Influence of Speciation of Thorium on Toxic Effects to Green Algae Chlorella pyrenoidosa
Abstract
:1. Introduction
2. Results and Discussion
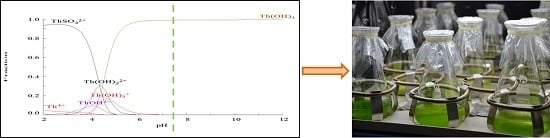
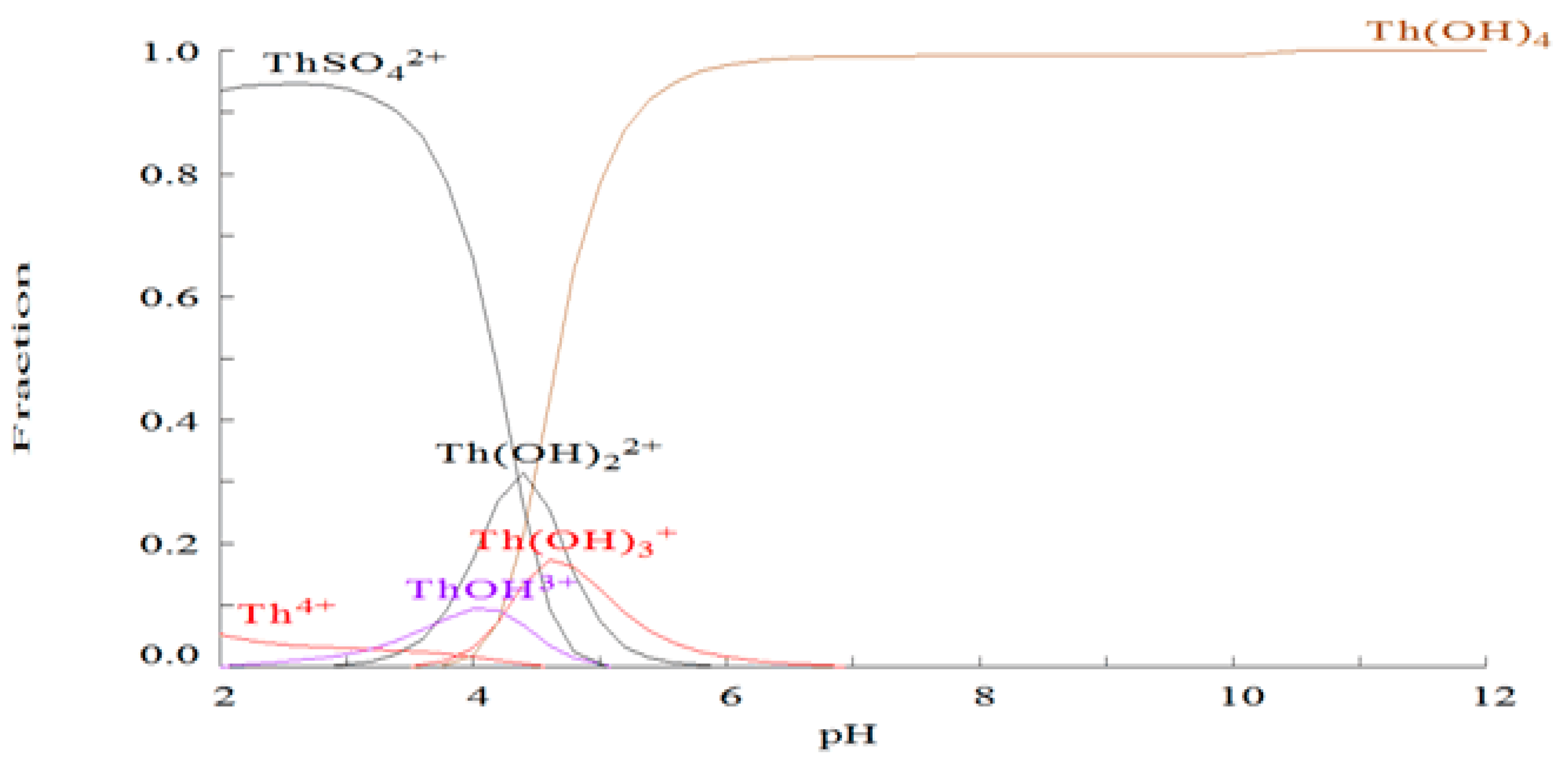
2.1. Chemical Species of Th in the Media
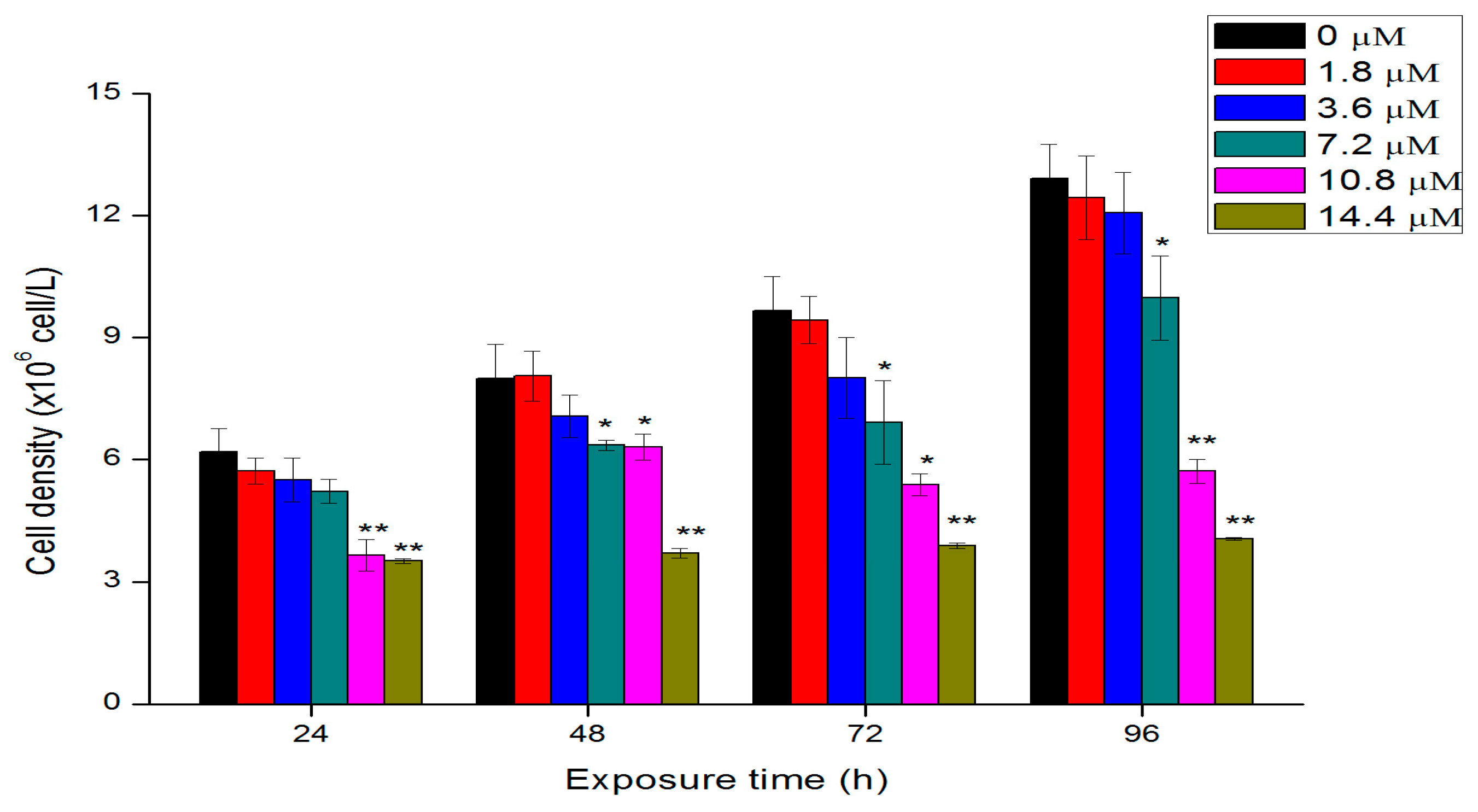
2.2. Effects of Th on Algal Growth
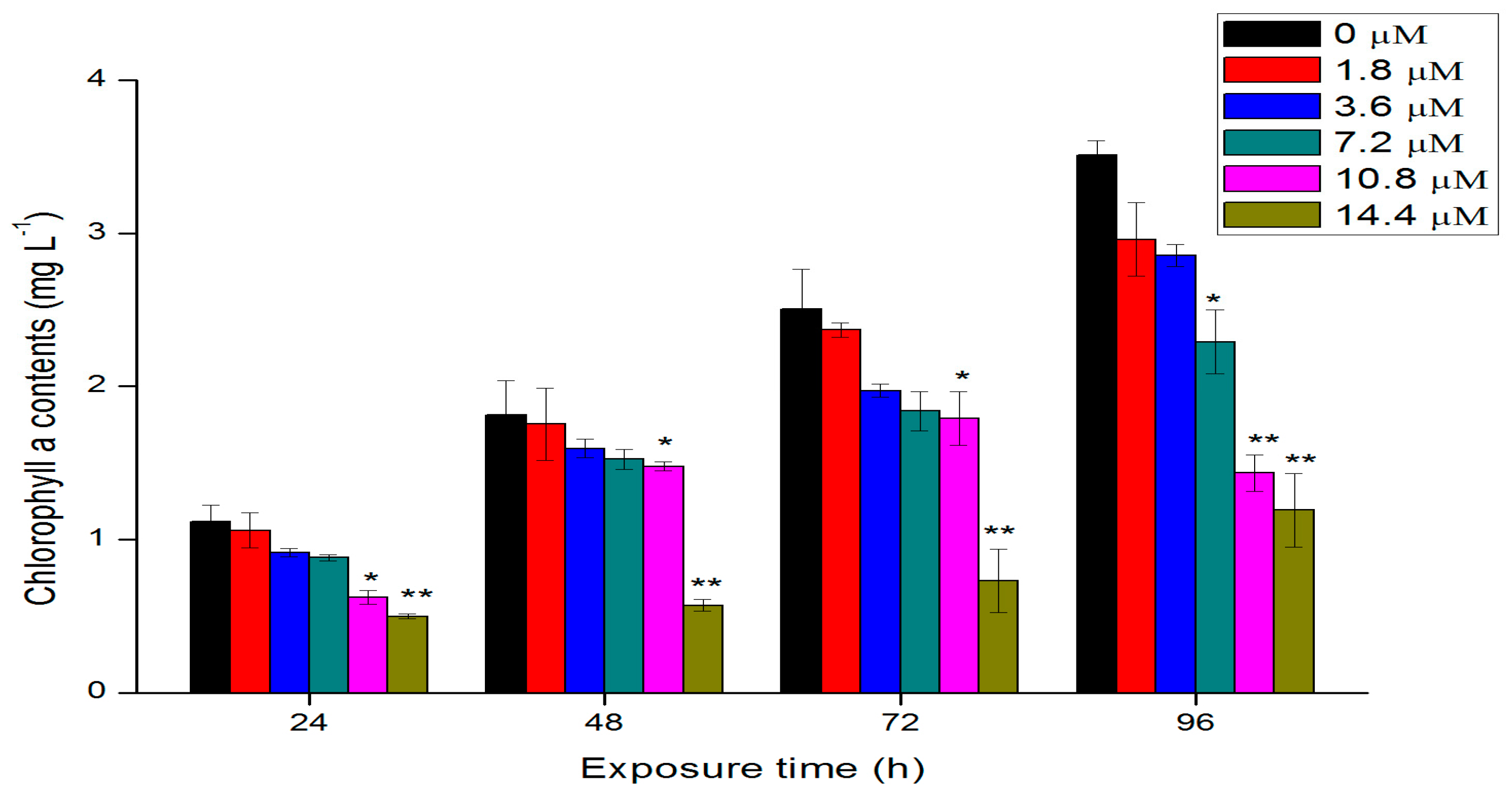
2.3. Effects of Th on Chlorophyll a Contents
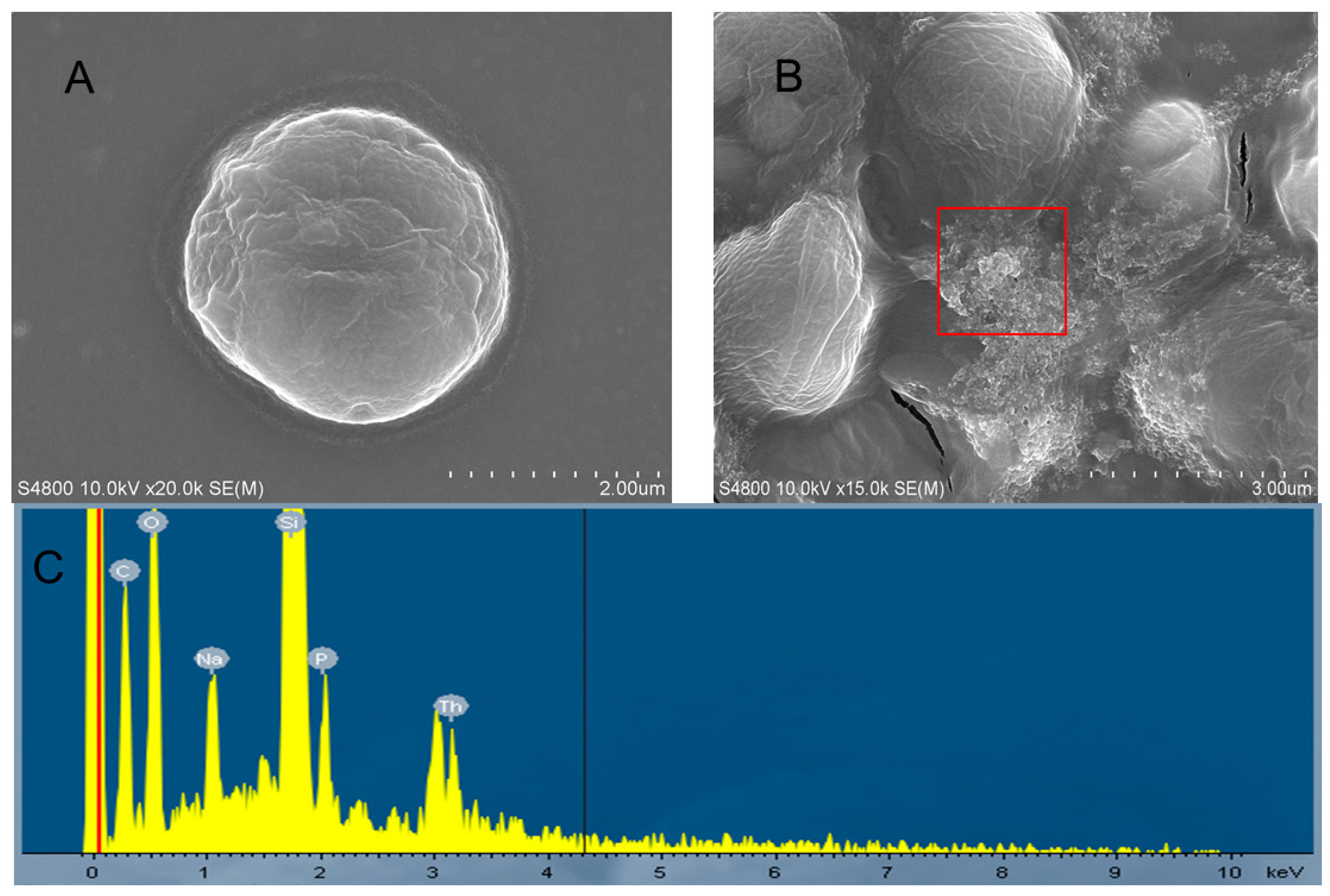
2.4. Morphological Changes
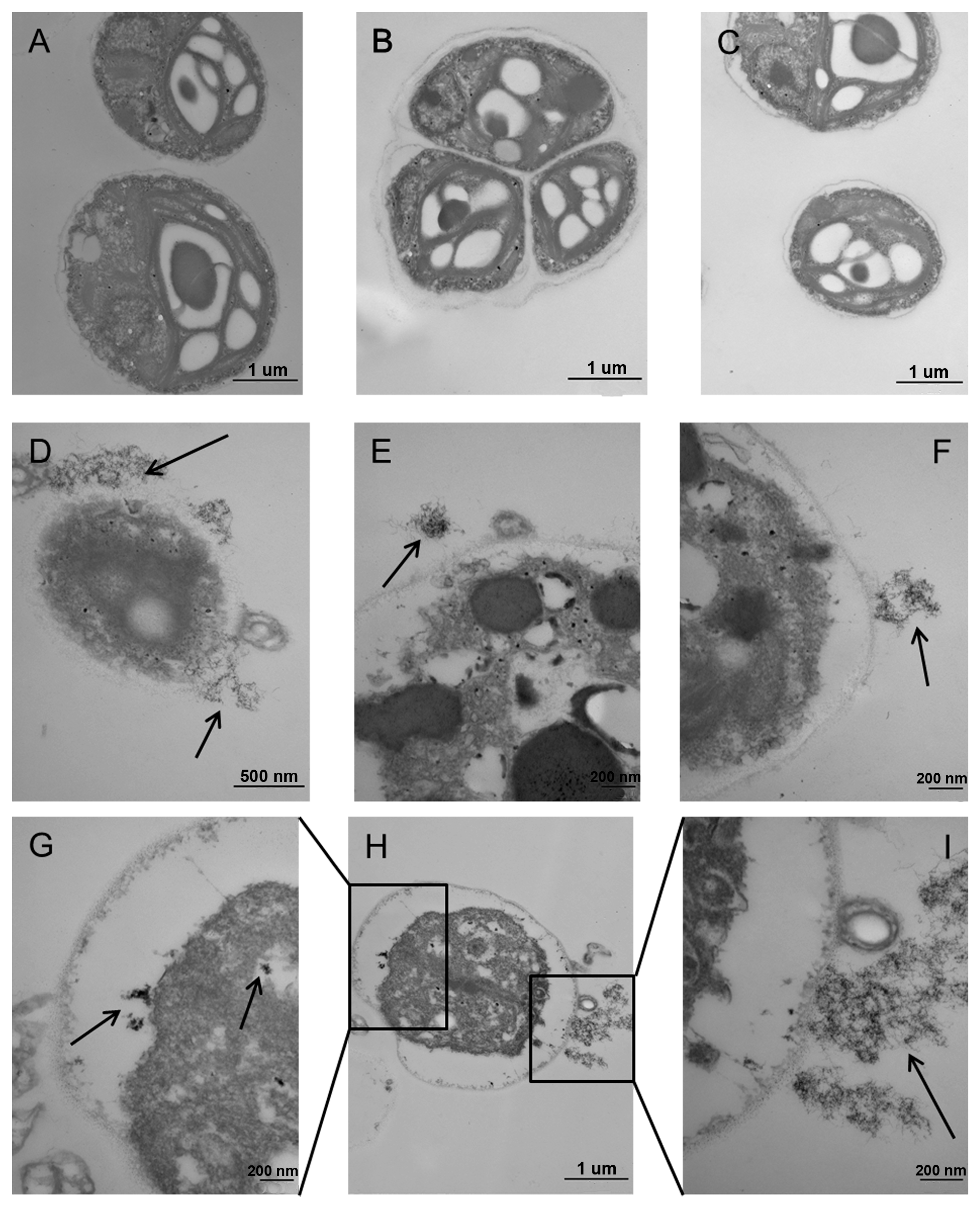
2.5. Ultrastructural Alterations
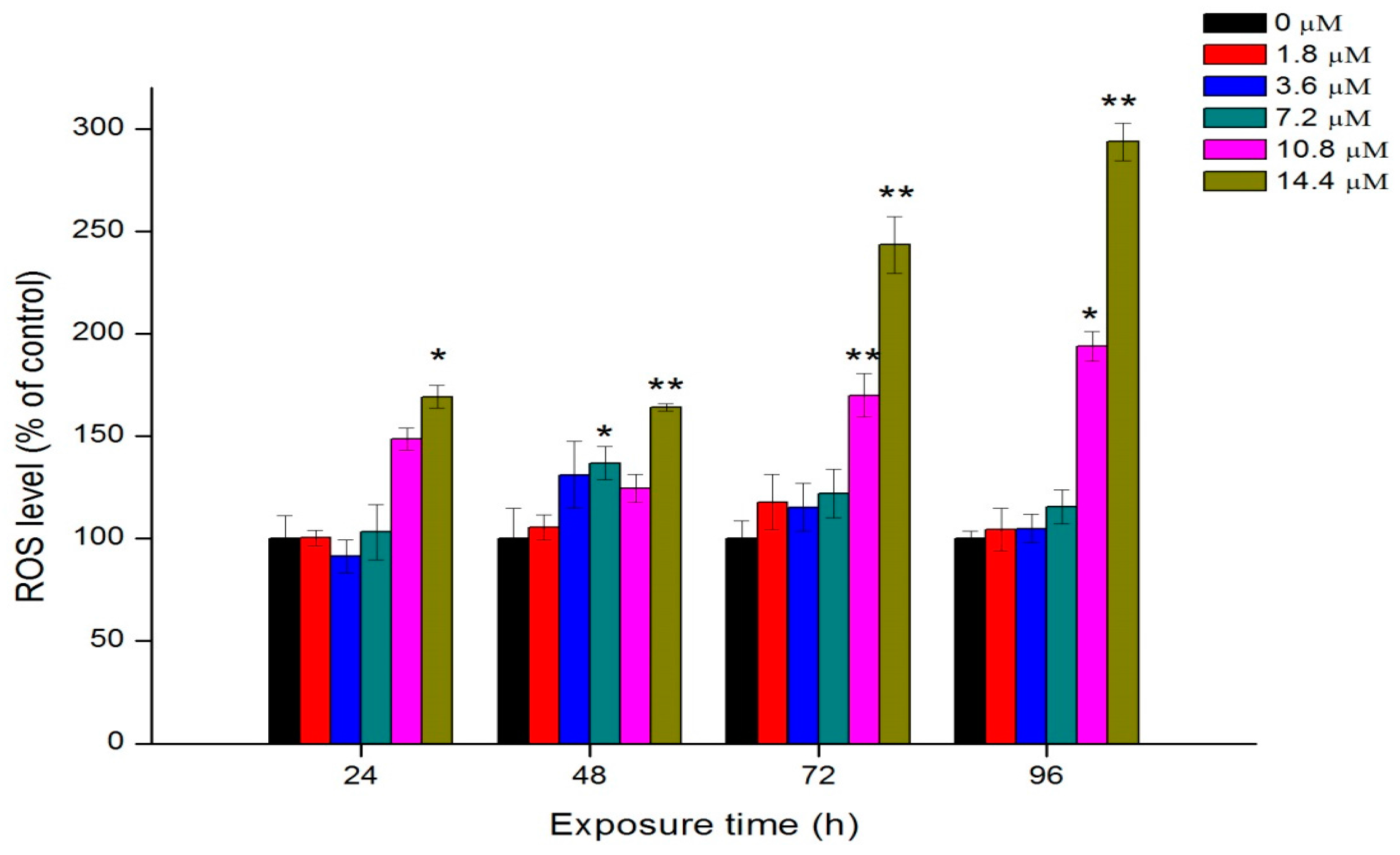
2.6. Oxidative Stress
3. Materials and Methods
3.1. Materials and Chlorella pyrenoidosa Culture
3.2. Algal Growth Assays
3.3. Chlorophyll a Fluorescence Measurements
3.4. Th Speciation in the Cultured Medium
3.5. SEM And TEM Observations
3.6. Oxidative Stress
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Casartelli, E.A.; Miekeley, N. Determination of thorium and light rare-earth elements in soil water and its high molecular mass organic fractions by inductively coupled plasma mass spectrometry and on-line-coupled size-exclusion