, C.J.; Witztum, J.L.; Lassmann, H. Oxidative damage in multiple sclerosis lesions. Brain 2011, 134, 1914–1924. [Google Scholar] [CrossRef] [PubMed]
- Pappolla, M.A.; Omar, R.A.; Kim, K.S.; Robakis, N.K. Immunohistochemical evidence of oxidative stress in alzheimer’s disease. Am. J. Pathol. 1992, 140, 621–628. [Google Scholar] [PubMed]
- Alam, Z.I.; Jenner, A.; Daniel, S.E.; Lees, A.J.; Cairns, N.; Marsden, C.D.; Jenner, P.; Halliwell, B. Oxidative DNA damage in the parkinsonian brain: An apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J. Neurochem. 1997, 69, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, S.J.; Workman, J.; Hart, P.E.; Mangiarini, L.; Mahal, A.; Bates, G.; Cooper, J.M.; Schapira, A.H. Mitochondrial dysfunction and free radical damage in the huntington R6/2 transgenic mouse. Ann. Neurol. 2000, 47, 80–86. [Google Scholar] [CrossRef]
- Rosen, D.R.; Siddique, T.; Patterson, D.; Figlewicz, D.A.; Sapp, P.; Hentati, A.; Donaldson, D.; Goto, J.; O’Regan, J.P.; Deng, H.X.; et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993, 362, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Steinman, L. No quiet surrender: Molecular guardians in multiple sclerosis brain. J. Clin. Investig. 2015, 125, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Licht-Mayer, S.; Wimmer, I.; Traffehn, S.; Metz, I.; Brück, W.; Bauer, J.; Bradl, M.; Lassmann, H. Cell type-specific Nrf2 expression in multiple sclerosis lesions. Acta Neuropathol. 2015, 130, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Van Horssen, J.; Schreibelt, G.; Bö, L.; Montagne, L.; Drukarch, B.; van Muiswinkel, F.L.; de Vries, H.E. NAD(P)H: Quinone oxidoreductase 1 expression in multiple sclerosis lesions. Free Radic. Biol. Med. 2006, 41, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Holley, J.E.; Newcombe, J.; Winyard, P.G.; Gutowski, N.J. Peroxiredoxin V in multiple sclerosis lesions: Predominant expression by astrocytes. Mult. Scler. 2007, 13, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.T.; Sharma, R.; Lim, J.L.; Haider, L.; Frischer, J.M.; Drexhage, J.; Mahad, D.; Bradl, M.; van Horssen, J.; Lassmann, H. Nadph oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain 2012, 135, 886–899. [Google Scholar] [CrossRef] [PubMed]
- Wood, Z.A.; Poole, L.B.; Hantgan, R.R.; Karplus, P.A. Dimers to doughnuts: Redox-sensitive oligomerization of 2-cysteine peroxiredoxins. Biochemistry 2002, 41, 5493–5504. [Google Scholar] [CrossRef] [PubMed]
- Knoops, B.; Argyropoulou, V.; Becker, S.; Ferte, L.; Kuznetsova, O. Multiple roles of peroxiredoxins in inflammation. Mol. Cells 2016, 39, 60–64. [Google Scholar] [PubMed]
- Perkins, A.; Nelson, K.J.; Parsonage, D.; Poole, L.B.; Karplus, P.A. Peroxiredoxins: Guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem. Sci. 2015, 40, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Wood, Z.A.; Schröder, E.; Robin Harris, J.; Poole, L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003, 28, 32–40. [Google Scholar] [CrossRef]
- Peskin, A.V.; Low, F.M.; Paton, L.N.; Maghzal, G.J.; Hampton, M.B.; Winterbourn, C.C. The high reactivity of peroxiredoxin 2 with H2O2 is not reflected in its reaction with other oxidants and thiol reagents. J. Biol. Chem. 2007, 282, 11885–11892. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Lee, I.K.; Kim, G.W.; Kim, B.U.; Han, Y.-H.; Yu, D.-Y.; Park, H.S.; Kim, K.Y.; Lee, J.S.; Choi, C.; et al. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature 2005, 435, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Sobotta, M.C.; Liou, W.; Stocker, S.; Talwar, D.; Oehler, M.; Ruppert, T.; Scharf, A.N.; Dick, T.P. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat. Chem. Biol. 2015, 11, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Shichita, T.; Hasegawa, E.; Kimura, A.; Morita, R.; Sakaguchi, R.; Takada, I.; Sekiya, T.; Ooboshi, H.; Kitazono, T.; Yanagawa, T.; et al. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat. Med. 2012, 18, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Giulian, D.; Woodward, J.; Young, D.G.; Krebs, J.F.; Lachman, L.B. Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. J. Neurosci. 1988, 8, 2485–2490. [Google Scholar] [PubMed]
