Morphological Evaluation of Tumor-Infiltrating Lymphocytes (TILs) to Investigate Invasive Breast Cancer Immunogenicity, Reveal Lymphocytic Networks and Help Relapse Prediction: A Retrospective Study
Abstract
1. Introduction
2. Results
2.1. Quantification and Distribution of TIL Subsets
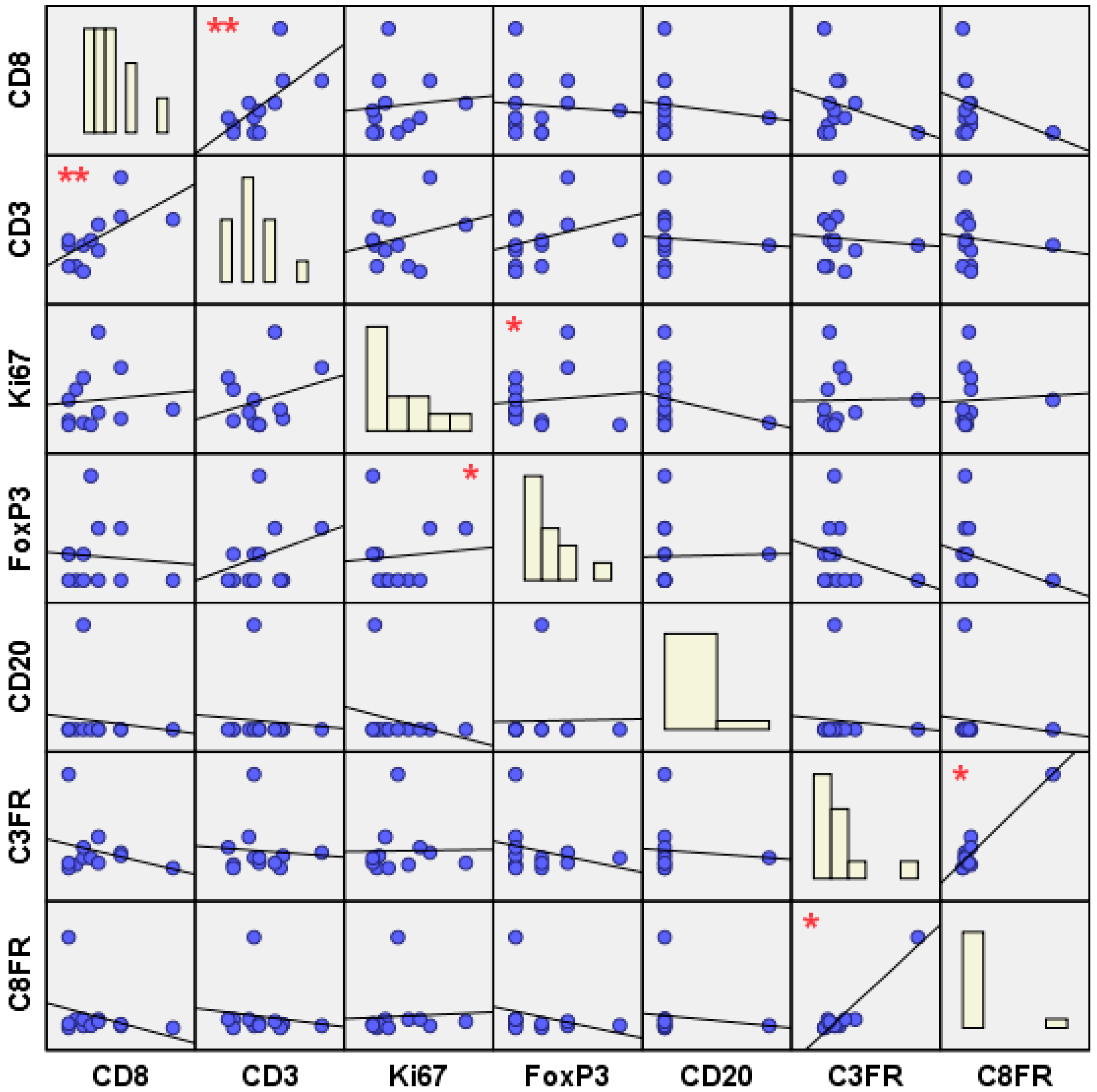
2.2. Topological and Clinical Correlations between Different TIL Subsets
2.3. Topological and Clinical Correlations within TIL Subsets
3. Discussion
3.1. Quantification and Distribution of TIL Subsets
3.2. Topological and Clinical TIL Inter-/Intra-Subtype Correlations
3.2.1. The Effector TILs
3.2.2. The Suppressor TILs
3.2.3. The Effector to Suppressor TIL Ratios
4. Materials and Methods
4.1. Cohort and Sample Selection
4.2. Tissue Micro Arrays (TMA)
4.3. Immunohistochemistry
4.4. Ethics Statement
4.5. Statistical Analysis
5. Conclusions
- (1)
- Patients without relapse exhibited several significant correlations of both suppressor (CD3, CD8) and effector (FoxP3) TILs, suggesting that the presence of strong lymphocytic networks might have a role in maintaining the tumor lymphocytic balance, meaning that a less wired and connected tumor immune microenvironment might be more prone to relapse.
