Ficus umbellata Vahl. (Moraceae) Stem Bark Extracts Exert Antitumor Activities In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
2.1. Preliminary Phytochemical Analysis
2.2. Cytotoxicity of F. umbellata Extracts
2.3. Effects on Cell Death Mechanism
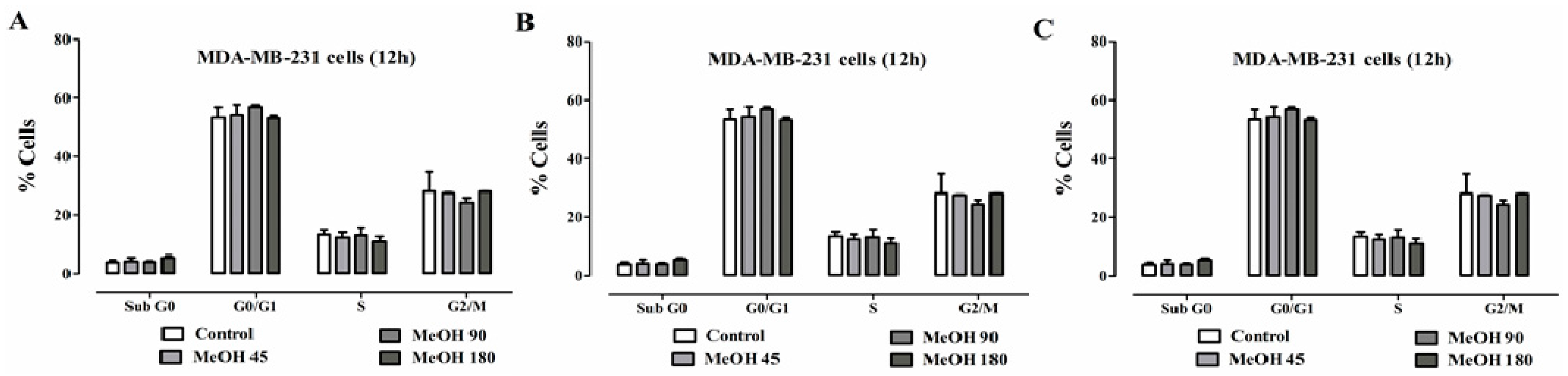
2.4. Effects on Cell Cycle
2.5. Effects on Cell Migration and Invasion
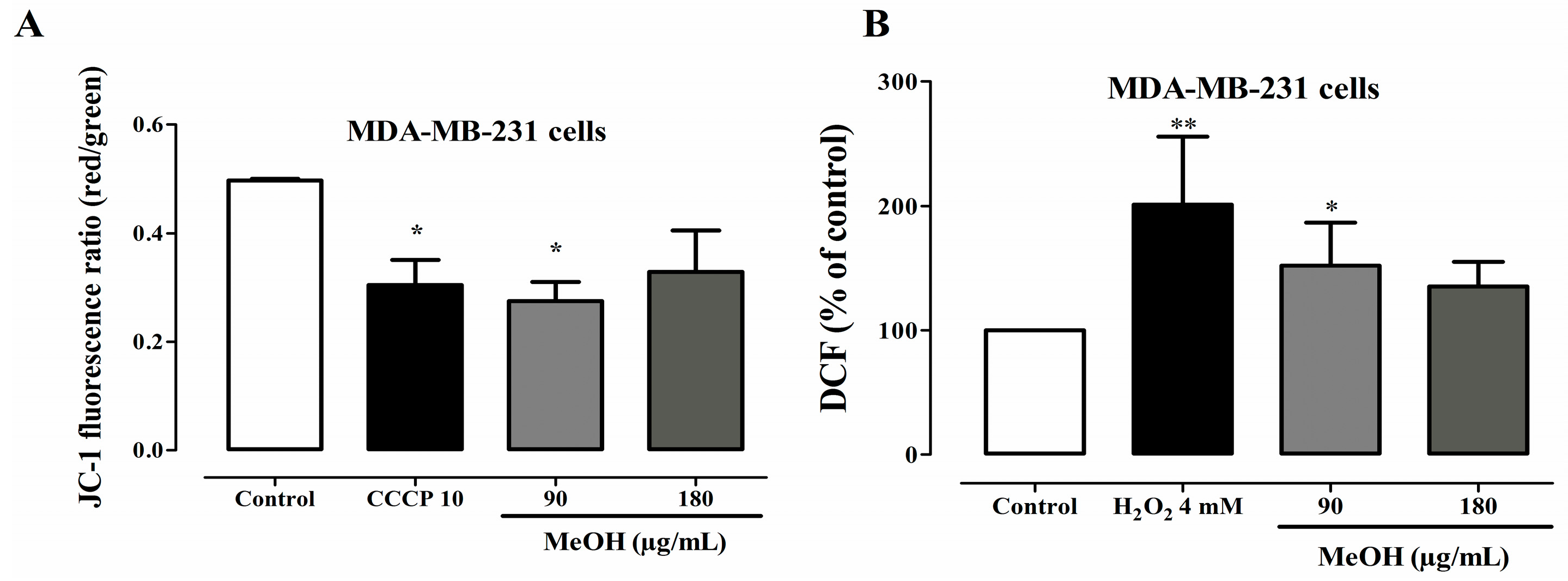
2.6. Effects on Mitochondrial Transmembrane Potential
2.7. Effects on ROS Levels
2.8. Effects on Bcl-2 Family Protein Expression
2.9. Effects on Caspases Activity
2.10. Effects on Body Weight and Survival
2.11. Effects on Ovarian Tumors
2.12. Histomorphological Analysis of Estrogen Target Organs
2.13. Effects of F. umbellata Treatment on Relative Organ Weights
2.14. Effects of F. umbellata Extracts on Various Toxicological Parameters
2.15. In Vivo Antioxidant Activities of F. umbellata Extracts
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Preparation of Extracts and Isolation of 7-Methoxycoumarin
4.4. Preliminary Phytochemical Investigations
4.5. Cell Lines
4.6. Cell Culture
4.7. Animals
4.8. Cell Viability Assay
4.9. Morphological Identification for Cell Death
4.10. Cell Death Mechanism by Cytometry
4.11. Cell Cycle Analysis
4.12. Wound-Healing Assay
4.13. Cell Invasion Assay
4.14. Measurement of the Mitochondrial Transmembrane Potential
4.15. Reactive Oxygen Species (ROS) Detection
4.16. Determination of Caspase Activities
4.17. Western Blot Analysis
4.18. DMBA- Induced Carcinogenesis in Rats
4.19. Histological Analysis
4.20. Biochemical Analysis
