Upregulation of Phosphatidylinositol 3-Kinase (PI3K) Enhances Ethylene Biosynthesis and Accelerates Flower Senescence in Transgenic Nicotiana tabacum L.
Abstract
:1. Introduction
2. Results
2.1. Overexpression of S. lycopersicum PI3K in Nicotiana tabacum
2.2. PI3K Transcript Levels in Detached Tobacco Flowers and Immunoblot Analysis PI3K in Tobacco Flowers
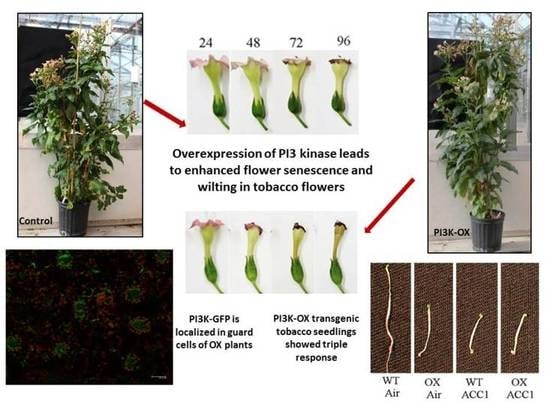
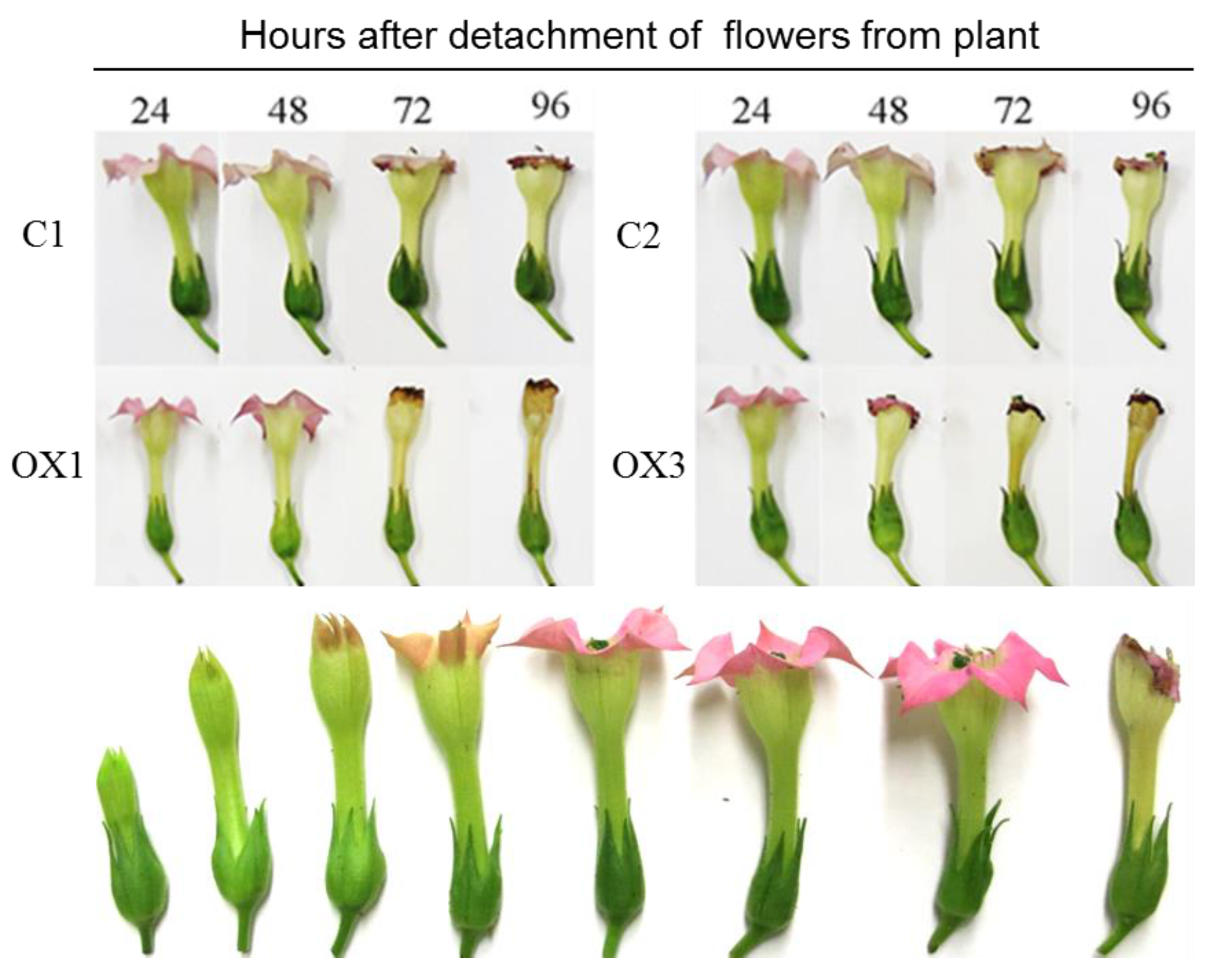
2.3. PI3K Overexpression Accelerates Tobacco Flower Senescence and Reduces Flower Lifespan
2.4. Transgenic Tobacco Flowers Produced More Ethylene
2.5. PI3K Overexpression Affects Transcript Levels of Ethylene Biosynthesis-Related Genes
2.6. PI3K-OX Tobacco Seedlings Displays Triple Response
2.7. Subcellular Localization of PI3K in Tobacco Seedlings
3. Discussion
3.1. PI3K in Flower Senescence
3.2. Proposed Mode of Action of PI3K in Ethylene Signal Transduction
4. Materials and Methods
4.1. Cloning of PI3K and Vector Construction
4.2. Agrobacterium tumefaciens-Mediated Stable Transformation of Tobacco
4.3. Gene Expression Analyses
4.4. Immunoblot Analysis
4.5. Ethylene Triple Response Assay
4.6. Measurement of Ethylene and Carbon Dioxide Production
4.7. Confocal Microscopy
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACO | 1-aminocyclopropane-1-carboxylic acid oxidase |
| ACC | 1-aminocyclopropane-1-carboxylic acid |
| PA | Phosphatidic acid |
| PI | Phosphatidylinositol |
| PI3K | Phosphatidylinositol 3-kinase |
| OX | Overexpression |
| PI3-P | Phosphatidylinositol 3-phosphate |
| PLD | Phospholipase D |
| WT | Wild type |
References
- Zimmermann, P.; Zentgraf, U. The correlation between oxidative stress and leaf senescence during plant development. Cell Mol. Biol. Lett. 2005, 10, 515–534. [Google Scholar] [PubMed]
- Noodén, L.D.; Penney, J.P. Correlative controls of senescence and plant death in Arabidopsis Thaliana (Brassicaceae). J. Exp. Bot. 2001, 52, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Bleecker, A.B.; Kende, H. Ethylene: A gaseous signal molecule in plants. Ann. Rev. Cell. Dev. Biol. 2000, 16, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.S. Ethylene in Plant Growth, Development, and Senescence. In Plant Hormones; Davies, P.J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 486–508. [Google Scholar]
- Aharoni, N.; Lieberman, M. Ethylene as a regulator of senescence in tobacco leaf discs. Plant Physiol. 1979, 64, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, N.; Sopory, S.K. Chemical signaling under abiotic stress environment in plants. Plant Signal. Behav. 2008, 3, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Botha, M-L.; Whitehead, C.S. The effect of polyamines on ethylene synthesis during normal and pollination-induced senescence of Petunia hybrida L. flowers. Planta 1992, 188, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.E.; Bleecker, A.B. Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science 1995, 270, 1809–1811. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, F.I.; Esch, J.J.; Hall, A.E.; Binder, B.M.; Schaller, G.E.; Bleecker, A.B. A copper cofactor for the ethylene receptor etr1 from Arabidopsis. Science 1999, 283, 996–998. [Google Scholar]