- Yong, V.W.; Moumdjian, R.; Yong, F.P.; Ruijs, T.C.; Freedman, M.S.; Cashman, N.; Antel, J.P. Gamma-interferon promotes proliferation of adult human astrocytes in vitro and reactive gliosis in the adult mouse brain in vivo. Proc. Natl. Acad. Sci. USA 1991, 88, 7016–7020. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.T.; Wimmer, I.; Höftberger, R.; Gerlach, S.; Haider, L.; Zrzavy, T.; Hametner, S.; Mahad, D.; Binder, C.J.; Krumbholz, M.; et al. Disease-specific molecular events in cortical multiple sclerosis lesions. Brain 2013, 136, 1799–1815. [Google Scholar] [CrossRef] [PubMed]
- Karplus, P.A. A primer on peroxiredoxin biochemistry. Free Radic. Biol. Med. 2015, 80, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Low, F.M.; Hampton, M.B.; Peskin, A.V.; Winterbourn, C.C. Peroxiredoxin 2 functions as a noncatalytic scavenger of low-level hydrogen peroxide in the erythrocyte. Blood 2007, 109, 2611–2617. [Google Scholar] [CrossRef] [PubMed]
- Salzano, S.; Checconi, P.; Hanschmann, E.-M.; Lillig, C.H.; Bowler, L.D.; Chan, P.; Vaudry, D.; Mengozzi, M.; Coppo, L.; Sacre, S.; et al. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc. Natl. Acad. Sci. USA 2014, 111, 12157–12162. [Google Scholar] [CrossRef] [PubMed]
- Navikas, V.; Link, H. Review: Cytokines and the pathogenesis of multiple sclerosis. J. Neurosci. Res. 1996, 45, 322–333. [Google Scholar] [CrossRef]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The imageJ ecosystem: An open platform for biomedical image analysis. Mol. Rep. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Levison, S.W.; Ting, J.P. Comparison and quantitation of la antigen expression on cultured macroglia and ameboid microglia from lewis rat cerebral cortex: Analyses and implications. J. Neuroimmunol. 1989, 25, 63–74. [Google Scholar] [CrossRef]

| Case | Age | Sex | Cause of Death | Inflammatory Circumstances Perimortal |
|---|---|---|---|---|
| MS-01 | 74 | female | pneumonia | pneumonia |
| MS-02 | 63 | male | unknown | unknown |
| MS-03 | 57 | male | pneumonia | sepsis |
| MS-04 | 51 | female | circulatory collapse | no |
| MS-05 | 49 | male | unknown | unknown |
| MS-06 | 49 | male | drug abuse | no |
| MS-07 | 47 | female | unknown | unknown |
| MS-08 | 44 | female | unknown | unknown |
| MS-09 | 41 | male | pulmonary embolism | no |
| MS-10 | 34 | male | seizure | unknown |
| CON-01 | 67 | female | adult respiratory distress syndrome | pneumonia |
| CON-02 | 63 | female | cardiovascular failure | no |
| CON-03 | 56 | male | lung cancer | unknown |
| CON-04 | 49 | male | multiple organ failure | sepsis |
| CON-05 | 48 | female | cardiovascular failure | no |
| CON-06 | 40 | female | cardiovascular failure | no |
| CON-07 | 35 | female | pulmonary embolism | no |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voigt, D.; Scheidt, U.; Derfuss, T.; Brück, W.; Junker, A. Expression of the Antioxidative Enzyme Peroxiredoxin 2 in Multiple Sclerosis Lesions in Relation to Inflammation. Int. J. Mol. Sci. 2017, 18, 760. https://doi.org/10.3390/ijms18040760
Voigt D, Scheidt U, Derfuss T, Brück W, Junker A. Expression of the Antioxidative Enzyme Peroxiredoxin 2 in Multiple Sclerosis Lesions in Relation to Inflammation. International Journal of Molecular Sciences. 2017; 18(4):760. https://doi.org/10.3390/ijms18040760
Chicago/Turabian StyleVoigt, David, Uta Scheidt, Tobias Derfuss, Wolfgang Brück, and Andreas Junker. 2017. "Expression of the Antioxidative Enzyme Peroxiredoxin 2 in Multiple Sclerosis Lesions in Relation to Inflammation" International Journal of Molecular Sciences 18, no. 4: 760. https://doi.org/10.3390/ijms18040760