4.21. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AE | Aqueous extract of F. umbellata |
| BW | Body weight |
| CC50 | CC50, cytotoxic concentration for 50% of the cells |
| DCM | Dichloromethane |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMBA | 7,12-Dimethylbenz(a)anththracene |
| DMSO | Dimethylsulfoxide |
| ER | Estrogen receptor |
| FBS | Fetal bovine serum |
| FCS | Fetal calf serum |
| HEPES | 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid |
| Hb | Hemoglobin |
| Ht | Hematocrit |
| MDA | Malondialdehyde |
| MCH | Mean corpuscular hemoglobin |
| MCHC | Mean corpuscular hemoglobin concentration |
| MCV | Mean corpuscular volume |
| MeOH | Methanol |
| NHC | National Herbarium of Cameroon |
| NOR | Normal control |
| RBC | Red blood cell |
| RPMI | Roswell Park Memorial Institute medium |
| ROS | Reactive oxygen species |
| SEM | Standard error of mean |
| TAM | Tamoxifen |
References
- Gennari, C.; Castoldi, D.; Sharon, O. Natural products with taxol-like anti-tumor activity: Synthetic approaches to eleutherobin and dictyostatin. Pure Appl. Chem. 2007, 79, 173–180. [Google Scholar] [CrossRef]
- Cancer Report Worldwide. Crisis of cancer impact worldwide exposed. Press Release WCD. 2014. Available online: http://www.worldcancerday.org/wcd-2014-resources/press-release-wcd-2014.
- Sankaranarayanan, R.; Ferlay, J. Worldwide burden of gynaecological cancer: The size of the problem. Best Pract. Res. Clin. Obs. Gynaecol 2006, 20, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013; Available online: http://globocan.iarc.fr (accessed on 9 October 2014).