- (2)
- Inter-subtype lymphocytic correlations (between CD3–CD8, FoxP3–Ki67, C3FR–C8FR) were significant only in the non-relapse group, while intra-subtype lymphocytic correlations (within CD3, Ki67) were found to be significant in both clinical groups. This may suggest that in particular the presence of strong inter-subtype lymphocytic networks might play a role in preventing breast cancer recurrence.
- (3)
- Moreover, the non-relapse group exhibited tumor heterogeneity in terms of distribution of lymphocytic networks. In fact, a significant positive correlation was found between CD3+ and CD8+ T-cells at the tumor center, whereas C3FR and C8FR were found to be positively correlated at the invasive edge of the tumor. This may suggest the presence of two different protective networks: a protective “effector” TIL network (CD3–CD8) at the tumor center, as well as a protective “effector/suppressor” TIL balance (C3FR–C8FR) at the tumor margin, possibly to control relapse-initiating CSCs and EMT.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baldassarre, G.; Belletti, B. Molecular biology of breast tumors and prognosis. F1000Res 2016, 5, 711. [Google Scholar] [CrossRef] [PubMed]
- Alkatout, I.; Order, B.; Klapper, W.; Weigel, M.T.; Jonat, W.; Schaefer, F.K.; Mundhenke, C.; Wenners, A. Surgical impact of new treatments in breast cancer. Minerva Ginecol. 2013, 65, 363–383. [Google Scholar] [PubMed]
- Mathot, L.; Stenninger, J. Behavior of seeds and soil in the mechanism of metastasis: A deeper understanding. Cancer Sci. 2012, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Luen, S.J.; Savas, P.; Fox, S.B.; Salgado, R.; Loi, S. Tumour-infiltrating lymphocytes and the emerging role of immunotherapy in breast cancer. Pathology 2017, 49, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Kepp, O.; Kroemer, G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat. Rev. Clin. Oncol. 2011, 8, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Stoll, G.; Enot, D.; Mlecnik, B.; Galon, J.; Zitvogel, L.; Kroemer, G. Immune-related gene signatures predict the outcome of neoadjuvant chemotherapy. Oncoimmunology 2014, 3, e27884. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Kang, M.; Baek, J.H.; Lee, J.I.; Ha, S.Y. Clinical impact of tumor-infiltrating lymphocytes for survival in curatively resected stage iv colon cancer with isolated liver or lung metastasis. Ann. Surg. Oncol. 2013, 20, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Kocián, P.; Šedivcová, M.; Drgáč, J.; Cerná, K.; Hoch, J.; Kodet, R.; Bartůňková, J.; Špíšek, R.; Fialová, A. Tumor-infiltrating lymphocytes and dendritic cells in human colorectal cancer: Their relationship to kras mutational status and disease recurrence. Hum. Immunol. 2011, 72, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, T.; Ye, J.; Li, H.; Huang, J.; Li, X.; Wu, B.; Huang, X.; Hou, J. Tumor-infiltrating lymphocytes predict response to chemotherapy in patients with advance non-small cell lung cancer. Cancer Immunol. Immunother. 