chromatography. Anal. Bioanal. Chem. 2003, 377, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Malanca, A.; Pessina, V.; Dallara, G. Assessment of the natural radioactivity in the brazilian state of rio grande do norte. Health Phys. 1993, 65, 298. [Google Scholar] [CrossRef] [PubMed]
- Ivanovich, M.; Harmon, R.S. Uranium-series disequilibrium: Applications to earth, marine, and environmental science. Geochim. Cosmochim. Acta 1993, 57, 4327–4328. [Google Scholar]
- Hughart, J.L.; Bashor, M.M. Industrial Chemicals and Terrorism: Human Health Threat Analysis, Mitigation and Prevention; Bureau of Justice Statistics: Washington, DC, USA, 1969. [Google Scholar]
- Howell, R.W. Patient exposures and consequent risks from nuclear medicine procedures. Health Phys. 2011, 100, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.S.; Duarte, I.M.; Paiva, A.V.; Yunes, S.N.; Almeida, C.E. The role of chemical interactions between thorium, cerium, and lanthanum in lymphocyte toxicity. Arch. Environ. Occup. Health 2014, 69, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Allred, B.E.; Rupert, P.B.; Gauny, S.S.; An, D.D.; Ralston, C.Y.; Sturzbecher-Hoehne, M.; Strong, R.K.; Abergel, R.J. Siderocalin-mediated