Comparative Analysis of Different Platelet Lysates and Platelet Rich Preparations to Stimulate Tendon Cell Biology: An In Vitro Study
Abstract
:1. Introduction
2. Results
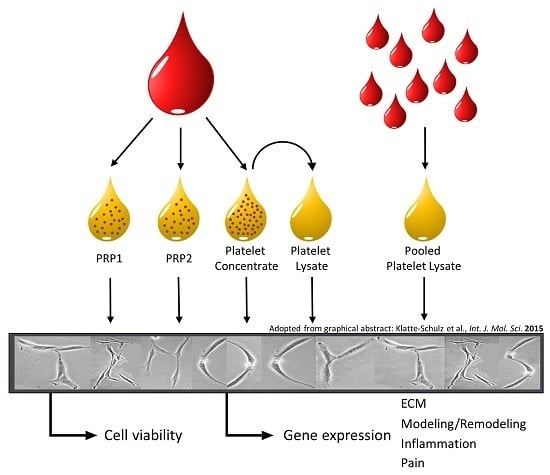
2.1. Characterization of Blood Products
2.2. Cell Stimulation
3. Discussion
4. Materials and Methods
4.1. Preparation of Human Blood Products
4.2. Platelet Concentrate (PC)/Plasma Lysate (PL)/Allo-PL Preparation
4.3. Standard PRP Preparations and Human Serum (HS) Control
4.4. Growth Factor Quantification
4.5. Growth Factor Release from Blood Products
4.6. Human Tenocyte-Like Cells
4.7. Cell Stimulation
4.8. Gene Expression Analysis
4.9. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fenwick, S.A.; Hazleman, B.L.; Riley, G.P. The vasculature and its role in the damaged and healing tendon. Arthritis Res. 2002, 4, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pufe, T.; Petersen, W.J.; Mentlein, R.; Tillmann, B.N. The role of vasculature and angiogenesis for the pathogenesis of degenerative tendons disease. Scand. J. Med. Sci. Sports 2005, 15, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Docheva, D.; Muller, S.A.; Majewski, M.; Evans, C.H. Biologics for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef] [PubMed]
- Halpern, B.C.; Chaudhury, S.; Rodeo, S.A. The role of platelet-rich plasma in inducing musculoskeletal tissue healing. HSS J. 2012, 8, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, J.H. PRP Treatment Efficacy for Tendinopathy: A Review of Basic Science Studies. BioMed Res. Int. 2016, 2016, 9103792. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, L.V.; Mohammed, H.O.; Miller, B.J.; McDermott, W.G.; Jacobson, M.S.; Santangelo, K.S.; Fortier, L.A. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J. Orthop. Res. 2007, 25, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Mazzocca, A.D.; McCarthy, M.B.; Chowaniec, D.M.; Dugdale, E.M.; Hansen, D.; Cote, M.P.; Bradley, J.P.; Romeo, A.A.; Arciero, R.A.; Beitzel, K. The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am. J. Sports Med. 2012, 40, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qiu, Y.; Triffitt, J.; Carr, A.; Xia, Z.; Sabokbar, A. Proliferation and differentiation of human tenocytes in response to platelet rich plasma: An in vitro and in vivo study. J. Orthop. Res. 2012, 30, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Ochsner, P.E.; Liu, F.; Fluckiger, R.; Evans, C.H. Accelerated healing of the rat Achilles tendon in response to autologous conditioned serum. Am. J. Sports Med. 2009, 37, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Dallaudiere, B.; Lempicki, M.; Pesquer, L.; Louedec, L.; Preux, P.M.; Meyer, P.; Hummel, V.; Larbi, A.; Deschamps, L.; Journe, C.; et al. Efficacy of intra-tendinous injection of platelet-rich plasma in treating tendinosis: Comprehensive assessment of a rat model. Eur. Radiol. 2013, 23, 2830–2837. [Google Scholar] [CrossRef] [PubMed]
- Spang, J.T.; Tischer, T.; Salzmann, G.M.; Winkler, T.; Burgkart, R.; Wexel, G.; Imhoff, A.B. Platelet concentrate vs. saline in a rat patellar tendon healing model. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Lyras, D.N.; Kazakos, K.; Verettas, D.; Polychronidis, A.; Tryfonidis, M.; Botaitis, S.; Agrogiannis, G.; Simopoulos, C.; Kokka, A.; Patsouris, E. The influence of platelet-rich plasma on angiogenesis during the early phase of tendon healing. Foot Ankle Int. 2009, 30, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Molloy, T.; Wang, Y.; Murrell, G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003, 33, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Maffulli, N. Muscle and tendon injuries: The role of biological interventions to promote and assist healing and recovery. Arthroscopy 2015, 31, 999–1015. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Del Buono, A. Platelet plasma rich products in musculoskeletal medicine: Any evidence? Surgeon 2012, 10, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Peerbooms, J.C.; Sluimer, J.; Bruijn, D.J.; Gosens, T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: Platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am. J. Sports Med. 2010, 38, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Gosens, T.; Peerbooms, J.C.; van Laar, W.; den Oudsten, B.L. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: A double-blind randomized controlled trial with 2-year follow-up. Am. J. Sports Med. 2011, 39, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Sampson, S.; Reed, M.; Silvers, H.; Meng, M.; Mandelbaum, B. Injection of platelet-rich plasma in patients with primary and secondary knee osteoarthritis: A pilot study. Am. J. Phys. Med. Rehabil. 2010, 89, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Wang-Saegusa, A.; Cugat, R.; Ares, O.; Seijas, R.; Cusco, X.; Garcia-Balletbo, M. Infiltration of plasma rich in growth factors for osteoarthritis of the knee short-term effects on function and quality of life. Arch. Orthop. Trauma Surg. 2011, 131, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Kon, E.; Di Matteo, B.; Di Martino, A.; Tesei, G.; Pelotti, P.; Cenacchi, A.; Marcacci, M. Platelet-rich plasma injections for the treatment of refractory Achilles tendinopathy: Results at 4 years. Blood Transfus. 2014, 12, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Randelli, P.; Arrigoni, P.; Ragone, V.; Aliprandi, A.; Cabitza, P. Platelet rich plasma in arthroscopic rotator cuff repair: A prospective RCT study, 2-year follow-up. J. Shoulder Elbow Surg. 2011, 20, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Kaux, J.F.; Croisier, J.L.; Bruyere, O.; De La Cruz, C.R.; Forthomme, B.; Brabant, G.; Lapraille, S.; Lonneux, V.; Noel, D.; Le Goff, C.; et al. One injection of platelet-rich plasma associated to a submaximal eccentric protocol to treat chronic jumper’s knee. J. Sports Med. Phys. Fit. 2015, 55, 953–961. [Google Scholar]
- Foster, T.E.; Puskas, B.L.; Mandelbaum, B.R.; Gerhardt, M.B.; Rodeo, S.A. Platelet-rich plasma: From basic science to clinical applications. Am. J. Sports Med. 2009, 37, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Kushida, S.; Kakudo, N.; Morimoto, N.; Hara, T.; Ogawa, T.; Mitsui, T.; Kusumoto, K. Platelet and growth factor concentrations in activated platelet-rich plasma: A comparison of seven commercial separation systems. J. Artif. Organs 2014, 17, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Castillo, T.N.; Pouliot, M.A.; Kim, H.J.; Dragoo, J.L. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am. J. Sports Med. 2011, 39, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Kruger, J.P.; Hondke, S.; Endres, M.; Pruss, A.; Siclari, A.; Kaps, C. Human platelet-rich plasma stimulates migration and chondrogenic differentiation of human subchondral progenitor cells. J. Orthop. Res. 2012, 30, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Mori, M.; Cervio, M.; Riva, F.; Liakos, I.; Athanassiou, A.; Saporito, F.; et al. Platelet lysate embedded scaffolds for skin regeneration. Expert Opin. Drug Deliv. 2015, 12, 525–545. [Google Scholar] [CrossRef] [PubMed]