2012, 61, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, M.; Adam, J.; Stoll, G.; Louvet, E.; Chaba, K.; Poirier-Colame, V.; Sauvat, A.; Senovilla, L.; Vacchelli, E.; Bloy, N.; et al. The ratio of CD8+/FOXP3 T lymphocytes infiltrating breast tissues predicts the relapse of ductal carcinoma in situ. Oncoimmunology 2016, 5, e1218106. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Loibl, S.; Noske, A.; Roller, M.; Müller, B.M.; Komor, M.; Budczies, J.; Darb-Esfahani, S.; Kronenwett, R.; Hanusch, C.; et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 2010, 28, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; von Minckwitz, G.; Brase, J.C.; Sinn, B.V.; Gade, S.; Kronenwett, R.; Pfitzner, B.M.; Salat, C.; Loi, S.; Schmitt, W.D.; et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J. Clin. Oncol. 2015, 33, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Ingold Heppner, B.; Loibl, S.; Denkert, C. Tumor-infiltrating lymphocytes: A promising biomarker in breast cancer. Breast Care 2016, 11, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.V.; Mathieu, M.C.; Guarneri, V.; Conte, P.; Delaloge, S.; Andre, F.; Goubar, A. Prognostic and predictive value of tumor-infiltrating lymphocytes in two phase III randomized adjuvant breast cancer trials. Ann. Oncol. 2015, 26, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.V.; Criscitiello, C.; Goubar, A.; Viale, G.; Conte, P.; Guarneri, V.; Ficarra, G.; Mathieu, M.C.; Delaloge, S.; Curigliano, G.; et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: A retrospective multicenter study. Ann. Oncol. 2015, 26, 1518. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Sasano, H.; Tamaki, K.; Hirakawa, H.; Takahashi, Y.; Nakagawa, S.; Watanabe, G.; Tada, H.; Suzuki, A.; Ohuchi, N.; et al. Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: A retrospective multicenter study. Breast Cancer Res. 2015, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Kashiwagi, S.; Goto, W.; Kurata, K.; Noda, S.; Takashima, T.; Onoda, N.; Tanaka, S.; Ohsawa, M.; Hirakawa, K. Tumour-infiltrating CD8 to FOXP3 lymphocyte ratio in predicting treatment responses to neoadjuvant chemotherapy of aggressive breast cancer. Br. J. Surg. 2016, 103, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILS) in breast cancer: Recommendations by an international TILS working group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Denkert, C.; Campbell, C.; Savas, P.; Nuciforo, P.; Nucifero, P.; Aura, C.; de Azambuja, E.; Eidtmann, H.; Ellis, C.E.; et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: A secondary analysis of the neoaltto trial. JAMA Oncol. 