Dexmedetomidine Prevents Lipopolysaccharide-Induced MicroRNA Expression in the Adult Rat Brain
Abstract
:1. Introduction
2. Results
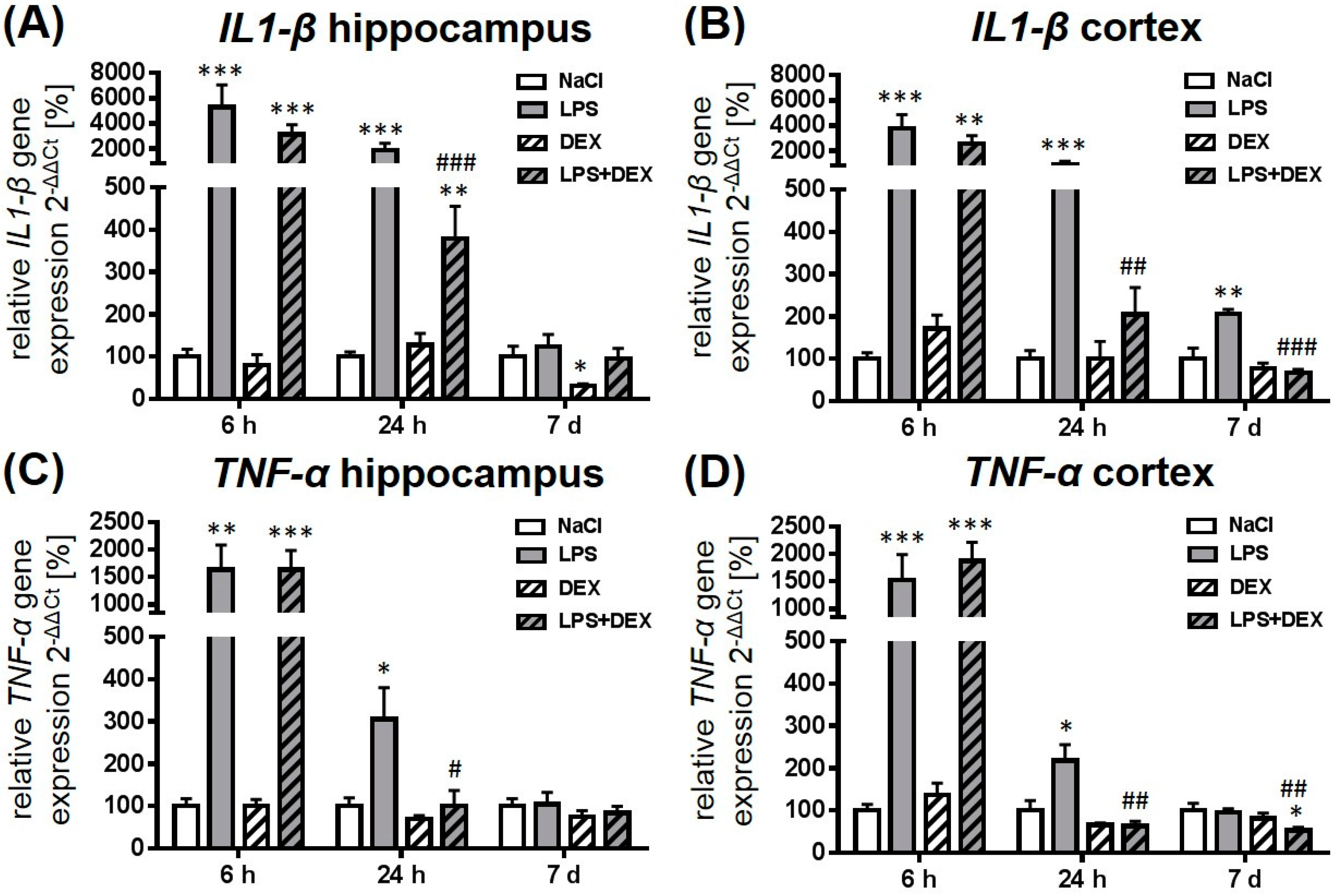
2.1. Dexmedetomidine-Attenuated, LPS-Induced IL1-β and TNF-α Gene Expression in the Hippocampus and Cortex
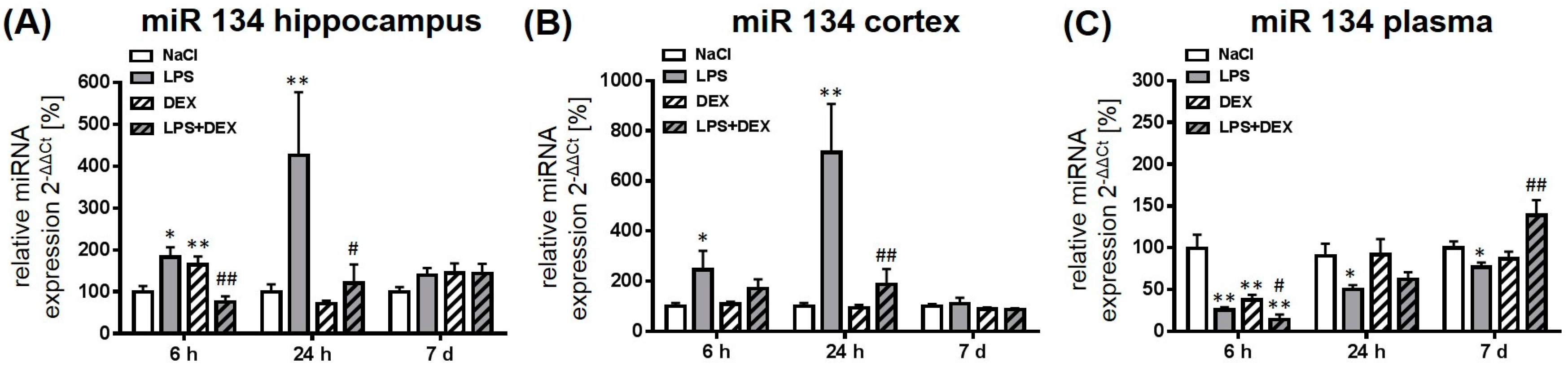
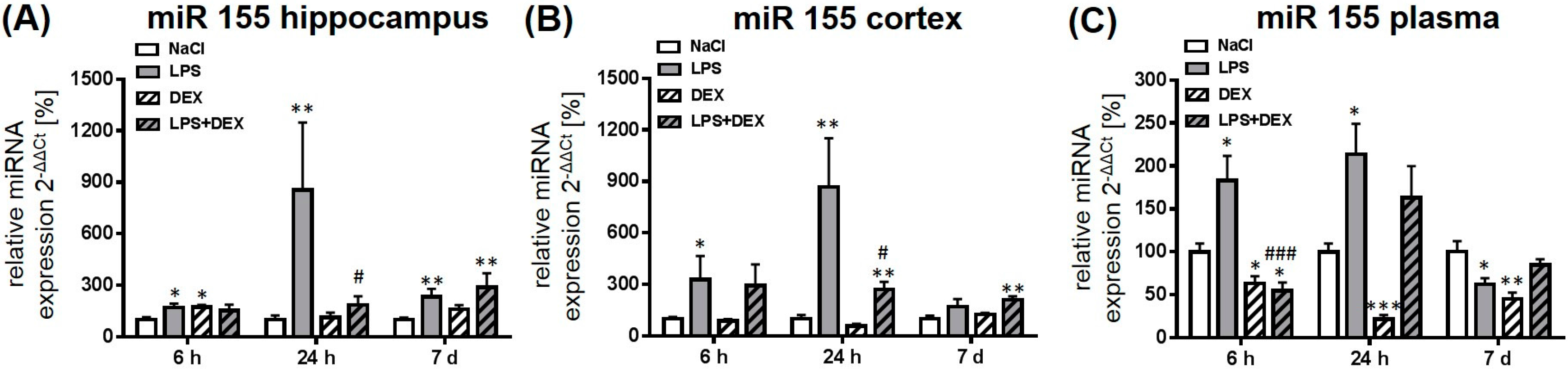
2.2. Dexmedetomidine Modulated the miRNA Expression in the Hippocampus, Cortex, and Plasma of LPS Treated Rats
2.2.1. Expression of MicroRNA 124
2.2.2. Expression of MicroRNA 132
2.2.3. Expression of MicroRNA 134
2.2.4. Expression of MicroRNA 155
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Tissue Preparation
4.3. RNA Extraction and Semiquantitative Real Time PCR
4.4. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| α-7-nAChR | α-7 Nicotinic acetylcholine receptor |
| ALS | Amyotrophic Lateral Sclerosis |
| cDNA | Complementary DNA |
| CNS | Central Nervous System |
| DEX | Dexmedetomidine |
| EDTA | Ethylene-Diamine-Tetra-Acetic acid |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
| i.p. | Intraperitoneal |
| IL1 | Interleukin 1 |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-Activated Protein Kinase |
| MCI | Mild Cognitive Impairment |
| miRNA | MicroRNA |
| M-MLV | Moloney Murine Leukemia Virus |
| mRNA | messengerRNA |
| MS | Multiple Sclerosis |
| MyD88 | Myeloid Differentiation Factor 88 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PCR | Polymerase Chain Reaction |
| qPCR | Quantitative Polymerase Chain Reaction |
| SHIP1 | Phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase 1 |
| SOCS1 | Suppressor of cytokine signaling 1 |
| TLR | Toll Like Receptor |
| TNF-α | Tumor necrosis factor-α |
References
- Biesmans, S.; Meert, T.F.; Bouwknecht, J.A.; Acton, P.D.; Davoodi, N.; de Haes, P.; Kuijlaars, J.; Langlois, X.; Matthews, L.J.; Ver Donck, L.; et al. Systemic immune activation leads to neuroinflammation and sickness behavior in mice. Mediators Inflamm. 2013. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, Y.K.; Yuk, D.Y.; Choi, D.Y.; Ban, S.B.; Oh, K.W.; Hong, J.T. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of β-amyloid generation. J. Neuroinflammation 2008, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, A.R.; Cibelli, M.; White, J.P.; Nagy, I.; Maze, M.; Ma, D. Systemic inflammation enhances surgery-induced cognitive dysfunction in mice. Neurosci. Lett. 2011, 498, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Bossu, P.; Cutuli, D.; Palladino, I.; Caporali, P.; Angelucci, F.; Laricchiuta, D.; Gelfo, F.; de Bartolo, P.; Caltagirone, C.; Petrosini, L. A single intraperitoneal injection of endotoxin in rats induces long-lasting modifications in behavior and brain protein levels of TNF-α and IL-18. J. Neuroinflamm. 