recognition, sensitization, and cellular uptake of actinides. Proc. Natl. Acad. Sci. USA 2015, 112, 10342. [Google Scholar] [CrossRef] [PubMed]
- Evseeva, T.; Geras’kin, S.; Majstrenko, T.; Brown, J.; Belykh, E. Comparative estimation of 232 th and stable Ce (III) toxicity and detoxification pathways in freshwater alga chlorella vulgaris. Chemosphere 2010, 81, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- De Queiroz, J.C.; Ferreira, A.C.D.M.; da Costa, A.C.A. The growth of Monoraphidium sp. and Scenedesmus sp. Cells in the presence of thorium. Sci. World J. 2012, 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Borgmann, U.; Couillard, Y.; Doyle, P.; Dixon, D.G. Toxicity of sixty-three metals and metalloids to Hyalella azteca at two levels of water hardness. Environ. Toxicol. Chem. 2005, 24, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Correa, L.M.; Kochhann, D.; Becker, A.G.; Pavanato, M.A.; Llesuy, S.F.; Loro, V.L.; Raabe, A.; Mesko, M.F.; Flores, E.M.; Dressler, V.L. Biochemistry, cytogenetics and bioaccumulation in silver catfish (Rhamdia quelen) exposed to different thorium concentrations. Aquat. Toxicol. 