2015, 1, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Dushyanthen, S.; Beavis, P.A.; Salgado, R.; Denkert, C.; Savas, P.; Combs, S.; Rimm, D.L.; Giltnane, J.M.; Estrada, M.V.; et al. Ras/mapk activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: Therapeutic cooperation between mek and PD-1/PD-L1 immune checkpoint inhibitors. Clin. Cancer Res. 2016, 22, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Senovilla, L.; Galluzzi, L.; André, F.; Zitvogel, L. Natural and therapy-induced immunosurveillance in breast cancer. Nat. Med. 2015, 21, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, S.; Asano, Y.; Goto, W.; Takada, K.; Takahashi, K.; Noda, S.; Takashima, T.; Onoda, N.; Tomita, S.; Ohsawa, M.; et al. Use of tumor-infiltrating lymphocytes (TILS) to predict the treatment response to eribulin chemotherapy in breast cancer. PLoS ONE 2017, 12, e0170634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Schiller, G.D.; Gill, P.G.; Coventry, B.J. Lymphoid cell infiltration during breast cancer growth: A syngeneic rat model. Immunol. Cell Biol. 1998, 76, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Pikarsky, E.; Karin, M.; Coussens, L.M.; Chen, Y.C.; El-Omar, E.M.; Trinchieri, G.; Dubinett, S.M.; Mao, J.T.; Szabo, E.; et al. Cancer and inflammation: Promise for biologic therapy. J. Immunother. 2010, 33, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Murri, A.M.; Hilmy, M.; Bell, J.; Wilson, C.; McNicol, A.M.; Lannigan, A.; Doughty, J.C.; McMillan, D.C. The relationship between the systemic inflammatory response, tumour proliferative activity, T-lymphocytic and macrophage infiltration, microvessel density and survival in patients with primary operable breast cancer. Br. J. Cancer 2008, 99, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Zlobec, I.; Lugli, A. Invasive front of colorectal cancer: Dynamic interface of pro-/anti-tumor factors. World J. Gastroenterol. 2009, 15, 5898–5906. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Coussens, L.M. Inflammation and breast cancer. Balancing immune response: Crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Shiao, S.L.; Ganesan, A.P.; Rugo, H.S.; Coussens, L.M. Immune microenvironments in solid tumors: New targets for therapy. Genes Dev. 2011, 25, 2559–2572. [Google Scholar] [CrossRef] [PubMed]
- Alkatout, I.; Hübner, F.; Wenners, A.; Hedderich, J.; Wiedermann, M.; Sánchez, C.; Röcken, C.; Mathiak, M.; Maass, N.; Klapper, W. In situ localization of tumor cells associated with the epithelial-mesenchymal transition marker snail and the prognostic impact of lymphocytes in the tumor microenvironment in invasive ductal breast cancer. Exp. Mol. Pathol. 2017, 102, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.P.; Roth, A.; Goya, R.; Oloumi, A.; Ha, G.; Zhao, Y.; Turashvili, G.; Ding, J.; Tse, K.; Haffari, G.; et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 2012, 486, 395–399. [Google Scholar] [CrossRef] [PubMed]