- Vanderhyden, B.C. Loss of ovarian function and the risk of ovarian cancer. Cell Tiss. Res. 2005, 322, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Stakleff, K.D.; Von Gruenigen, V.E. Rodent models for ovarian cancer research. Int. J. Gynecol. Cancer 2003, 13, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.A.; Kimler, B.F.; Fabian, C.J.; Petroff, B.K. Characterization of a preclinical model of simultaneous breast and ovarian cancer progression. Carcnogenesis 2007, 28, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Jobsen, J.J.; Van-der-Palen, J.; Brinkhuis, M.; Ong, F.; Struikmans, H. Sequence of radiotherapy and chemotherapy in breast cancer after breast-conserving surgery. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, M.O.; Kavan, P.; Miller, W.H., Jr.; Panasci, L.; Assouline, S.; Johnson, N.; Cohen, V.; Patenaude, F.; Pollak, M.; Jagoe, R.T.; et al. Systemic cancer therapy: Achievements and challenges that lie ahead. Front. Pharmacol 2013, 4, 57. [Google Scholar] [CrossRef] [PubMed]
- Kingham, T.P.; Alatise, O.I.; Vanderpuye, V.; Casper, C.; Abantanga, F.A.; Kamara, T.B.; Olopade, O.I.; Habeebu, M.; Abdulkareem, F.B.; Denny, V. Treatment of cancer in sub-Saharan Africa. Lancet Oncol. 2013, 14, 158–167. [Google Scholar] [CrossRef]
- Pui, C.H.; Evans, W.E.N. Treatment of acute lymphoblastic leukemia. Eng. J. Med. 2006, 354, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Efferth, T. Pharmacogenomics of Cameroonian traditional herbal medicine for cancer therapy. J. Ethnopharmacol. 2011, 137, 752–766. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A. Editorial: Current advances in cancer prevention and treatment by natural products. Curr. Pharm. Biotechnol. 2012, 13, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Block, K.A. Broad-spectrum integrative design for cancer prevention and therapy: The challenge ahead. Semin. Cancer Biol. 2015, 35, 1–4. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, Traditional Medicine. Fact Sheet 134. 2003–2005. Available online: http://www.pharmpress.com/files/docs/sample%20chapter(2) (accessed on 28 July 2012).
- Arbonnier, M. Arbres, Arbustes et Lianes des Zones Sèches d’Afrique de l’Ouest, Quæ RD 10 ed.; Service des Publications Scientifiques: Paris, France, 2009; pp. 396–416. [Google Scholar]
- Guarrera, P.M. Traditional phytotherapy in Central Italy (Marche, Abruzzo, and Latium). Fitoterapia 2005, 76, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Rubnov, S.; Kashman, Y.; Rabinowitz, R.; Schlesinger, M.; Mechoulam, R. Suppressors of cancer cell proliferation from fig (Ficus carica) resin: Isolation and structure elucidation. J. Nat. Prod. 2001, 64, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Zingue, S.; Michel, T.; Tchatchou, J.; Magne Nde, C.B.; Winter, E.; Monchot, A.; Awounfack, C.F.; Djiogue, S.; Clyne, C.; Fernandez, X.; et al. Estrogenic effects of Ficus umbellata Vahl. (Moraceae) extracts and their ability to alleviate some menopausal symptoms induced by ovariectomy in Wistar rats. J. Ethnopharmacol. 2016, 179, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Weyermann, J.; Lochmann, D.; Zimmer, A. A practical note on the use of cytotoxicity assays. Int. J. Pharm. 2005, 288, 369–376. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, T.A.; Ramalho, R.M.; Luo, X.; Ramalhete, C.; Rodrigues, C.M.; Ferreira, M.J. Isoflavones as apoptosis inducers in human hepatoma huh-7 cells. Phytother. Res. 2011, 25, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.; Whitehead, S.A. Phytoestrogens and breast cancer promoters or protectors? Endocr. Relat. Cancer 2006, 13, 995–1015. [Google Scholar] [CrossRef] [PubMed]