2012, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Cibelli, M.; Fidalgo, A.R.; Terrando, N.; Ma, D.; Monaco, C.; Feldmann, M.; Takata, M.; Lever, I.J.; Nanchahal, J.; Fanselow, M.S.; et al. Role of interleukin-1β in postoperative cognitive dysfunction. Ann. Neurol. 2010, 68, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Terrando, N.; Monaco, C.; Ma, D.; Foxwell, B.M.; Feldmann, M.; Maze, M. Tumor necrosis factor-α triggers a cytokine cascade yielding postoperative cognitive decline. Proc. Natl. Acad. Sci. USA 2010, 107, 20518–20522. [Google Scholar] [CrossRef] [PubMed]
- Ning, Q.; Liu, Z.; Wang, X.; Zhang, R.; Zhang, J.; Yang, M.; Sun, H.; Han, F.; Zhao, W.; Zhang, X. Neurodegenerative changes and neuroapoptosis induced by systemic lipopolysaccharide administration are reversed by dexmedetomidine treatment in mice. Neurol. Res. 2017, 39, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Abildstrom, H.; Rasmussen, L.S.; Rentowl, P.; Hanning, C.D.; Rasmussen, H.; Kristensen, P.A.; Moller, J.T. Cognitive dysfunction 1–2 years after non-cardiac surgery in the elderly. Ispocd group. International study of post-operative cognitive dysfunction. Acta Anaesthesiol. Scand. 2000, 44, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Caza, N.; Taha, R.; Qi, Y.; Blaise, G. The effects of surgery and anesthesia on memory and cognition. Prog. Brain Res. 2008, 169, 409–422. [Google Scholar] [PubMed]
- Pisani, M.A.; Kong, S.Y.; Kasl, S.V.; Murphy, T.E.; Araujo, K.L.; Van Ness, P.H. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am. J. Respir. Crit. Care Med. 2009, 180, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Yildizeli, B.; Ozyurtkan, M.O.; Batirel, H.F.; Kuscu, K.; Bekiroglu, N.; Yuksel, M. Factors associated with postoperative delirium after thoracic surgery. Ann. Thorac. Surg. 2005, 79, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Afonso, J.; Reis, F. Dexmedetomidine: Current role in anesthesia and intensive care. Rev. Bras. Anestesiol. 2012, 62, 118–133. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, H.; Mi, W.; Wang, T.; He, Y.; Zhang, X.; Ma, X.; Li, H. Effects of dexmedetomidine on anesthesia recovery period and postoperative cognitive function of patients after robot-assisted laparoscopic radical cystectomy. Int. J. Clin. Exp. Med. 2015, 8, 11388–11395. [Google Scholar] [PubMed]