- West, N.R.; Milne, K.; Truong, P.T.; Macpherson, N.; Nelson, B.H.; Watson, P.H. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res. 2011, 13, R126. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Van Eenoo, F.; Rouas, G.; Francis, P.; Crown, J.P.; Hitre, E.; et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: Big 02–98. J. Clin. Oncol. 2013, 31, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Qu, Q.; Chen, X.; Huang, O.; Wu, J.; Shen, K. The prognostic value of tumor-infiltrating lymphocytes in breast cancer: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0152500. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S.; Kumar, S.; Konwar, R.; Srivastava, A.N.; Makker, A.; Goel, M.M. Presence of CD3+ tumor infiltrating lymphocytes is significantly associated with good prognosis in infiltrating ductal carcinoma of breast. Indian J. Cancer 2013, 50, 239–244. [Google Scholar] [PubMed]
- Ali, H.R.; Provenzano, E.; Dawson, S.J.; Blows, F.M.; Liu, B.; Shah, M.; Earl, H.M.; Poole, C.J.; Hiller, L.; Dunn, J.A.; et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann. Oncol. 2014, 25, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lachapelle, J.; Leung, S.; Gao, D.; Foulkes, W.D.; Nielsen, T.O. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012, 14, R48. [Google Scholar] [CrossRef] [PubMed]
- Alkatout, I.; Wiedermann, M.; Bauer, M.; Wenners, A.; Jonat, W.; Klapper, W. Transcription factors associated with epithelial-mesenchymal transition and cancer stem cells in the tumor centre and margin of invasive breast cancer. Exp. Mol. Pathol. 2013, 94, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Ghebeh, H.; Barhoush, E.; Tulbah, A.; Elkum, N.; Al-Tweigeri, T.; Dermime, S. FOXP3+ tregs and B7-H1+/PD-1+ T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: Implication for immunotherapy. BMC Cancer 2008, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Droeser, R.; Zlobec, I.; Kilic, E.; Güth, U.; Heberer, M.; Spagnoli, G.; Oertli, D.; Tapia, C. Differential pattern and prognostic significance of CD4+, FOXP3+ and IL-17+ tumor infiltrating lymphocytes in ductal and lobular breast cancers. BMC Cancer 2012, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Bense, R.D.; Sotiriou, C.; Piccart-Gebhart, M.J.; Haanen, J.B.; van Vugt, M.A.; de Vries, E.G.; Schröder, C.P.; Fehrmann, R.S. Relevance of tumor-infiltrating immune cell composition and functionality for disease outcome in breast cancer. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Michiels, S.; Salgado, R.; Sirtaine, N.; Jose, V.; Fumagalli, D.; Kellokumpu-Lehtinen, P.L.; Bono, P.; Kataja, V.; Desmedt, C.; et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the finher trial. Ann. Oncol. 2014, 25, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Gray, R.J.; Demaria, S.; Goldstein, L.; Perez, E.A.; Shulman, L.N.; Martino, S.; Wang, M.; Jones, V.E.; Saphner, T.J.; et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J. Clin. Oncol. 2014, 32, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S.; Kumar, S.; Konwar, R.; Makker, A.; Negi, M.P.; Goel, M.M. CD3+, CD4+ & CD8+ tumour infiltrating lymphocytes (TILS) are predictors of favourable survival outcome in infiltrating ductal carcinoma of breast. Indian J. Med. Res. 2014, 140, 361–369. [Google Scholar] [PubMed]