- Del Fante, C.; Perotti, C.; Bonferoni, M.C.; Rossi, S.; Sandri, G.; Ferrari, F.; Scudeller, L.; Caramella, C.M. Platelet lysate mucohadesive formulation to treat oral mucositis in graft versus host disease patients: A new therapeutic approach. AAPS PharmSciTech 2011, 12, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Pezzotta, S.; Del Fante, C.; Scudeller, L.; Cervio, M.; Antoniazzi, E.R.; Perotti, C. Autologous platelet lysate for treatment of refractory ocular GVHD. Bone Marrow Transplant. 2012, 47, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- Dellera, E.; Bonferoni, M.C.; Sandri, G.; Rossi, S.; Ferrari, F.; Del Fante, C.; Perotti, C.; Grisoli, P.; Caramella, C. Development of chitosan oleate ionic micelles loaded with silver sulfadiazine to be associated with platelet lysate for application in wound healing. Eur. J. Pharm. Biopharm. 2014, 88, 643–650. [Google Scholar] [CrossRef] [PubMed]
- El Backly, R.; Ulivi, V.; Tonachini, L.; Cancedda, R.; Descalzi, F.; Mastrogiacomo, M. Platelet lysate induces in vitro wound healing of human keratinocytes associated with a strong proinflammatory response. Tissue Eng. Part A 2011, 17, 1787–1800. [Google Scholar] [CrossRef] [PubMed]
- Ranzato, E.; Mazzucco, L.; Patrone, M.; Burlando, B. Platelet lysate promotes in vitro wound scratch closure of human dermal fibroblasts: Different roles of cell calcium, P38, ERK and PI3K/AKT. J. Cell. Mol. Med. 2009, 13, 2030–2038. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Strunk, D.; Koh, M.B.; Schallmoser, K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 2016, 76, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Schallmoser, K.; Bartmann, C.; Rohde, E.; Reinisch, A.; Kashofer, K.; Stadelmeyer, E.; Drexler, C.; Lanzer, G.; Linkesch, W.; Strunk, D. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion 2007, 47, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Strunk, D.; Lozano, M.; Marks, D.C.; Loh, Y.S.; Gstraunthaler, G.; Schennach, H.; Rohde, E.; Laner-Plamberger, S.; Oller, M.; Nystedt, J.; et al. International Forum on GMP-grade human platelet lysate for cell propagation: Summary. Vox Sang. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Agostini, F.; Chieregato, K.; Amati, E.; Durante, C.; Rassu, M.; Ruggeri, M.; Sella, S.; Lombardi, E.; Mazzucato, M.; et al. The production method affects the efficacy of platelet derivatives to expand mesenchymal stromal cells in vitro. J. Transl. Med. 2017, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Muraglia, A.; Nguyen, V.T.; Nardini, M.; Mogni, M.; Coviello, D.; Dozin, B.; Strada, P.; Baldelli, I.; Formica, M.; Cancedda, R.; et al. Culture Medium Supplements Derived from Human Platelet and Plasma: Cell Commitment and Proliferation Support. Front. Bioeng. Biotechnol. 2017, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Cancedda, R.; Descalzi, F. Platelet lysate activates quiescent cell proliferation and reprogramming in human articular cartilage: Involvement of hypoxia inducible factor 1. J. Tissue Eng. Regen. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Chen, M.S.; Chou, M.L.; Lin, H.C.; Seghatchian, J.; Burnouf, T. Comparison of three human platelet lysates used as supplements for in vitro expansion of corneal endothelium cells. Transfus. Apher. Sci. 2017, 56, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Del Bue, M.; Ricco, S.; Conti, V.; Merli, E.; Ramoni, R.; Grolli, S. Platelet lysate promotes in vitro proliferation of equine mesenchymal stem cells and tenocytes. Vet. Res. Commun. 2007, 31 (Suppl. S1), 289–292. [Google Scholar] [CrossRef] [PubMed]