- Liu, Y.; Ma, L.; Gao, M.; Guo, W.; Ma, Y. Dexmedetomidine reduces postoperative delirium after joint replacement in elderly patients with mild cognitive impairment. Aging Clin. Exp. Res. 2016, 28, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.; Muzyk, A.J.; Bucklin, M.H.; Brudney, S.; Gagliardi, J.P. Defining the role of dexmedetomidine in the prevention of delirium in the intensive care unit. Biomed. Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Pasin, L.; Landoni, G.; Nardelli, P.; Belletti, A.; di Prima, A.L.; Taddeo, D.; Isella, F.; Zangrillo, A. Dexmedetomidine reduces the risk of delirium, agitation and confusion in critically ill patients: A meta-analysis of randomized controlled trials. J. Cardiothorac. Vasc. Anesth. 2014, 28, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.; Amaral, A. Dexmedetomidine to reduce intubation time in patients with agitated delirium. JAMA 2016, 316, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Pandharipande, P.P.; Pun, B.T.; Herr, D.L.; Maze, M.; Girard, T.D.; Miller, R.R.; Shintani, A.K.; Thompson, J.L.; Jackson, J.C.; Deppen, S.A.; et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: The mends randomized controlled trial. JAMA 2007, 298, 2644–2653. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Liu, H.Y.; Liu, S.L.; Ji, F.H. Dexmedetomidine-fentanyl compared with midazolam-fentanyl for conscious sedation in patients undergoing lumbar disc surgery. Clin. Ther. 2016, 38, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Riker, R.R.; Shehabi, Y.; Bokesch, P.M.; Ceraso, D.; Wisemandle, W.; Koura, F.; Whitten, P.; Margolis, B.D.; Byrne, D.W.; Ely, E.W.; et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: A randomized trial. JAMA 2009, 301, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Y.; Wang, Y.; Ning, Q.; Zhang, Y.; Gong, C.; Zhao, W.; Jing, G.; Wang, Q. Dexmedetomidine attenuates inflammatory reaction in the lung tissues of septic mice by activating cholinergic anti-inflammatory pathway. Int. Immunopharmacol. 2016, 35, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Peng, K.; Meng, X.W.; Ji, F.H. Attenuation of neuroinflammation by dexmedetomidine is associated with activation of a cholinergic anti-inflammatory pathway in a rat tibial fracture model. Brain Res. 2016, 1644, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Davis, J.R.; Wu, Z.L.; Faez Abdelgawad, A. Dexmedetomidine attenuates lipopolysaccharide induced MCP-1 expression in primary astrocyte. Biomed. Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Wang, Y.L.; Wang, C.Y.; Chen, C. Dexmedetomidine attenuates lipopolysaccharide-induced proinflammatory response in primary microglia. J. Surg. Res. 2013, 179, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, Y.; Zhou, C.; Huang, L.; Dong, Z. Dexmedetomidine provides neuroprotection: Impact on ketamine-induced neuroapoptosis in the developing rat brain. Acta Anaesthesiol. Scand. 