- Liu, F.; Lang, R.; Zhao, J.; Zhang, X.; Pringle, G.A.; Fan, Y.; Yin, D.; Gu, F.; Yao, Z.; Fu, L. CD8+ cytotoxic T cell and FOXP3+ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res. Treat. 2011, 130, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Gao, Z.; Cai, Z.; Wang, M.; He, J. Clinicopathological and prognostic significance of FOXP3+ tumor infiltrating lymphocytes in patients with breast cancer: A meta-analysis. BMC Cancer 2015, 15, 727. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xu, D.; Liu, Z.; Shi, M.; Zhao, P.; Fu, B.; Zhang, Z.; Yang, H.; Zhang, H.; Zhou, C.; et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 2007, 132, 2328–2339. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Joshi, K.; Wig, J.D.; Arora, S.K. Intratumoral FOXP3 expression in infiltrating breast carcinoma: Its association with clinicopathologic parameters and angiogenesis. Acta Oncol. 2007, 46, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Chikazawa, N.; Tasaka, T.; Wada, J.; Yamasaki, A.; Kitaura, Y.; Sozaki, M.; Tanaka, M.; Onishi, H.; Morisaki, T.; et al. Intratumoral CD8+ T/FOXP3+ cell ratio is a predictive marker for survival in patients with colorectal cancer. Cancer Immunol. Immunother. 2010, 59, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Frigola, X.; Bonne-Annee, S.; Mercader, M.; Kuntz, S.M.; Krambeck, A.E.; Sengupta, S.; Dong, H.; Cheville, J.C.; Lohse, C.M.; et al. Tumor-infiltrating Foxp3-Cd4+Cd25+ T cells predict poor survival in renal cell carcinoma. Clin. Cancer Res. 2007, 13, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Wolf, A.M.; Rumpold, H.; Fiegl, H.; Zeimet, A.G.; Muller-Holzner, E.; Deibl, M.; Gastl, G.; Gunsilius, E.; Marth, C. The expression of the regulatory T cell-specific forkhead box transcription factor FOXP3 is associated with poor prognosis in ovarian cancer. Clin. Cancer Res. 2005, 11, 8326–8331. [Google Scholar] [CrossRef] [PubMed]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Aldrich, A.J.; McDonnell, E.; Cheng, Q.; Aggarwal, A.; Patel, P.; Williams, M.M.; Boczkowski, D.; Lyerly, H.K.; Morse, M.A.; et al. Immunologic targeting of FOXP3 in inflammatory breast cancer cells. PLoS ONE 2013, 8, e53150. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A.; Rego, R.L.; Ansell, S.M.; Knutson, K.L.; Foster, N.R.; Sargent, D.J. Intraepithelial effector (CD3+)/regulatory (FOXP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology 2009, 137, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.P.; Campa, M.J.; Sperlazza, J.; Conlon, D.; Joshi, M.B.; Harpole, D.H.; Patz, E.F. Tumor infiltrating FOXP3+ regulatory T-cells are associated with recurrence in pathologic stage 1 NSCLC patients. Cancer 2006, 107, 2866–2872. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Qiu, S.J.; Fan, J.; Zhou, J.; Wang, X.Y.; Xiao, Y.S.; Xu, Y.; Li, Y.W.; Tang, Z.Y. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J. Clin. Oncol. 2007, 25, 2586–2593. [Google Scholar] [CrossRef] [PubMed]