- Clark, K.L.; Larsen, P.B.; Wang, X.; Chang, C. Association of the Arabidopsis CTR1 Raf-like kinase with the ETR1 and ERS ethylene receptors. Proc. Natl. Acad. Sci. USA 1998, 95, 5401–5406. [Google Scholar] [CrossRef] [PubMed]
- Cancel, J.D.; Larsen, P.B. Loss-of-function mutations in the ethylene receptor ETR1 cause enhanced sensitivity and exaggerated response to ethylene in Arabidopsis. Plant Physiol. 2002, 129, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Yoon, G.M.; Shemansky, J.M.; Lin, D.Y.; Ying, Z.I.; Chang, J.; Garrett, W.M.; Kessenbrock, M.; Groth, G.; Tucker, M.L.; et al. CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 19486–19491. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Chen, Y.F.; Randlett, M.D.; Zhao, X.C.; Findell, J.L.; Kieber, J.J.; Schaller, G.E. Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J. Biol. Chem. 2003, 278, 34725–34732. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, A.N.; Alonso, J.M. Ethylene signaling and response pathway: A unique signaling cascade with a multitude of inputs and outputs. Physiol. Plant. 2003, 123, 195–206. [Google Scholar] [CrossRef]
- Raynaud, F.I.; Eccles, S.; Clarke, P.A.; Hayes, A.; Nutley, B.; Alix, S.; Henley, A.; di Stefano, F.; Ahmad, Z.; Guillard, S.; et al. Pharmacologic characterization of a potent inhibitor of class I phosphatidylinositide 3-kinases. Cancer Res. 2007, 67, 5840–5850. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, E.S.; Choi, Y.; Hwang, I.; Staiger, C.J.; Chung, Y.Y.; Lee, Y. The Arabidopsis phosphatidylinositol 3-kinase is important for pollen development. Plant Physiol. 2008, 147, 1886–1897. [Google Scholar] [CrossRef] [PubMed]
- Foster, F.M.; Traer, C.J.; Abraham, S.M.; Fry, M.J. The phosphoinositide (PI) 3-kinase family. J. Cell Sci. 2003, 116, 3037–3040. [Google Scholar] [CrossRef] [PubMed]
- Boss, W.F.; Im, Y.J. Phosphoinositide signaling. Annu. Rev. Plant Biol. 2012, 63, 409–429. [Google Scholar] [CrossRef] [PubMed]
- Welters, P.; Takegawa, K.; Emr, S.D.; Chrispeels, M.J. AtVPS34, a phosphatidylinositol 3-kinase of Arabidopsis thaliana, is an essential protein with homology to a calcium-dependent lipid binding domain. Proc. Natl. Acad. Sci. USA 1994, 91, 11398–11402. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Kim, Y.W.; Kwak, J.M.; Hwang, J.U.; Young, J.; Schroeder, J.I.; Hwang, I.; Lee, Y. Phosphatidylinositol 3- and 4-phosphate are required for normal stomatal movements. Plant Cell 2002, 14, 2399–2412. [Google Scholar] [CrossRef] [PubMed]
- Dove, S.K.; Lloyd, C.W.; Drøbak, B.K. Identification of a phosphatidylinositol 3-hydroxy kinase in plant cells: Association with the cytoskeleton. Biochem. J. 1994, 303, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, J.E.M.; van Leeuwen, W.; Tobena-Santamaria, R.; Laxalt, A.M.; Jones, D.R.; Divecha, N.; Gadella, T.W.J.; Munnik, T. Visualization of PtdIns3P dynamics in living plant cells. Plant J. 2006, 47, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Eu, Y.J.; Yoo, C.M.; Kim, Y.W.; Pih, K.T.; Jin, J.B.; Kim, S.J.; Stenmark, H.; Hwan, I.H. Trafficking