2008, 88, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Kochhann, D.; Pavanato, M.A.; Llesuy, S.F.; Correa, L.M.; Riffel, A.P.K.; Loro, V.L.; Mesko, M.F.; Flores, É.M.; Dressler, V.L.; Baldisserotto, B. Bioaccumulation and oxidative stress parameters in silver catfish (Rhamdia quelen) exposed to different thorium concentrations. Chemosphere 2009, 77, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, J.; Peng, C.; Ding, Y.; He, X.; Zhang, P.; Li, N.; Lan, T.; Wang, D.; Zhang, Z. Toxicity of cerium and thorium on daphnia magna. Ecotoxicol. Environ. Saf. 2016, 134, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Mernagh, T.P.; Miezitis, Y. A Review of the Geochemical Processes Controlling the Distribution of Thorium in the Earth’s Crust and Australia’s Thorium Resources; Geoscience Australia: Canberra, Australia, 2007. [Google Scholar]
- Higashi, S. Determination of the solubility of thorium hydroxide. Bull. Inst. Chem. Res. Kyoto Univ. 1959, 37, 200–206. [Google Scholar]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Talarek, M.; Bralska, M.; Zambrzycka, E. The effect of lead on the growth, content of primary metabolites, and antioxidant response of green alga acutodesmus obliquus (chlorophyceae). Environ. Sci. Pollut. Res. 2015, 22, 19112–19123. [Google Scholar] [CrossRef] [PubMed]
- Lamai, C.; Kruatrachue, M.; Pokethitiyook, P.; Upatham, E.S.; Soonthornsarathool, V. Toxicity and accumulation of lead and cadmium in the filamentous green alga cladophora fracta (O.F. Muller ex vahl) kutzing: A laboratory study. Scienceasia 2005, 31, 121–127. [Google Scholar] [CrossRef]
- Sathasivam, R.; Ebenezer, V.; Guo, R.; Ki, J.S. Physiological and biochemical responses of the freshwater green algae closterium ehrenbergii to the common disinfectant chlorine. Ecotoxicol. Environ. Saf. 2016, 133, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhou, K.; Yang, K.; Lin, D. Heteroagglomeration of oxide nanoparticles with algal cells: Effects of particle type, ionic strength and ph. Environ. Sci. Technol. 2014, 49, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Ji, J.; Yang, K.; Lin, D.; Wu, F. Systematic and quantitative investigation of the mechanism of carbon nanotubes’ toxicity toward algae. Environ. Sci. Technol. 2012, 46, 8458–8466. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cao, X.; Liu, X.; Wang, Z.; Zhang, C.; White, J.C.; Xing, B. Interactions of cuo nanoparticles with the algae Chlorella pyrenoidosa: Adhesion, uptake and toxicity. Nanotoxicology 2016, 10, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.B.; Von, D.K.F.; Hofmann, T.; Baalousha, M.; Ottofuelling, S.; Baun, A. Algal testing of titanium dioxide nanoparticles—Testing considerations, inhibitory effects and modification of cadmium bioavailability. Toxicology 2010, 269, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Jie, L.; Wei, L.; Yang, J.; Chernick, M.; Hinton, D.E. Developmental toxicity from exposure to various forms of mercury compounds in medaka fish (Oryzias latipes) embryos. PeerJ 2016, 4, e2282. [Google Scholar]
- Lei, C.; Zhang, L.; Yang, K.; Zhu, L.; Lin, D. Toxicity of iron-based nanoparticles to green algae: Effects of particle size, crystal phase, oxidation state and environmental aging. Environ. Pollut. 2016, 218, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cao, X.; Wang, Z.; Dai, Y.; Xing, B. Mechanistic understanding toward the toxicity of graphene-family materials to freshwater algae. Water Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lei, C.; Chen, J.; Yang, K.; Zhu, L.; Lin, D. Effect of natural and synthetic surface coatings on the toxicity of multiwalled carbon nanotubes toward green algae. Carbon 2015, 83, 198–207. [Google Scholar] [CrossRef]
- Test, B.R.M. Oecd guideline for the testing of chemicals. Mortality 1997, 1, 10. [Google Scholar]
- Jeffrey, S.T.; Humphrey, G. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Puigdomenech, I. Program MEDUSA (Make Equilibrium Diagrams Using Sophisticated Algorithms); Royal Institute of Technology, Inorganic Chemistry: Stockolm, Sweden, 2010. [Google Scholar]
- Xia, B.; Chen, B.; Sun, X.; Qu, K.; Ma, F.; Du, M. Interaction of tio 2 nanoparticles with the marine microalga nitzschia closterium: Growth inhibition, oxidative stress and internalization. Sci. Total Environ. 2015, 508, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Saison, C.; Perreault, F.; Daigle, J.-C.; Fortin, C.; Claverie, J.; Morin, M.; Popovic, R. Effect of core–Shell copper oxide nanoparticles on cell culture morphology and photosynthesis (photosystem II energy distribution) in the green alga, chlamydomonas reinhardtii. Aquat. Toxicol. 2010, 96, 109–114. [Google Scholar] [CrossRef] [PubMed]