- Steffen, U.S.; Weber, B.; Siegers, C. Antitumor-activities of coumarin, 7-hydroxy-coumarin and its glucuronide in several human tumor cell lines. Res. Commun. Mol. Pathol. Pharmacol. 1998, 99, 193–206. [Google Scholar]
- Ovadje, P.; Roma, A.; Steckle, M.; Nicoletti, L.; Arnason, J.T.; Pandey, S. Advances in the research and development of natural health products as main stream cancer therapeutics. Evid. Based Complement. Alternat. Med. 2015, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Boik, J. Natural Compounds. In Cancer Therapy; Farnell, S., Ed.; Oregon Medical Press: Minneapolis, MN, USA, 2001. [Google Scholar]
- Cotter, T.G. Apoptosis and cancer: The genesis of a research field. Nat. Rev. Cancer 2009, 7, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Kirkin, V.; Joos, S.; Zornig, M. The role of Bcl-2 family members in tumorigenesis. Biochim. Biophys. Acta 2004, 1644, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Kiechle, F.L.; Zhang, X.B. Apoptosis: Biochemical aspects and clinical implications. Clin. Chim. Acta 2002, 326, 27–45. [Google Scholar] [CrossRef]
- Riedl, S.J.; Shi, Y.G. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004, 5, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Seol, D.W. The role of mitochondria in apoptosis. BMB Rep. 2008, 41, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Banjerdpongchai, R.; Wudtiwai, B.; Pompimon, W. Stigmalactam from Orophea enterocarpa induces human cancer cell apoptosis via a mitochondrial pathway. Asian Pac. J. Cancer Prev. 2014, 15, 10397–10400. [Google Scholar] [CrossRef] [PubMed]
- Billen, L.P.; Shamas-Din, A.; Andrews, D.W. Bid: A Bax-like BH3 protein. Oncogene 2008, 27, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Kim, M.O.; Lee, J.D.; Choi, Y.H.; Kim, G.Y. Rosmarinic acid sensitizes cell death through suppression of TNF-alpha-induced NF-kappaB activation and ROS generation in human leukemia U937 cells. Cancer Lett. 2009, 288, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Salvesen, G.S.; Duckett, C.S. IAP proteins: Blocking the road to death’s door. Nat. Rev. Mol. Cell Biol. 2002, 3, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Sayers, T.J. Targeting the extrinsic apoptosis signaling pathway for cancer therapy. Cancer Immunol. Immunother. 2011, 60, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ling, Y.; Chen, Y.; Li, C.L.; Feng, F.; You, Q.D.; Lu, N.; Guo, Q.L. Flavonoid baicalein suppresses adhesion, migration and invasion of MDA-MB-231 human breast cancer cells. Cancer Lett. 2010, 297, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Bardia, A.; Wittner, B.S.; Stott, S.L.; Smas, M.E.; Ting, D.T.; Isakoff, S.J.; Ciciliano, J.C.; Wells, M.N.; Shah, A.M.; et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013, 339, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Nakajima, M.; Yokoi, T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005, 227, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Russo, J.; Russo, I.H. Atlas and histologic classification of Tumors of rat mammary gland. J. Mamm. Gland. Biol. Neoplasia 2000, 5, 187–200. [Google Scholar] [CrossRef]
