Therapeutic Effect of Cilostazol Ophthalmic Nanodispersions on Retinal Dysfunction in Streptozotocin-Induced Diabetic Rats
Abstract
:1. Introduction
2. Results
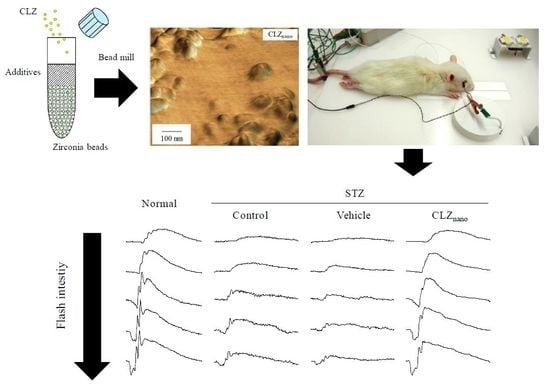
2.1. Changes in Retinal Function in STZ Rats
2.2. Preventive Effect of CLZnano Instillation on Retinal Disorders in STZ Rats
2.3. Corneal Stimulation by CLZnano Instillation
3. Discussion
4. Materials and Methods
4.1. Reagents and Animals
4.2. Preparation of Ophthalmic CLZ Nanodispersions
4.3. Measurement of CLZ by HPLC
4.4. Measurement of Plasma Glucose and Insulin
4.5. Measurement of CLZ Content in Blood and Retina
4.6. Measurement of VEGF
4.7. Measurement of ERG
4.8. Morphology of Rat Retina
4.9. Measurement of In Vitro Corneal Epithelial Stimulation by CLZnano
4.10. Measurement of In Vivo Corneal Toxicity by CLZnano
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Abs | Absorbance |
| BAC | Benzalkonium Chloride |
| CLZ | Cilostazol |
| DR | Diabetic Retinopathy |
| ERG | Electroretinogram |
| ET | Endotheline |
| HCE-T | Human Corneal Epithelial Cell Line |
| H.E. | Hematoxylin and Eosin |
| HPβCD | 2-Hydroxypropyl-β-Cyclodextrin |
| OPs | Oscillatory Potentials |
| MC | Methylcellulose |
| STZ rat | Streptozotocin-Induced Diabetic Rat |
| VEGF | Vascular Endothelial Growth Factor |
References
- Miller, J.W.; Adamis, A.P.; Aiello, L.P. Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes Metab. Rev. 1997, 13, 37–50. [Google Scholar] [CrossRef]
- Cheung, N.; Wong, T.Y. Diabetic retinopathy and systemic vascular complications. Prog. Retin. Eye Res. 2008, 27, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Trick, G.L.; Burde, R.M.; Gordon, M.O.; Kilo, C.; Santiago, J.V. Retinocortical conduction time in diabetics with abnormal pattern reversal electroretinograms and visual evoked potentials. Doc. Ophthalmol. 1988, 70, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Wolff, B.E.; Bearse, M.A., Jr.; Schneck, M.E.; Barez, S.; Adams, A.J. Multifocal VEP (mfVEP) reveals abnormal neuronal delays in diabetes. Doc. Ophthalmol. 2010, 121, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Falsini, B.; Porciatti, V.; Scalia, G.; Caputo, S.; Minnella, A.; di Leo, M.A.; Ghirlanda, G. Steady-state pattern electroretinogram in insulin-dependent diabetics with no or minimal retinopathy. Doc. Ophthalmol. 1989, 73, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, D.; Aranda, M.L.; Rosenstein, R.E. Enriched environment protects the optic nerve from early diabetes-induced damage in adult rats. PLoS ONE 2015, 10, e0136637. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, D.C.; Pasquini, L.A.; Dorfman, D.; Aldana Marcos, H.J.; Rosenstein, R.E. Early distal axonopathy of the visual pathway in experimental diabetes. Am. J. Pathol. 2012, 180, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, D.C.; Pasquini, L.A.; Dorfman, D.; Aldana Marcos, H.J.; Rosenstein, R.E. Ischemic conditioning protects from axoglial alterations of the optic pathway induced by experimental diabetes in rats. PLoS ONE 2012, 7, e51966. [Google Scholar] [CrossRef] [PubMed]
- Chapman, T.M.; Goa, K.L. Cilostazol: A review of its use in intermittent claudication. Am. J. Cardiovasc. Drugs 2003, 3, 117–138. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Gotoh, F.; Fukuuchi, Y.; Amano, T.; Uematsu, D.; Kawamura, J.; Yamawaki, T.; Itoh, N.; Obara, K.; Muramatsu, K. Effects of a selective inhibitor of cyclic AMP phosphodiesterase on the pial microcirculation in feline cerebral ischemia. Stroke 1989, 20, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.U.; Cho, Y.J.; Koo, J.S.; Bae, H.J.; Lee, Y.S.; Hong, K.S.; Lee, J.H.; Kim, J.S. Cilostazol prevents the progression of the symptomatic intracranial arterial stenosis: The multicenter double-blind placebo-controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke 2005, 36, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Kim, Y.S.; Kawamori, R.; Yamasaki, Y. The phosphodiesterase inhibitor cilostazol induces regression of carotid atherosclerosis in subjects with type 2 diabetes mellitus. Circulation 2010, 121, 2584–2591. [Google Scholar] [CrossRef] [PubMed]