- Preston, C.C.; Maurer, M.J.; Oberg, A.L.; Visscher, D.W.; Kalli, K.R.; Hartmann, L.C.; Goode, E.L.; Knutson, K.L. The ratios of CD8+ T cells to CD4+CD25+ FOXP3+ and FOXP3− T cells correlate with poor clinical outcome in human serous ovarian cancer. PLoS ONE 2013, 8, e80063. [Google Scholar] [CrossRef] [PubMed]
- Böcker, W. Who classification of breast tumors and tumors of the female genital organs: Pathology and genetics. Verh. Dtsch. Ges. Pathol. 2002, 86, 116–119. [Google Scholar] [PubMed]
- Hammond, M.E.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American society of clinical oncology/college of american pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007, 25, 118–145. [Google Scholar] [CrossRef] [PubMed]
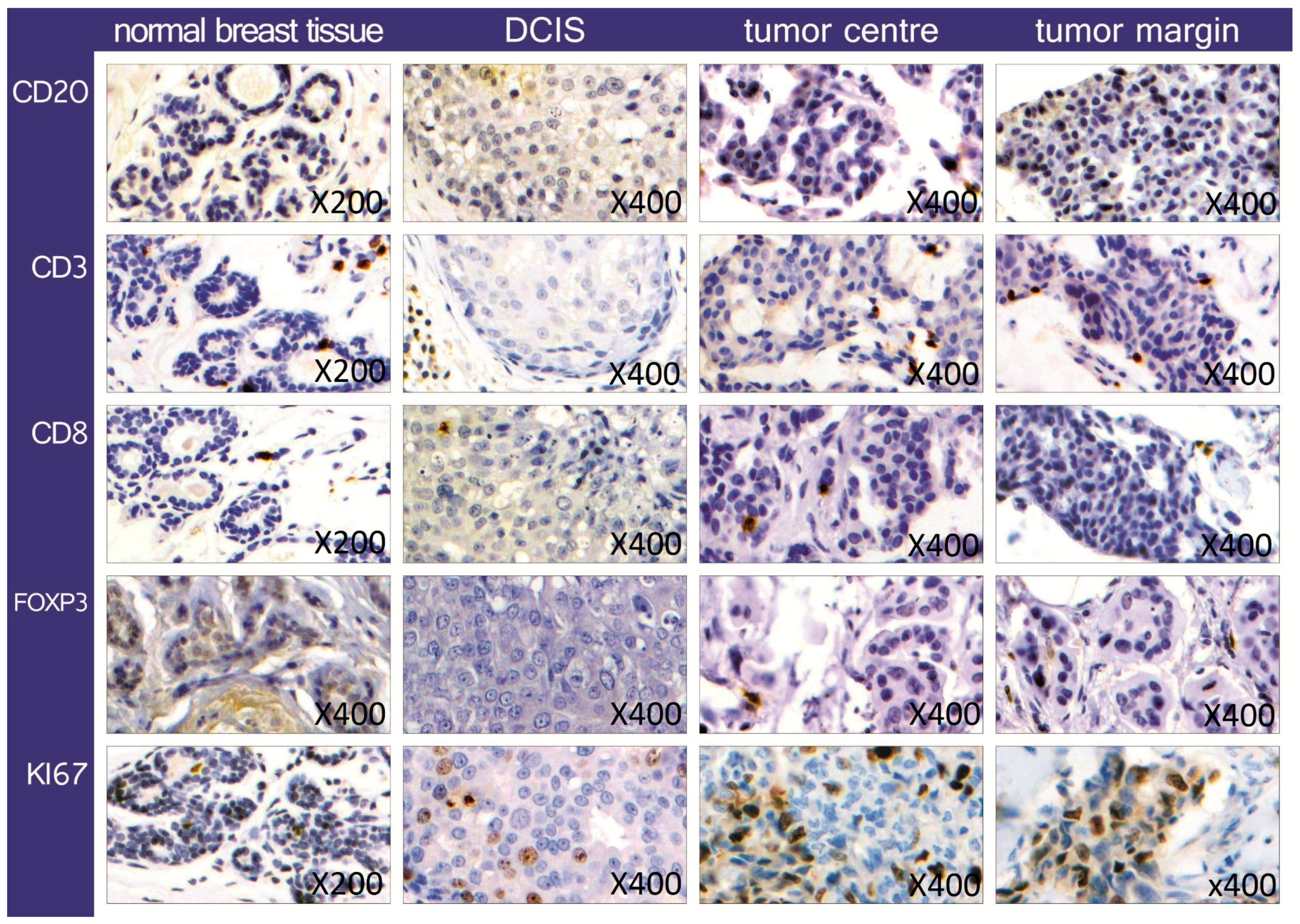
| Invasive Breast Cancer Samples | CD3 | CD8 | CD20 | FoxP3 | Ki67 | CD3/FoxP3 | CD8/FoxP3 |
|---|---|---|---|---|---|---|---|
| All samples (n = 62) | 3.8 | 1.58 | 0.15 | 0.54 | 11.77 | 3.73 | 1.78 |
| Clinical groups | |||||||
| R (n = 26) | 3.67 | 1.06 | 0.1 | 0.58 | 12.46 | 3.6 | 0.82 |
| N (n = 36) | 3.88 | 1.98 | 0.18 | 0.52 | 11.31 | 3.82 | 2.74 |
| p 0.825 | p 0.137 | p 0.447 | p 0.801 | p 0.753 | p 0.886 | p 0.334 | |
| Topological groups | |||||||
| M (n = 31) | 4.27 | 1.82 | 0.07 | 0.69 | 10.84 | 3.14 | 2.31 |
| C (n = 31) | 3.26 | 1.27 | 0.23 | 0.38 | 12.81 | 4.74 | 1.02 |
| p 0.263 | p 0.409 | p 0.127 | p 0.232 | p 0.580 | p 0.298 | p 0.524 | |
| Subgroups | |||||||
| RM (n = 13) | 4.02 | 1.14 | 0 | 0.84 | 11.42 | 2.7 | 0.76 |
| NM (n = 18) | 4.45 | 2.32 | 0.12 | 0.6 | 10.46 | 3.46 | 3.63 |
| p 0.882 | p 0.245 | p 0.163 | p 0.560 | p 0.844 | p 0.620 | p 0.401 | |
| RC (n = 13) | 3.3 | 0.98 | 0.2 | 0.33 | 13.59 | 5.11 | 0.9 |
| NC (n = 18) | 3.23 | 1.5 | 0.25 | 0.42 | 12.28 | 4.46 | 1.29 |
| p 0.944 | p 0.521 | p 0.779 | p 0.806 | p 0.816 | p 0.855 | p 0.634 |
| Significant Correlations | Pearson Correlation (r Coefficient) | Significance (2-Tailed) Value | Correlation’s Sample Size |
|---|---|---|---|
| Inter-subtype correlations | |||
| CD3–CD8 | 0.392 | 0.009 ** | 43 |
| CD3c–CD8c | 0.496 | 0.031 * | 19 |
| CD3n–CD3n | 0.469 | 0.016 * | 26 |
| FoxP3–Ki67 | 0.337 | 0.024 * | 45 |
| FoxP3c–Ki67c | 0.803 | 0.000 ** | 21 |
| FoxP3c–Ki67m | 0.457 | 0.043 * | 20 |
| FoxP3n–Ki67n | 0.55 | 0.003 ** | 27 |
| FoxP3nc–Ki67nc | 0.887 | 0.000 ** | 12 |
| FoxP3nm–Ki67nc | 0.582 | 0.037 * | 13 |
| C3FR–C8FR | 0.56 | 0.013 * | 19 |
| C3FRm–C8FRm | 0.884 | 0.000 ** | 12 |