2014, 58, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Wei, Y.; Chen, Y.; Zhang, X.; Gong, Z.; Jiang, Y.; Gong, Q.; Zhou, L.; Wang, H.; Xie, Y. Dexmedetomidine attenuates propofol-induce neuroapoptosis partly via the activation of the PI3k/AKT/GSK3β pathway in the hippocampus of neonatal rats. Environ. Toxicol. Pharmacol. 2017, 52, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Sifringer, M.; von Haefen, C.; Krain, M.; Paeschke, N.; Bendix, I.; Buhrer, C.; Spies, C.D.; Endesfelder, S. Neuroprotective effect of dexmedetomidine on hyperoxia-induced toxicity in the neonatal rat brain. Oxid. Med. Cell. Longev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Endesfelder, S.; Makki, H.; von Haefen, C.; Spies, C.D.; Buhrer, C.; Sifringer, M. Neuroprotective effects of dexmedetomidine against hyperoxia-induced injury in the developing rat brain. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.L.; Zhang, W.; Liu, M.Z.; Zhou, Y.B.; Zhang, J.M.; Han, L.; Peng, Y.M.; Jiang, J.H.; Wang, Q.D. Dexmedetomidine improves early postoperative cognitive dysfunction in aged mice. Eur. J. Pharmacol. 2015, 746, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, D.; Kawano, T.; Nishigaki, A.; Aoyama, B.; Tateiwa, H.; Shigematsu-Locatelli, M.; Locatelli, F.M.; Yokoyama, M. Preventive effects of dexmedetomidine on the development of cognitive dysfunction following systemic inflammation in aged rats. J. Anesth. 2017, 31, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mrnas are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.; Silahtaroglu, A.; Moller, M.; Christensen, M.; Rath, M.F.; Skryabin, B.; Tommerup, N.; Kauppinen, S. MicroRNA expression in the adult mouse central nervous system. RNA 2008, 14, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Fineberg, S.K.; Kosik, K.S.; Davidson, B.L. MicroRNAs potentiate neural development. Neuron 2009, 64, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Bushati, N.; Cohen, S.M. MicroRNA functions. Annu. Rev. Cell Dev. Biol 2007, 23, 175–205. [Google Scholar] [CrossRef] [PubMed]
- Thounaojam, M.C.; Kaushik, D.K.; Basu, A. MicroRNAs in the brain: It’s regulatory role in neuroinflammation. Mol. Neurobiol. 2013, 47, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Yu, G.F.; Jin, S.Y.; Zhang, W.H.; Lei, D.X.; Zhou, S.L.; Song, X.R. Activation of α2 adrenoceptor attenuates lipopolysaccharide-induced hepatic injury. Int. J. Clin. Exp. Pathol. 2015, 8, 10752–10759. [Google Scholar] [PubMed]
