The Plant Growth-Promoting Fungus (PGPF) Alternaria sp. A13 Markedly Enhances Salvia miltiorrhiza Root Growth and Active Ingredient Accumulation under Greenhouse and Field Conditions
Abstract
:1. Introduction
2. Results
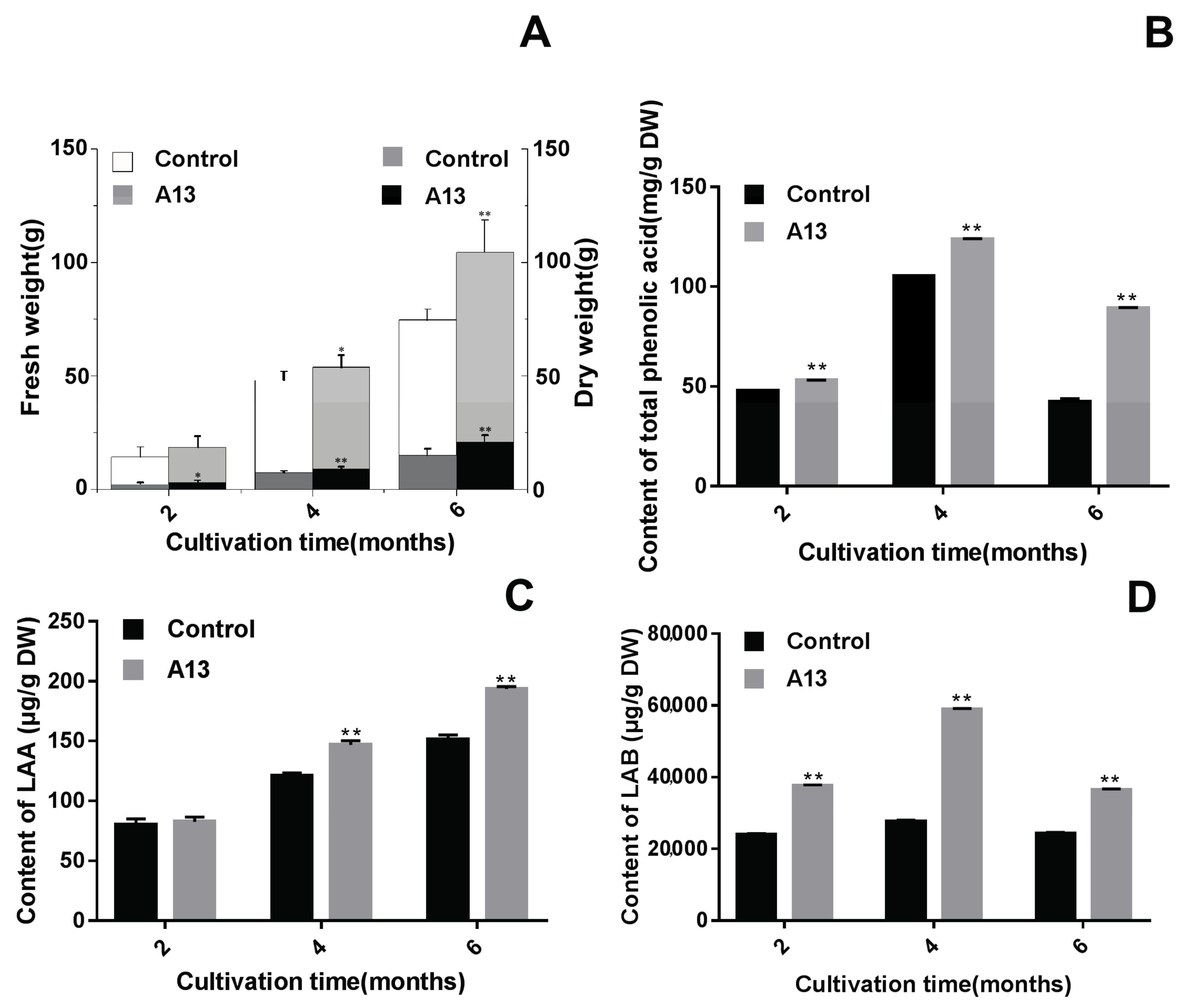
2.1. Screening for the Optimal PGPF under Greenhouse Conditions
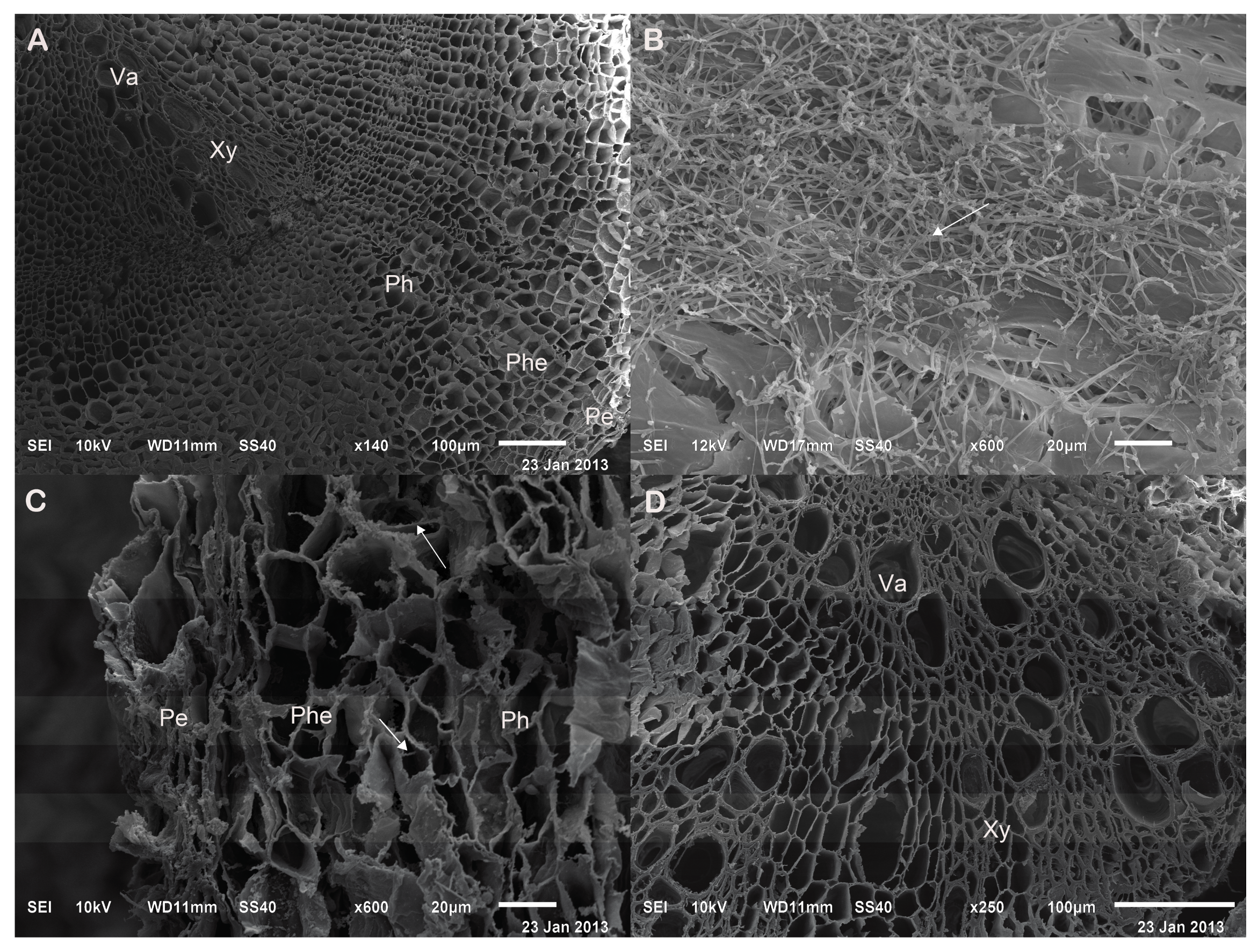