- Sellberg, F.; Berglund, E.; Ronaghi, M.; Strandberg, G.; Lof, H.; Sommar, P.; Lubenow, N.; Knutson, F.; Berglund, D. Composition of growth factors and cytokines in lysates obtained from fresh versus stored pathogen-inactivated platelet units. Transfus. Apher. Sci. 2016, 55, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Kruger, J.P.; Freymannx, U.; Vetterlein, S.; Neumann, K.; Endres, M.; Kaps, C. Bioactive factors in platelet-rich plasma obtained by apheresis. Transfus. Med. Hemother. 2013, 40, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Y.; Niu, X.; Lin, Q.; Zhao, B.; Wang, Y.; Zhu, L. An in situ photocrosslinkable platelet rich plasma—Complexed hydrogel glue with growth factor controlled release ability to promote cartilage defect repair. Acta Biomater. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Fluckiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Fujioka-Kobayashi, M.; Sculean, A.; Chappuis, V.; Buser, D.; Schaller, B.; Dori, F.; Miron, R.J. Effects of platelet rich plasma (PRP) on human gingival fibroblast, osteoblast and periodontal ligament cell behaviour. BMC Oral Health 2017, 17, 91. [Google Scholar] [CrossRef]
- Sundman, E.A.; Cole, B.J.; Fortier, L.A. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am. J. Sports Med. 2011, 39, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Magalon, J.; Bausset, O.; Serratrice, N.; Giraudo, L.; Aboudou, H.; Veran, J.; Magalon, G.; Dignat-Georges, F.; Sabatier, F. Characterization and comparison of 5 platelet-rich plasma preparations in a single-donor model. Arthroscopy 2014, 30, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Giusti, I.; D’Ascenzo, S.; Manco, A.; Di Stefano, G.; Di Francesco, M.; Rughetti, A.; Dal Mas, A.; Properzi, G.; Calvisi, V.; Dolo, V. Platelet concentration in platelet-rich plasma affects tenocyte behavior in vitro. BioMed Res. Int. 2014, 2014, 630870. [Google Scholar] [CrossRef] [PubMed]
- Sadoghi, P.; Lohberger, B.; Aigner, B.; Kaltenegger, H.; Friesenbichler, J.; Wolf, M.; Sununu, T.; Leithner, A.; Vavken, P. Effect of platelet-rich plasma on the biologic activity of the human rotator-cuff fibroblasts: A controlled in vitro study. J. Orthop. Res. 2013, 31, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Arslan, E.; Nellesen, T.; Bayer, A.; Prescher, A.; Lippross, S.; Nebelung, S.; Jahr, H.; Jaeger, C.; Huebner, W.D.; Fischer, H.; et al. Effect of platelet mediator concentrate (PMC) on Achilles tenocytes: An in vitro study. BMC Musculoskelet. Disord. 2016, 17, 307. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.H.; Kim, J.E.; Yoon, K.S.; Shin, S. Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. Am. J. Sports Med. 2012, 40, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- De Mos, M.; van der Windt, A.E.; Jahr, H.; van Schie, H.T.; Weinans, H.; Verhaar, J.A.; van Osch, G.J. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am. J. Sports Med. 2008, 36, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Maffulli, N.; Ewen, S.W.; Waterston, S.W.; Reaper, J.; Barrass, V. Tenocytes from ruptured and tendinopathic achilles tendons produce greater quantities of type III collagen than tenocytes from normal achilles tendons. An in vitro model of human tendon healing. Am. J. Sports Med. 2000, 28, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, R.; Chyung, J.H.; Murtaugh, L.C.; Brent, A.E.; Rosen, V.; Olson, E.N.; Lassar, A.; Tabin, C.J. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 2001, 128, 3855–3866. [Google Scholar] [PubMed]