- Chen, Y.; Miao, L.; Yao, Y.; Wu, W.; Wu, X.; Gong, C.; Qiu, L.; Chen, J. Dexmedetomidine ameliorate CLP-induced rat intestinal injury via inhibition of inflammation. Mediators Inflamm. 2015. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, Y.; Huang, H.; Zhu, Y.; Zhang, Y.; Lu, F.; Zhou, C.; Huang, L.; Li, X.; Zhou, C. Dexmedetomidine inhibits inflammatory reaction in lung tissues of septic rats by suppressing TLR4/NF-κB pathway. Mediators Inflamm. 2013, 2013, 562154. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Hu, B.; Li, Z.; Li, J. Dexmedetomidine controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Inflammation 2014, 37, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Sheinerman, K.S.; Tsivinsky, V.G.; Abdullah, L.; Crawford, F.; Umansky, S.R. Plasma microRNA biomarkers for detection of mild cognitive impairment: Biomarker validation study. Aging 2013, 5, 925–938. [Google Scholar] [CrossRef] [PubMed]
- Soreq, H.; Wolf, Y. Neurimmirs: MicroRNAs in the neuroimmune interface. Trends Mol. Med. 2011, 17, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, R.; Li, X.; Chen, J. Preoperative serum microRNA-155 expression independently predicts postoperative cognitive dysfunction after laparoscopic surgery for colon cancer. Med. Sci. Monit. 2016, 22, 4503–4508. [Google Scholar] [CrossRef] [PubMed]
- Woodbury, M.E.; Freilich, R.W.; Cheng, C.J.; Asai, H.; Ikezu, S.; Boucher, J.D.; Slack, F.; Ikezu, T. miR-155 is essential for inflammation-induced hippocampal neurogenic dysfunction. J. Neurosci. 2015, 35, 9764–9781. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, Y.; Jiang, R.; Nie, C.; Zeng, Z.; Zhao, N.; Huang, C.; Shao, Q.; Ding, C.; Qing, C.; et al. miR-132 inhibits lipopolysaccharide-induced inflammation in alveolar macrophages by the cholinergic anti-inflammatory pathway. Exp. Lung Res. 2015, 41, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Taganov, K.D.; Boldin, M.P.; Chang, K.J.; Baltimore, D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.M.; Taganov, K.D.; Boldin, M.P.; Cheng, G.; Baltimore, D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. USA 2007, 104, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Q.; Gui, H.; Xu, D.P.; Yang, Y.L.; Su, D.F.; Liu, X. MicroRNA-124 mediates the cholinergic anti-inflammatory action through inhibiting the production of pro-inflammatory cytokines. Cell Res. 2013, 23, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qin, Z.; Li, Q.; Wan, J.J.; Cheng, M.H.; Wang, P.Y.; Su, D.F.; Yu, J.G.; Liu, X. MicroRNA-124 negatively regulates LPS-induced TNF-α production in mouse macrophages by decreasing protein stability. Acta Pharmacol. Sin. 2016, 37, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Shaked, I.; Meerson, A.; Wolf, Y.; Avni, R.; Greenberg, D.; Gilboa-Geffen, A.; Soreq, H. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity 2009, 31, 965–973. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A. Boosting the brain’s ability to block inflammation via microRNA-132. Immunity 2009, 31, 854–855. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.F.; Karelina, K.; Sakamoto, K.; Wayman, G.A.; Impey, S.; Obrietan, K. miRNA-132: A dynamic regulator of cognitive capacity. Brain Struct. Funct. 2013, 218, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.F.; Sakamoto, K.; Wayman, G.A.; Impey, S.; Obrietan, K. Transgenic miR132 alters neuronal spine density and impairs novel object recognition memory. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.L.; Tamagnini, F.; Narduzzo, K.E.; Howarth, J.L.; Lee, Y.B.; Wong, L.F.; Brown, M.W.; Warburton, E.C.; Bashir, Z.I.; Uney, J.B. MicroRNA-132 regulates recognition memory and synaptic plasticity in the perirhinal cortex. Eur. J. Neurosci. 2012, 36, 2941–2948. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.F.; Sakamoto, K.; Aten, S.; Snider, K.H.; Loeser, J.; Hesse, A.M.; Page, C.E.; Pelz, C.; Arthur, J.S.; Impey, S.; et al. Targeted deletion of miR-132/-212 impairs memory and alters the hippocampal transcriptome. Learn Mem. 2016, 23, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Yamakuchi, M. MicroRNA regulation of sirt1. Front. Physiol. 2012, 3, 68. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, W.Y.; Mao, Y.W.; Graff, J.; Guan, J.S.; Pan, L.; Mak, G.; Kim, D.; Su, S.C.; Tsai, L.H. A novel pathway regulates memory and plasticity via sirt1 and miR-134. Nature 2010, 466, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Quinn, S.R.; Mangan, N.E.; Caffrey, B.E.; Gantier, M.P.; Williams, B.R.; Hertzog, P.J.; McCoy, C.E.; O’Neill, L.A. The role of ETS2 transcription factor in the induction of microRNA-155 (miR-155) by lipopolysaccharide and its targeting by interleukin-10. J. Biol. Chem. 2014, 289, 4316–4325. [Google Scholar] [CrossRef] [PubMed]
- Ceppi, M.; Pereira, P.M.; Dunand-Sauthier, I.; Barras, E.; Reith, W.; Santos, M.A.; Pierre, P. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc. Natl. Acad. Sci. USA 2009, 106, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Tili, E.; Michaille, J.J.; Cimino, A.; Costinean, S.; Dumitru, C.D.; Adair, B.; Fabbri, M.; Alder, H.; Liu, C.G.; Calin, G.A.; et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-α stimulation and their possible roles in regulating the response to endotoxin shock. J. Immunol. 2007, 179, 5082–5089. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, A.; Lee, I.; Hood, L.; Galas, D.; Wang, K. Extracellular microRNA: A new source of biomarkers. Mutat. Res. 2011, 717, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Zhou, H.; Zhang, R.; Song, M.; Yu, L.; Wang, L.; Liu, Z.; Zhang, Q.; Cui, D.; Wang, X.; et al. Serum miR-206 and miR-132 as potential circulating biomarkers for mild cognitive impairment. J. Alzheimers Dis. 2015, 45, 721–731. [Google Scholar] [PubMed]