of phosphatidylinositol 3-phosphate from the trans-Golgi network to the lumen of the central vacuole in plant cells. Plant Cell 2001, 13, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Bunney, T.D.; Watkins, P.A.; Beven, A.F.; Shaw, P.J.; Hernandez, L.E.; Lomonossoff, G.P.; Shanks, M.; Peart, J.; Drobak, B.K. Association of phosphatidylinositol 3-kinase with nuclear transcription sites in higher plants. Plant Cell 2000, 12, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Gao, X.Q.; Zhao, X.Y.; Zhu, D.Z.; Zhou, L.Z.; Zhang, X.S. Arabidopsis AtVPS15 is essential for pollen development and germination through modulating phosphatidylinositol 3-phosphate formation. Plant Mol. Biol. 2011, 77, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Gillooly, D.J.; Morrow, I.C.; Lindsay, M.; Gould, R.; Bryant, N.J.; Gaullier, J.M.; Parton, R.G.; Stenmark, H. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 2000, 19, 4577–4588. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Jung, J.Y.; Park, J.; Hwang, J.U.; Kim, Y.W.; Hwang, I.; Lee, Y. A role for phosphatidylinositol 3-phosphate in abscisic acid-induced reactive oxygen species generation in guard cells. Plant Physiol. 2003, 132, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Strohm, A.K.; Baldwin, K.L.; Masson, P.H. Multiple roles for membrane-associated protein trafficking and signaling in gravitropism. Front. Plant Sci. 2012, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, K.; Paliyath, G. Microarray analysis of ripening-regulated gene expression and its modulation by 1-MCP and hexanal. Plant Physiol. Biochem. 2011a, 49, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, A.; Murachi, S.; Okunishi, H.; Shiomi, S.; Nakano, R.; Kubo, Y.; Inaba, A. differential expression and internal feedback regulation of l-aminocyclopropane-l-carboxylate synthase, 1-aminocyclopropane-l-carboxylate oxidase and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol. 1998, 118, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, J.A.; Zhang, X.S.; Nair, H.; O’Neill, S.D. Temporal and spatial regulation of 1-aminocyclopropane-1-carboxylate oxidase in the pollination-induced senescence of orchid flowers. Plant Physiol. 1993, 103, 31–39. [Google Scholar] [CrossRef]
- Woltering, E.J.; van Doorn, W.G. Role of ethylene in senescence of petals—Morphological and taxonomical relationships. J. Exp. Bot. 1988, 39, 1605–1616. [Google Scholar] [CrossRef]
- Henskens, J.A.M.; Rouwendal, G.J.A.; Ten-Have, A.; Woltering, E.J. Molecular cloning of two different ACC synthase PCR fragments in carnation flowers and organ specific expression of the corresponding genes. Plant Mol. Biol. 1994, 26, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Satoh, S.; Shibuya, K.; Waki, K.; Ichimura, K. Mechanism of senescence in carnation flowers. Acta Hortic. 2005, 669, 191–198. [Google Scholar] [CrossRef]