- Gibellini, L.; Pinti, M.; Nasi, M.; Montagna, J.P.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cooper, E.L.; Cossarizza, A. Quercetin and Cancer Chemoprevention (Review). Evid. Based Complement. Altern. Med. 2011. [Google Scholar] [CrossRef] [PubMed]
- Zingue, S.; Cisilotto, J.; Tueche, A.B.; Bishayee, A.; Mefegue, F.A.; Sandjo, L.P.; Magne Nde, C.B.; Winter, E.; Michel, T.; Ndinteh, D.T.; et al. Crateva adansonii DC, an African ethnomedicinal plant, exerts cytotoxicity in vitro and prevents experimental mammary tumorigenesis in vivo. J. Ethnopharmacol. 2016, 190, 183–199. [Google Scholar] [CrossRef] [PubMed]
- McGuire, W.L. Hormone receptors; their role in predicting prognosis and response to endocrine therapy. Semin. Oncol. 1978, 5, 428. [Google Scholar] [PubMed]
- Howell, J.S.; Marchant, J.; Orr, J.W. The induction of ovarian tumours in mice with 9: 10-dimethyl-1: 2-benzanthracene. Br. J. Cancer 1954, 8, 635. [Google Scholar] [CrossRef] [PubMed]
- Engelbreth-Holm, J.; Lefevre, H. Acceleration of the development of leukemias and mammary carcinomas in mice by 9:10-dimethyl-1:2-benzanthracene. Cancer Res. 1941, 1, 102–108. [Google Scholar]
- Jull, J.W.; Streeter, D.J.; Statherland, L. The mechanism of induction of ovarian tumors in the mouse by 7,12-dimethylbenz (alpha) anthracene. Effect of steroid hormones and carcinogen concentration in vivo. J. Nat. Cancer Inst. 1966, 37, 409–420. [Google Scholar] [PubMed]
- Taguchi, O.; Michael, S.D.; Nishizuka, Y. Rapid Induction of Ovarian Granulosa Cell Tumors by 7,12-Dimethylbenz(fl)anthracene in Neonatally Estrogenized Mice. Cancer Res. 1988, 48, 425–429. [Google Scholar] [PubMed]
- Sak, K. Site-specific anticancer effects of dietary flavonoid quercetin. Nutr. Cancer 2014, 66, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Huang, T.; Rice, M.S.; Rimm, E.B.; Tworoger, S.S. Intake of dietary flavonoids and risk of epithelial ovarian cancer. Am. J. Clin. Nutr. 2014, 100, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- So, F.V.; Guthrie, N.; Chambers, A.F.; Carroll, K.K. Inhibition of proliferation of estrogen receptor-positive MCF-7 human breast cancer cells by flavonoïds in the presence and absence of excess of estrogen. Cancer Lett. 1997, 112, 127–133. [Google Scholar] [CrossRef]
- Nordberg, J.; Arner, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Welch, J.T. Advances in the preparation of biologically active organofluorine compounds. Tetrahedron 1987, 43, 3123–3197. [Google Scholar] [CrossRef]
- Kuper, H.; Tzonou, A.; kaklamani, E.; Hsieh, C.C.; Lagiou, P.; Adami, H.O. Tobacco smoking alcohol consumption and their interaction in the causation of hepetocellular carcinoma. Int. J. Cancer 2000, 85, 498–502. [Google Scholar] [CrossRef]
- Odebiyi, A.; Sofowora, A.E. Phytochemical screening of Nigerian medical plants part II. Lloydia 1978, 41, 234–246. [Google Scholar] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scanving effects on superoxide radicals. J. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Becker, K.; Abel, H.; Pawelzik, E. Nutrient contents, rumen protein degradability and antinutritional factors in some colour and white flowering cultivars of Vicia fababeans. J. Sci. Food. Agric. 1997, 75, 511–520. [Google Scholar] [CrossRef]