- Avansini, S.H.; de Sousa Lima, B.P.; Secolin, R.; Santos, M.L.; Coan, A.C.; Vieira, A.S.; Torres, F.R.; Carvalho, B.S.; Alvim, M.K.; Morita, M.E.; et al. MicroRNA hsa-miR-134 is a circulating biomarker for mesial temporal lobe epilepsy. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Balcells, I.; Cirera, S.; Busk, P.K. Specific and sensitive quantitative RT-PCR of miRNAs with DNA primers. BMC Biotechnol. 2011, 11, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busk, P.K. A tool for design of primers for microRNA-specific quantitative RT-qPCR. BMC Bioinformatics 2014, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


| IL1-β NM_031512 | F | aacaaaaatgcctcgtgctgtct | TNF-α NM_012675 | F | tcgagtgacaagcccgtagc |
| R | tgttggcttatgttctgtccattg | R | ctcagccactccagctgctc | ||
| P | 6-fam-acccatgtgagctga aagctctccacc-tamra | P | 6-fam-cgtcgtagcaaacca ccaagcaga-tamra | ||
| GAPDH NM_017008 | F | gatgctggtgctgagtatgtcgt | RT-primer | caggtccagttttttttttttttt | |
| R | tcaggtgagccccagcct | ||||
| P | 6-fam-tctactggcgtcttc accaccatggaga-tamra | ||||
| miR 103 MIMAT0000824 | F | gcagagcagcattgtacag | miR 134 MIMAT0000840 | F | gcagtgtgactggttgac |
| R | ggtccagtttttttttttttttcatag | R | cagtttttttttttttttcccctct | ||
| miR 124 MIMAT0004728 | F | gcagcgtgttcacagc | miR 155 MIMAT0030409 | F | cgcagttaatgctaattgtgatag |
| R | tccagtttttttttttttttcaaggt | R | aggtccagtttttttttttttttacc | ||
| miR 132 MIMAT0008381 | F | gcagtaacagtctacagcca | snU6RNA NR_004394 | F | atacagagaagattagcatggcc |
| R | gtccagtttttttttttttttcgac | R | cgaatttgcgtgtcatccttg | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paeschke, N.; Von Haefen, C.; Endesfelder, S.; Sifringer, M.; Spies, C.D. Dexmedetomidine Prevents Lipopolysaccharide-Induced MicroRNA Expression in the Adult Rat Brain. Int. J. Mol. Sci. 2017, 18, 1830. https://doi.org/10.3390/ijms18091830
Paeschke N, Von Haefen C, Endesfelder S, Sifringer M, Spies CD. Dexmedetomidine Prevents Lipopolysaccharide-Induced MicroRNA Expression in the Adult Rat Brain. International Journal of Molecular Sciences. 2017; 18(9):1830. https://doi.org/10.3390/ijms18091830
Chicago/Turabian StylePaeschke, Nadine, Clarissa Von Haefen, Stefanie Endesfelder, Marco Sifringer, and Claudia D. Spies. 2017. "Dexmedetomidine Prevents Lipopolysaccharide-Induced MicroRNA Expression in the Adult Rat Brain" International Journal of Molecular Sciences 18, no. 9: 1830. https://doi.org/10.3390/ijms18091830