- Hotta, H.; Ito, H.; Kagitani, F.; Sato, A. Cilostazol, a selective cAMP phosphodiesterase inhibitor, dilates retinal arterioles and increases retinal and choroidal blood flow in rats. Eur. J. Pharmacol. 1998, 344, 49–52. [Google Scholar] [CrossRef]
- Asasutjarit, R.; Thanasanchokpibull, S.; Fuongfuchat, A.; Veeranodha, S. Optimization and evaluation of thermoresponsive diclofenac sodium ophthalmic in situ gels. Int. J. Pharm. 2011, 411, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Rafie, F.; Javadzadeh, Y.; Javadzadeh, A.R.; Ghavidel, L.A.; Jafari, B.; Moogooee, M.; Davaran, S. In vivo evaluation of novel nanoparticles containing dexa-methasone for ocular drug delivery on rabbit eye. Curr. Eye Res. 2010, 35, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Diebold, Y.; Jarrín, M.; Sáez, V.; Carvalho, E.L.; Orea, M.; Calonge, M.; Seijo, B.; Alonso, M.J. Ocular drug delivery by liposome-chitosan nanoparticle complexes (LCS-NP). Biomaterials 2007, 28, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Hao, J.L.; Wang, S.; Zheng, Y.; Zhang, W.S. Nanoparticles in the ocular drug delivery. Int. J. Ophthalmol. 2013, 6, 390–396. [Google Scholar] [PubMed]
- Rahul, M.; Mohita, U.; Sanat, M. Design considerations for chemotherapeutic drug nanocarriers. Pharm. Anal. Acta 2014, 5, 279. [Google Scholar]
- Gupta, H.; Aqil, M.; Khar, R.K.; Ali, A.; Bhatnagar, A.; Mittal, G. Biodegradable levofloxacin nanoparticles for sustained ocular drug delivery. J. Drug Target 2011, 19, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, K.; Watanabe, A.; Suzuki, K.; Inagi, T.; Terada, H.; Makino, K. Enhanced transdermal permeability of estradiol using combination of PLGA nanoparticles system and iontophoresis. Colloids Surf. B Biointerfaces 2012, 97, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, K.; Terashima, H.; Suzuki, K.; Inagi, T.; Terada, H.; Makino, K. Enhanced transdermal delivery of indomethacin-loaded PLGA nanoparticles by iontophoresis. Colloids Surf. B Biointerfaces 2011, 88, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, K.; Terashima, H.; Suzuki, K.; Inagi, T.; Terada, H.; Makino, K. Enhanced transdermal delivery of indomethacin using combination of PLGA nanoparticles and iontophoresis in vivo. Colloids Surf. B Biointerfaces 2012, 92, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y. Effect of solid nanoparticle of indomethacin on therapy for rheumatoid arthritis in adjuvant-induced arthritis rat. Biol. Pharm. Bull. 2014, 37, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y. A new preparation method for ophthalmic drug nanoparticles. Pharm. Anal. Acta 2014, 5, 6. [Google Scholar]
- Nagai, N.; Ono, H.; Hashino, M.; Ito, Y.; Okamoto, N.; Shimomura, Y. Improved corneal toxicity and permeability of tranilast by the preparation of ophthalmic formulations containing its nanoparticles. J. Oleo Sci. 2014, 63, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation reduces the corneal toxicity of indomethacin eye drops and enhances its corneal permeability. Toxicology 2014, 319, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y. Therapeutic effects of gel ointments containing tranilast nanoparticles on paw edema in adjuvant-induced arthritis rats. Biol. Pharm. Bull. 2014, 37, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yoshioka, C.; Mano, Y.; Tanabe, W.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation of disulfiram prolongs corneal residence time of the drug and reduces intraocular pressure. Exp. Eye Res. 2015, 132, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yoshioka, C.; Ito, Y. Topical Therapies for rheumatoid arthritis by gel ointments containing indomethacin nanoparticles in adjuvant-induced arthritis rat. J. Oleo Sci. 2015, 64, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yoshioka, C.; Tanabe, W.; Tanino, T.; Ito, Y.; Okamoto, N.; Shimomura, Y. Effects of ophthalmic formulations containing cilostazol nanoparticles on retinal vasoconstriction in rats injected with endothelin-1. Pharm. Anal. Acta 2015, 6, 4. [Google Scholar]