| C3FRnm–C8FRnm | 0.911 | 0.004 ** | 7 |
| Intra-subtype correlations | |||
| CD3c–CD3m | 0.647 | 0.001 ** | 22 |
| CD3rc–CD3rm | 0.694 | 0.038 * | 9 |
| CD3nc–CD3nm | 0.632 | 0.020 * | 13 |
| Ki67c–Ki67m | 0.778 | 0.000 ** | 26 |
| Ki67rc–Ki67rm | 0.858 | 0.001 ** | 10 |
| Ki67nc–Ki67nm | 0.724 | 0.002 ** | 16 |
| FoxP3c–FoxP3m | 0.618 | 0.006 ** | 18 |
| FoxP3nc–FoxP3nm | 0.738 | 0.010 ** | 11 |
| Parameters | R Group’s Absolute Frequency (n = 13) | R Group’s Relative Frequency % | NR Group’s Absolute Frequency (n = 18) | NR Group’s Relative Frequency % |
|---|---|---|---|---|
| TNM classification | ||||
| T1 | 13 | 100 | 18 | 100 |
| N0 | 10 | 76.9 | 18 | 100 |
| M0 | 11 | 84.6 | 16 | 88.9 |
| Histological type | ||||
| Ductal | 8 | 61.5 | 14 | 77.8 |
| Lobular | 2 | 15.4 | 3 | 16.7 |
| Other | 3 | 23.1 | 1 | 5.6 |
| Tumor grade | ||||
| ≤G2 | 8 | 61.5 | 12 | 66.7 |
| Receptor expression | ||||
| ER+ ≥ 3 | 7 | 53.8 | 15 | 83.3 |
| PR+ ≥ 3 | 6 | 46.1 | 12 | 66.7 |
| Her2neu ≥ 2 | 2 | 15.4 | 4 | 22.2 |
| Patients’ age | ||||
| Mean | 51 | 55 | ||
| Max | 68 | 72 | ||
| Min | 36 | 36 | ||
| Time of follow-up * | 99 | 54 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romagnoli, G.; Wiedermann, M.; Hübner, F.; Wenners, A.; Mathiak, M.; Röcken, C.; Maass, N.; Klapper, W.; Alkatout, I. Morphological Evaluation of Tumor-Infiltrating Lymphocytes (TILs) to Investigate Invasive Breast Cancer Immunogenicity, Reveal Lymphocytic Networks and Help Relapse Prediction: A Retrospective Study. Int. J. Mol. Sci. 2017, 18, 1936. https://doi.org/10.3390/ijms18091936
Romagnoli G, Wiedermann M, Hübner F, Wenners A, Mathiak M, Röcken C, Maass N, Klapper W, Alkatout I. Morphological Evaluation of Tumor-Infiltrating Lymphocytes (TILs) to Investigate Invasive Breast Cancer Immunogenicity, Reveal Lymphocytic Networks and Help Relapse Prediction: A Retrospective Study. International Journal of Molecular Sciences. 2017; 18(9):1936. https://doi.org/10.3390/ijms18091936
Chicago/Turabian StyleRomagnoli, Gloria, Meike Wiedermann, Friederike Hübner, Antonia Wenners, Micaela Mathiak, Christoph Röcken, Nicolai Maass, Wolfram Klapper, and Ibrahim Alkatout. 2017. "Morphological Evaluation of Tumor-Infiltrating Lymphocytes (TILs) to Investigate Invasive Breast Cancer Immunogenicity, Reveal Lymphocytic Networks and Help Relapse Prediction: A Retrospective Study" International Journal of Molecular Sciences 18, no. 9: 1936. https://doi.org/10.3390/ijms18091936
APA StyleRomagnoli, G., Wiedermann, M., Hübner, F., Wenners, A., Mathiak, M., Röcken, C., Maass, N., Klapper, W., & Alkatout, I. (2017). Morphological Evaluation of Tumor-Infiltrating Lymphocytes (TILs) to Investigate Invasive Breast Cancer Immunogenicity, Reveal Lymphocytic Networks and Help Relapse Prediction: A Retrospective Study. International Journal of Molecular Sciences, 18(9), 1936. https://doi.org/10.3390/ijms18091936