2.2. Verifying the Promoting Effects Induced by the Optimal PGPF in the Field Experiments
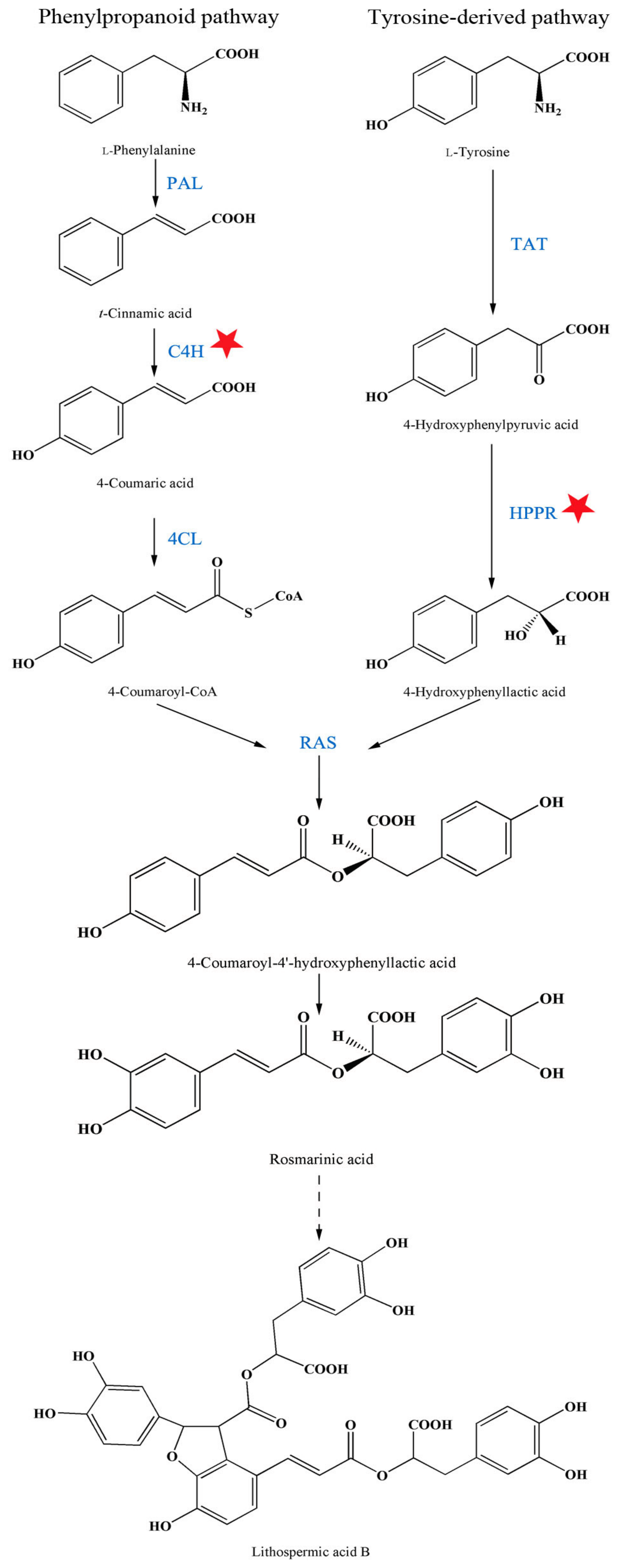
2.3. Influence of A13 on Phenolic Acid Biosynthetic Enzyme Activity
3. Discussion
3.1. Application of PGPF Improved Herb Yield and Quality
3.2. The Changing Law of Enzyme Activities to Guide Agronomic Measures for Active Components Accumulation
4. Materials and Methods
4.1. Experimental Design
4.2. Fungal Materials
4.3. Greenhouse and Field Experiments
4.4. Extraction and Analysis of the Active Ingredients
4.5. Enzyme Activity Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shen Nong’s Herbal Classic. Available online: http://www.theqi.com/cmed/oldbook/sn_herb/ (accessed on 30 August 2015).