- Alberton, P.; Popov, C.; Pragert, M.; Kohler, J.; Shukunami, C.; Schieker, M.; Docheva, D. Conversion of human bone marrow-derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem Cells Dev. 2012, 21, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Murchison, N.D.; Price, B.A.; Conner, D.A.; Keene, D.R.; Olson, E.N.; Tabin, C.J.; Schweitzer, R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development 2007, 134, 2697–2708. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Maffulli, N. Biology of tendon injury: Healing, modeling and remodeling. J. Musculoskelet. Neuronal Interact. 2006, 6, 181–190. [Google Scholar] [PubMed]
- Moojen, D.J.; Everts, P.A.; Schure, R.M.; Overdevest, E.P.; van Zundert, A.; Knape, J.T.; Castelein, R.M.; Creemers, L.B.; Dhert, W.J. Antimicrobial activity of platelet-leukocyte gel against Staphylococcus aureus. J. Orthop. Res. 2008, 26, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Borregaard, N.; Cowland, J.B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 1997, 89, 3503–3521. [Google Scholar] [PubMed]
- John, T.; Lodka, D.; Kohl, B.; Ertel, W.; Jammrath, J.; Conrad, C.; Stoll, C.; Busch, C.; Schulze-Tanzil, G. Effect of pro-inflammatory and immunoregulatory cytokines on human tenocytes. J. Orthop. Res. 2010, 28, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.Z.; Zhang, C.; Lin, X.J. Efficacy of platelet-rich plasma in arthroscopic repair of full-thickness rotator cuff tears: A meta-analysis. J. Shoulder Elbow Surg. 2015, 24, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, C.P.; Hou, Y.L.; Song, J.Q.; Cui, Z.; Yu, B. Are platelet concentrates an ideal biomaterial for arthroscopic rotator cuff repair? A meta-analysis of randomized controlled trials. Arthroscopy 2014, 30, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, B.M.; Jain, A.; Campbell, K.A.; Mascarenhas, R.; Romeo, A.A.; Verma, N.N.; Cole, B.J. Does the Use of Platelet-Rich Plasma at the Time of Surgery Improve Clinical Outcomes in Arthroscopic Rotator Cuff Repair When Compared With Control Cohorts? A Systematic Review of Meta-analyses. Arthroscopy 2016, 32, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Warth, R.J.; Dornan, G.J.; James, E.W.; Horan, M.P.; Millett, P.J. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: A meta-analysis and meta-regression. Arthroscopy 2015, 31, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Flury, M.; Rickenbacher, D.; Schwyzer, H.K.; Jung, C.; Schneider, M.M.; Stahnke, K.; Goldhahn, J.; Audige, L. Does Pure Platelet-Rich Plasma Affect Postoperative Clinical Outcomes After Arthroscopic Rotator Cuff Repair? A Randomized Controlled Trial. Am. J. Sports Med. 2016, 44, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Middleton, K.K.; Fu, F.H.; Im, H.J.; Wang, J.H. HGF mediates the anti-inflammatory effects of PRP on injured tendons. PLoS ONE 2013, 8, e67303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klatte-Schulz, F.; Pauly, S.; Scheibel, M.; Greiner, S.; Gerhardt, C.; Schmidmaier, G.; Wildemann, B. Influence of age on the cell biological characteristics and the stimulation potential of male human tenocyte-like cells. Eur. Cells Mater. 2012, 24, 74–89. [Google Scholar] [CrossRef]