- Guzmán, P.; Ecker, J.R. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 1990, 2, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Testerink, C.; Larsen, P.B.; van der Does, D.; van Himbergen, J.A.; Munnik, T. Phosphatidic acid binds to and inhibits the activity of Arabidopsis CTR1. J. Exp. Bot. 2007, 58, 3905–3914. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, M.; Gałgańska, H.; Nowak, W.; Sadowski, J. Exogenously induced expression of ethylene biosynthesis, ethylene perception, phospholipase D, and Rboh-oxidase genes in broccoli seedlings. J. Exp. Bot. 2010, 61, 3475–3491. [Google Scholar] [CrossRef] [PubMed]
- Paliyath, G.; Lynch, D.V.; Thompson, J.E. Regulation of membrane phospholipid catabolism in senescing carnation flowers. Physiologia Plant. 1987, 71, 503–511. [Google Scholar] [CrossRef]
- Paliyath, G.; Droillard, M.J. The mechanisms of membrane deterioration and disassembly during senescence. Plant Physiol. Biochem. 1992, 30, 789–812. [Google Scholar]
- Pinhero, R.G.; Almquist, K.C.; Novotna, Z.; Paliyath, G. Developmental regulation of phospholipase D in tomato fruits. Plant Physiol. Biochem. 2003, 41, 223–240. [Google Scholar] [CrossRef]
- Paliyath, G.; Tiwari, K.; Yuan, H.; Whitaker, B.D. Structural Deterioration in Produce: Phospholipase D, Membrane Deterioration, and Senescence. In Postharvest Biology and Technology of Fruits, Vegetables, and Flowers; Paliyath, D., Murr, D.P., Handa, A.K., Lurie, S., Eds.; Wiley-Blackwell Publishing: Ames, IO, USA, 2008; pp. 195–239. [Google Scholar]
- Tiwari, K.; Paliyath, G. Cloning, expression and functional characterization of the C2 domain from tomato phospholipase Dα. Plant Physiol. Biochem. 2011b, 49, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Leprince, A.S.; Magalhaes, N.; de Vos, D.; Bordenave, M.; Crilat, E.; Clément, G.; Meyer, C.; Munnik, T.; Savouré, A. Involvement of phosphatidylinositol 3-kinase in the regulation of proline catabolism in Arabidopsis thaliana. Frontiers. Plant Sci. 2015, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sol Genomics Network. Available online: http://solgenomics.net/ (accessed on 12 July 2017).
- Horsch, R.B.; Fraley, R.T.; Rogers, S.G.; Sanders, P.R.; Lloyd, A.; Hoffmann, N. Inheritance of functional foreign genes in plants. Science 1984, 223, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]








© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dek, M.S.P.; Padmanabhan, P.; Sherif, S.; Subramanian, J.; Paliyath, A.G. Upregulation of Phosphatidylinositol 3-Kinase (PI3K) Enhances Ethylene Biosynthesis and Accelerates Flower Senescence in Transgenic Nicotiana tabacum L. Int. J. Mol. Sci. 2017, 18, 1533. https://doi.org/10.3390/ijms18071533
Dek MSP, Padmanabhan P, Sherif S, Subramanian J, Paliyath AG. Upregulation of Phosphatidylinositol 3-Kinase (PI3K) Enhances Ethylene Biosynthesis and Accelerates Flower Senescence in Transgenic Nicotiana tabacum L. International Journal of Molecular Sciences. 2017; 18(7):1533. https://doi.org/10.3390/ijms18071533
Chicago/Turabian StyleDek, Mohd Sabri Pak, Priya Padmanabhan, Sherif Sherif, Jayasankar Subramanian, and And Gopinadhan Paliyath. 2017. "Upregulation of Phosphatidylinositol 3-Kinase (PI3K) Enhances Ethylene Biosynthesis and Accelerates Flower Senescence in Transgenic Nicotiana tabacum L." International Journal of Molecular Sciences 18, no. 7: 1533. https://doi.org/10.3390/ijms18071533