- Hazra, B.; Biswas, S.; Mandal, M. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement. Alternat. Med. 2008, 8, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Randall, R.J.; Lewis, A. The folin by oliver. Readings 1951, 193, 265–275. [Google Scholar]
- Faustino-Rocha, A.; Oliveira, P.A.; Pinho-Oliveira, J.; Teixeira-Guedes, C.; Soares-Maia, R.; da Costa, R.G.; Colaço, B.; Pires, M.J.; Colaço, J.; Ferreira, R.; et al. Estimation of rat mammary tumor volume using caliper and ultrasonography measurements. Lab. Animal 2013, 42, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gonal, A.G.; Bardwill, G.S.; David, M.M. Determination of serum proteins by the means of biuret reactions. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar]
- Misra, F. Determination of the Level of Superoxide Dismutase in Whole Blood; Yale Univ. Press: New Haven, CT, USA, 1972; pp. 101–109. [Google Scholar]
- Wilbur, K.M.; Bernheim, F.; Shapiro, O.W. Determination of lipid peroxidation. Arch. Biochem. Biophys. 1949, 24, 305–310. [Google Scholar]











| Chemical Names | Crystal Color | Structure, Molecular Weight and Formula |
|---|---|---|
| 7-Methoxycoumarin Methylumbelliferone 7-Methoxy-2H-chromen-2-one | White |  Molecular weight = 176.1 M Molecular formula = C10H8O3 |
| N° | Phytochemical Class | Concentration of F. umbellata Extract | |
|---|---|---|---|
| Aqueous | Methanolic | ||
| 1 | Total phenols | 540.21 ± 4.42 | 651.31 ± 12.03 |
| 2 | Flavonoids | 268.25 ± 35.55 | 545.33 ± 99.39 |
| 3 | Flavonols | 78.22 ± 6.11 | 180.15 ± 10.74 |
| 4 | Alkaloids | 60.21 ± 14.38 | 120.21 ± 6.04 |
| CC50 (µg/mL or µM) | |||||||
| A | MeOH | AE | FU-Hex | FU-DCM | FU-R | MC | |
| MCF-7 | 250 | >300 | >300 | 197 | >300 | >300 | |
| MDA-MB-231 | 180 | >300 | 190 | 180 | >300 | >300 | |
| NIH-3T3 | 297 | >300 | 270 | 215 | >300 | >300 | |
| CC50 (µg/mL) | |||||||
| B | SF-295 | 4T1 | HUVEC | MRC-5 | SK-MEL-28 | HCC 1954 | |
| MeOH | 237 | 283 | 449 | 378 | 185 | 192 | |
| FU-DCM | 252 | 245 | 440 | 370 | 176 | 162 | |
| Selectivity Index | |||||||
| C | HUVEC/MCF-7 | MRC-5/MDA-MB-231 | NIH-3T3/4T1 | ||||
| MeOH | 1.6 | 2.08 | 1.05 | ||||
| FU-DCM | 2.23 | 2.05 | 0.88 | ||||
| Items | Control | DMBA | EA 50 + DMBA | EA 200 + DMBA | MeOH 50 + DMBA | Tamox + DMBA |
|---|---|---|---|---|---|---|
| Number of rats with tumors/total rats | 0/10 | 6/10 | 4/10 | 1/10 | 1/10 | 4/10 |
| Tumor incidence (%) | 0 | 60 ### | 40 * | 10 *** | 10 *** | 40 * |
| Average tumor weight (g) | - | 1.73 ± 0.51 | 1.60 ± 0.95 | 0.73 ± 0.18 | 0.94 ± 0.27 | 0.83 ± 0.19 |
| % Inhibition related to tumor weight | - | - | 7.5 | 57.8 | 45.6 | 52 |
| Total tumor burden (g) | 0 | 17.32 | 16.04 | 7.30 | 9.39 | 8.28 |
| % Inhibition related to tumor burden | - | - | 7 | 58 | 46 | 52 |
| Organs (mg/kg) | Control | DMBA | EA 50 + DMBA | EA 200 + DMBA | MeOH 50 + DMBA | Tamox + DMBA |
|---|---|---|---|---|---|---|
| Uterus | 2703.87 ± 494.85 | 1887.56 ± 212.11 | 1618.97 ± 73.79 | 2158.32 ± 305.66 | 2566.01 ± 418.52 | 643.38 ± 25.45 * |
| Liver | 33,978.23 ± 608.21 | 36,983.76 ± 496.00 | 35,057.02 ± 1189.18 | 33,073.22 ± 1330.93 | 31,154.28 ± 535.92 ** | 32,068.64 ± 1801.93 * |
| Lungs | 7152.24 ± 548.77 | 6374.34 ± 263.17 | 6710.31 ± 540.57 | 7295.43 ± 488.55 | 6547.02 ± 426.91 | 6332.43 ± 347.95 |
| Spleen | 2115.80 ± 113.88 | 2021.21 ± 100.23 | 1964.29 ± 70.05 | 2841.28 ± 204.94 ** | 1903.22 ± 50.42 | 2389.72 ± 139.20 |