- Barber, A.J.; Lieth, E.; Khin, S.A.; Antonetti, D.A.; Buchanan, A.G.; Gardner, T.W. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J. Clin. Investig. 1998, 102, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Lecleire-Collet, A.; Audo, I.; Aout, M.; Girmens, J.F.; Sofroni, R.; Erginay, A.; Gargasson, J.F.; Mohand-Saïd, S.; Meas, T.; Guillausseau, P.J.; et al. Evaluation of retinal function and flicker light-induced retinal vascular response in normotensive patients with diabetes without retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2861–2867. [Google Scholar] [CrossRef] [PubMed]
- Hancock, H.A.; Kraft, T.W. Oscillatory potential analysis and ERGs of normal and diabetic rats. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1002–1008. [Google Scholar] [CrossRef]
- Li, Q.; Zemel, E.; Miller, B.; Perlman, I. Early retinal damage in experimental diabetes: Electroretinographical and morphological observations. Exp. Eye Res. 2002, 74, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Kohzaki, K.; Vingrys, A.J.; Bui, B.V. Early inner retinal dysfunction in streptozotocin-induced diabetic rats. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3595–3604. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.O.; Salama, H.A.; Ghorab, M.; Mahmoud, A.A. Nanoemulsions as potential ophthalmic delivery systems for orzolamide hydrochloride. Pharm. Sci. Tech. 2009, 10, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yoshioka, C.; Ito, Y.; Funakami, Y.; Nishikawa, H.; Kawabata, A. Intravenous administration of cilostazol nanoparticles ameliorates acute ischemic stroke in a cerebral ischemia/reperfusion-induced injury model. Int. J. Mol. Sci. 2015, 16, 29329–29344. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.L.; Phipps, J.A.; Wilkinson-Berka, J.L. Dysfunction of retinal neurons and glia during diabetes. Clin. Exp. Optom. 2005, 88, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.H.; Frank, R.N.; Kennedy, A.; Eliott, D.; Puklin, J.E.; Abrams, G.W. Vascular endothelial growth factor is present in glial cells of the retina and optic nerve of human subjects with nonproliferative diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 1997, 38, 36–47. [Google Scholar]
- Hiramatsu, N.; Deguchi, S.; Yoshioka, C.; Otake, H.; Yamamoto, N.; Nagai, N. Evaluation of retinal function in streptozotocin-induced diabetic rats by using the electroretinography and immunohistochemistry methods. Yakugaku Zasshi 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y.; Sasaki, S. Hyperglycemia enhances the production of amyloid β1–42 in the lenses of otsuka long-evans tokushima fatty rats, a model of human type 2 diabetes. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Majima, K.; Marunouchi, T. A study of the proliferating activity in lens epithelium and the identification of tissue-type stem cells. Med. Mol. Morphol. 2008, 41, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Yamada, M.; Yoshikawa, K.; Iino, M. Presented at the Ganka-Kaigyoui notameno Gimon·nanmon Kaiketusaku; Shindan to Chiryosha Co.: Tokyo, Japan, 2006; pp. 216–217. (In Japanese) [Google Scholar]







| Treatment | Body Weight (g) | Glucose (mg/dL) | Insulin (ng/dL) |
|---|---|---|---|
| Vehicle | 301.1 ± 8.68 | 273.2 ± 30.7 | N.D. |
| CLZnano | 299.5 ± 9.10 | 269.7 ± 29.8 | N.D. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, N.; Deguchi, S.; Otake, H.; Hiramatsu, N.; Yamamoto, N. Therapeutic Effect of Cilostazol Ophthalmic Nanodispersions on Retinal Dysfunction in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2017, 18, 1971. https://doi.org/10.3390/ijms18091971
Nagai N, Deguchi S, Otake H, Hiramatsu N, Yamamoto N. Therapeutic Effect of Cilostazol Ophthalmic Nanodispersions on Retinal Dysfunction in Streptozotocin-Induced Diabetic Rats. International Journal of Molecular Sciences. 2017; 18(9):1971. https://doi.org/10.3390/ijms18091971
Chicago/Turabian StyleNagai, Noriaki, Saori Deguchi, Hiroko Otake, Noriko Hiramatsu, and Naoki Yamamoto. 2017. "Therapeutic Effect of Cilostazol Ophthalmic Nanodispersions on Retinal Dysfunction in Streptozotocin-Induced Diabetic Rats" International Journal of Molecular Sciences 18, no. 9: 1971. https://doi.org/10.3390/ijms18091971