| Time (h) | EC50 (μM) | 95% CI (μM) |
|---|---|---|
| 24 | 18.5 | 10.9 26.7 |
| 48 | 16.7 | 14.7 21.7 |
| 72 | 11.8 | 10.2 14.1 |
| 96 | 10.4 | 7.9 15.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.; Ma, Y.; Ding, Y.; He, X.; Zhang, P.; Lan, T.; Wang, D.; Zhang, Z.; Zhang, Z. Influence of Speciation of Thorium on Toxic Effects to Green Algae Chlorella pyrenoidosa. Int. J. Mol. Sci. 2017, 18, 795. https://doi.org/10.3390/ijms18040795
Peng C, Ma Y, Ding Y, He X, Zhang P, Lan T, Wang D, Zhang Z, Zhang Z. Influence of Speciation of Thorium on Toxic Effects to Green Algae Chlorella pyrenoidosa. International Journal of Molecular Sciences. 2017; 18(4):795. https://doi.org/10.3390/ijms18040795
Chicago/Turabian StylePeng, Can, Yuhui Ma, Yayun Ding, Xiao He, Peng Zhang, Tu Lan, Dongqi Wang, Zhaohui Zhang, and Zhiyong Zhang. 2017. "Influence of Speciation of Thorium on Toxic Effects to Green Algae Chlorella pyrenoidosa" International Journal of Molecular Sciences 18, no. 4: 795. https://doi.org/10.3390/ijms18040795