| Adrenals | 256.92 ± 21.38 | 260.86 ± 19.99 | 297.01 ± 30.53 | 379.80 ± 36.63 * | 348.27 ± 36.42 | 344.08 ± 20.18 |
| Kidneys | 4993.25 ± 103.85 | 5500.42 ± 82.00 | 4952.97 ± 501.31 | 5709.14 ± 114.13 | 5208.32 ± 113.03 | 5753.71± 236.31 |
| Femur | 2734.37 ± 95.99 | 2990.48 ± 106.61 | 3019.59 ± 120.11 | 3013.05 ± 132.31 | 3262.95 ± 114.77 | 3163.50 ± 283.44 |
| Brain | 8426.09 ± 189.21 | 8580.18 ± 171.83 | 9045.49 ± 247.90 | 8900.86 ± 210.99 | 8309.45 ± 147.89 | 10,456.47 ± 376.26 *** |
| Items | Control | DMBA | EA 50 + DMBA | EA 200 + DMBA | MeOH 50 + DMBA | Tamox + DMBA |
|---|---|---|---|---|---|---|
| WBC (×103 µL−1) | 1.91 ± 0.34 | 2.51 ± 0.40 | 3.9 ± 0.86 | 3.21 ± 0.67 | 2.3 ± 0.32 | 2.15 ± 0.22 |
| Lymphocytes (%) | 61.21 ± 4.88 | 59.48 ± 3.83 | 58.23 ± 3.62 | 62.15 ± 2.91 | 62.08 ± 3.04 | 58.55 ± 2.35 |
| Monocytes (%) | 6.65 ± 0.42 | 7.33 ± 0.60 | 6.61 ± 0.42 | 6.91 ± 0.81 | 7.28 ± 0.74 | 6.75 ± 0.52 |
| Granulocytes (%) | 32.13 ± 4.56 | 33.18 ± 3.68 | 35.15 ± 3.28 | 30.93 ± 2.28 | 30.63 ± 2.35 | 34.7 ± 1.86 |
| RBC (×103 µL−1) | 7.22 ± 0.48 | 6.96 ± 0.40 # | 7.52 ± 0.23 | 7.60 ± 0.17 | 7.74 ± 0.18 | 7.28 ± 0.24 |
| Hematocrit (%) | 43.12 ± 3.43 | 42.16 ± 2.59 | 44.61 ± 1.39 | 45.1 ± 0.98 | 47.11 ± 1.17 | 43.73 ± 1.26 |
| MCV (fL) | 59.85 ± 1.12 | 60.51 ± 0.29 | 59.35 ± 0.54 | 59.38 ± 0.7 | 60.91 ± 0.39 | 60.16 ± 0.35 |
| Platelets (×103 µL−1) | 378.2 ± 41.73 | 408.5 ± 18.75 | 422.83 ± 20.45 | 425.66 ± 32.12 | 455 ± 38.09 | 478.16 ± 15.03 |
| MCH (pg) | 18.68 ± 0.32 | 19.3 ± 0.25 | 18.65 ± 0.23 | 18.73 ± 0.20 | 18.96 ± 0.16 | 18.3 ± 0.15* |
| Hemoglobin (g/dL) | 13.58 ± 1.08 | 13.51 ± 0.90 | 14.08 ± 0.52 | 14.28 ± 0.32 | 14.75 ± 0.42 | 13.41 ± 0.44 |
| MCHC (g/dL) | 31.31 ± 0.22 | 31.96 ± 0.28 | 31.48 ± 0.27 | 31.61 ± 0.43 | 31.23 ± 0.16 | 30.6 ± 0.22** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silihe, K.K.; Zingue, S.; Winter, E.; Awounfack, C.F.; Bishayee, A.; Desai, N.N.; João Mello, L., Jr.; Michel, T.; Tankeu, F.N.; Ndinteh, D.T.; et al. Ficus umbellata Vahl. (Moraceae) Stem Bark Extracts Exert Antitumor Activities In Vitro and In Vivo. Int. J. Mol. Sci. 2017, 18, 1073. https://doi.org/10.3390/ijms18061073
Silihe KK, Zingue S, Winter E, Awounfack CF, Bishayee A, Desai NN, João Mello L Jr., Michel T, Tankeu FN, Ndinteh DT, et al. Ficus umbellata Vahl. (Moraceae) Stem Bark Extracts Exert Antitumor Activities In Vitro and In Vivo. International Journal of Molecular Sciences. 2017; 18(6):1073. https://doi.org/10.3390/ijms18061073
Chicago/Turabian StyleSilihe, Kevine Kamga, Stéphane Zingue, Evelyn Winter, Charline Florence Awounfack, Anupam Bishayee, Nishil N. Desai, Leônidas João Mello, Jr., Thomas Michel, Francine Nzufo Tankeu, Derek Tantoh Ndinteh, and et al. 2017. "Ficus umbellata Vahl. (Moraceae) Stem Bark Extracts Exert Antitumor Activities In Vitro and In Vivo" International Journal of Molecular Sciences 18, no. 6: 1073. https://doi.org/10.3390/ijms18061073
APA StyleSilihe, K. K., Zingue, S., Winter, E., Awounfack, C. F., Bishayee, A., Desai, N. N., João Mello, L., Jr., Michel, T., Tankeu, F. N., Ndinteh, D. T., Honorine Riwom, S., Njamen, D., & Creczynski-Pasa, T. B. (2017). Ficus umbellata Vahl. (Moraceae) Stem Bark Extracts Exert Antitumor Activities In Vitro and In Vivo. International Journal of Molecular Sciences, 18(6), 1073. https://doi.org/10.3390/ijms18061073