- Xiong, G.; Lingampalli, N.; Koltsov, J.C.B.; Leung, L.L.; Bhutani, N.; Robinson, W.H.; Chu, C.R. Men and Women Differ in the Biochemical Composition of Platelet-Rich Plasma. Am. J. Sports Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Fekete, N.; Gadelorge, M.; Furst, D.; Maurer, C.; Dausend, J.; Fleury-Cappellesso, S.; Mailander, V.; Lotfi, R.; Ignatius, A.; Sensebe, L.; et al. Platelet lysate from whole blood-derived pooled platelet concentrates and apheresis-derived platelet concentrates for the isolation and expansion of human bone marrow mesenchymal stromal cells: Production process, content and identification of active components. Cytotherapy 2012, 14, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Pauly, S.; Klatte, F.; Strobel, C.; Schmidmaier, G.; Greiner, S.; Scheibel, M.; Wildemann, B. Characterization of tendon cell cultures of the human rotator cuff. Eur. Cells Mater. 2010, 20, 84–97. [Google Scholar] [CrossRef]






| Gene | Accession No. | Primer Sequence | Size (bp) |
|---|---|---|---|
| 18S RNA | NM_022551 | Forward: 5′ CGGAAAATAGCCTTTGCCATC 3′ | 107 |
| Reverse: 5′ AGTTCTCCCGCCCTCTTGGT 3′ | |||
| Col1A1 | NM_000088.3 | Forward: 5′ TGA CCT CAA GAT GTG CCA CT 3′ | 197 |
| Reverse: 5′ ACC AGA CAT GCC TCT TGT CC 3′ | |||
| Col3A1 | NM_000090.3 | Forward: 5′ GCT GGC ATC AAA GGA CAT CG 3′ | 199 |
| Reverse: 5′ TGT TAC CTC GAG GCC CTG GT 3′ | |||
| IL-1β | NM_000576 | Forward: 5′ TCC AGG AGA ATG ACC TGA GC 3′ | 111 |
| Reverse: 5′ GTG ATC GTA CAG GTG CAT CG 3′ | |||
| IL-6 | NM_000600 | Forward: 5′ TGA GGA GAC TTG CCT GGT GA 3′ | 188 |
| Reverse: 5′ TTG GGT CAG GGG TGG TTA TT 3′ | |||
| IL-10 | NM_000572 | Forward: 5′ TGA GAA CAG CTG CAC CCA CT 3′ | 164 |
| Reverse: 5′ GGC AAC CCA GGT AAC CCT TA 3′ | |||
| TNF-α | NM_000594 | Forward: 5′ AGC CCA TGT TGT AGC AAA CC 3′ | 133 |
| Reverse: 5′ GAG GTA CAG GCC CTC TGA TG 3′ | |||
| COX1 | NM_001271368 | Forward: 5′ CGT GTG TGT GAC CTG CTG AA 3′ | 193 |
| Reverse: 5′ TGC GGT ATT GGA ACT GGA CA 3′ | |||
| COX2 | NM_000963 | Forward: 5′ TAG AGC CCT TCC TCC TGT GC 3′ | 129 |
| Reverse: 5′ TGG GGA TCA GGG ATG AAC TT3′ | |||
| HGF | NM_000601 | Forward: 5′ CGC TGG GAG TAC TGT GCA AT 3′ | 116 |
| Reverse: 5′ GCC CCT GTA GCC TTC TCC TT 3′ | |||
| MMP-1 | NM_002421.3 | Forward: 5′ CAC GCC AGA TTT GCC AAG AG 3′ | 148 |
| Reverse: 5′ GTC CCG ATG ATC TCC CCT GA 3′ | |||
| MMP-2 | NM_004530 | Forward: 5′ TGG ATG ATG CCT TTG CTC GT 3′ | 156 |
| Reverse: 5′ CCA GGA GTC CGT CCT TAC CG 3′ | |||
| MMP-9 | NM_004994.2 | Forward: 5′ GGG ACG CAG ACA TCG TCA TC3′ | 150 |
| Reverse: 5′ GGG ACC ACA ACT CGT CAT CG 3′ | |||
| MMP-13 | NM_002427.3 | Forward: 5′ CCT TCC CAG TGG TGG TGA TG 3′ | 144 |
| Reverse: 5′ CGG AGC CTC TCA GTC ATG GA 3′ | |||
| SCX | Quantitect primer Assay Hs_SCXB_2_SG | Not available |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klatte-Schulz, F.; Schmidt, T.; Uckert, M.; Scheffler, S.; Kalus, U.; Rojewski, M.; Schrezenmeier, H.; Pruss, A.; Wildemann, B. Comparative Analysis of Different Platelet Lysates and Platelet Rich Preparations to Stimulate Tendon Cell Biology: An In Vitro Study. Int. J. Mol. Sci. 2018, 19, 212. https://doi.org/10.3390/ijms19010212
Klatte-Schulz F, Schmidt T, Uckert M, Scheffler S, Kalus U, Rojewski M, Schrezenmeier H, Pruss A, Wildemann B. Comparative Analysis of Different Platelet Lysates and Platelet Rich Preparations to Stimulate Tendon Cell Biology: An In Vitro Study. International Journal of Molecular Sciences. 2018; 19(1):212. https://doi.org/10.3390/ijms19010212
Chicago/Turabian StyleKlatte-Schulz, Franka, Tanja Schmidt, Melanie Uckert, Sven Scheffler, Ulrich Kalus, Markus Rojewski, Hubert Schrezenmeier, Axel Pruss, and Britt Wildemann. 2018. "Comparative Analysis of Different Platelet Lysates and Platelet Rich Preparations to Stimulate Tendon Cell Biology: An In Vitro Study" International Journal of Molecular Sciences 19, no. 1: 212. https://doi.org/10.3390/ijms19010212