- WHO Statistics. Available online: www.who.int/cardiovascular_diseases (accessed on 12 November 2016).
- Bomme, U.; Bauer, R.; Friedl, F.; Heuberger, H.; Heubl, G.; Torreslondoño, P.; Hummelsberger, J. Cultivating Chinese medicinal plants in Germany: A pilot project. J. Altern. Complement. Med. 2007, 13, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chena, F.; Chiu, F.C.; Lo, C.M. The effect of yeast elicitor on the growth and secondary metabolism of hairy root cultures of Salvia miltiorrhiza. Enzyme Microb. Tech. 2001, 28, 100–105. [Google Scholar] [CrossRef]
- Ling, H.; Lu, X.; Zhao, Y.; Zhang, S. The survey on the research of water-soluble components of Chinese medicine Danshen. Natl. Prod. Res. Dev. 1999, 11, 75–81. [Google Scholar]
- Petersen, M.; Abdullah, Y.; Benner, J.; Eberle, D.; Gehlen, K.; Hücherig, S.; Janiak, V.; Kyunghee, K.; Sander, M.; Weitzel, C. Evolution of rosmarinic acid biosynthesis. Phytochemistry 2009, 70, 1663–1679. [Google Scholar] [CrossRef] [PubMed]
- Ming, Q.; Han, T.; Li, W.; Zhang, Q.; Zhang, H.; Zheng, C.; Huang, F.; Rahman, K.; Qin, L. Tanshinone IIA and tanshinone I production by Trichoderma atroviride D16, an endophytic fungus in Salvia miltiorrhiza. Phytomedicine 2012, 19, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Ming, Q.; Su, C.; Zheng, C.; Jia, M.; Zhang, Q.; Zhang, H.; Rahman, K.; Han, T.; Qin, L. Elicitors from the endophytic fungus Trichoderma atroviride promote Salvia miltiorrhiza hairy root growth and tanshinone biosynthesis. J. Exp. Bot. 2013, 64, 5687–5694. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Ng, J.; Shi, M.; Wu, S.J. Enhanced secondary metabolite (tanshinone) production of Salvia miltiorrhiza hairy roots in a novel root–bacteria coculture process. Appl. Microbiol. Biotechnol. 2007, 77, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.L.; Zhou, L.G.; Wu, J.Y. Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl. Microbiol. Biotechnol. 2010, 87, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. Int. 2017, 24, 3315–3335. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Rediers, H.; Ghequire, M.G.; Nguyen, H.H.; De Mot, R.; Vanderleyden, J.; Spaepen, S. The plant growth-promoting effect of the nitrogen-fixing endophyte Pseudomonas stutzeri A15. Arch. Microbiol. 2017, 199, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Weselowski, B.; Nathoo, N.; Eastman, A.W.; Macdonald, J.; Yuan, Z.C. Isolation, identification and characterization of Paenibacillus polymyxa CR1 with potentials for biopesticide, biofertilization, biomass degradation and biofuel production. BMC Microbiol. 2016, 16, 244. [Google Scholar] [CrossRef] [PubMed]
- Satheesan, J.; Narayanan, A.K.; Sakunthala, M. Induction of root colonization by Piriformospora indica leads to enhanced asiaticoside production in Centella asiatica. Mycorrhiza 2012, 22, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.S.; Lv, Y.L.; Zhao, Y.; Guo, S.X. Promoting role of an endophyte on the growth and contents of kinsenosides and flavonoids of Anoectochilus formosanus hayata, a rare and threatened medicinal orchidaceae plant. J. Zhejiang Univ. Sci. B 2013, 14, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Dai, G.J.; Yun-Tong, M.A.; Zhang, Q.; Chen, X.; Wan, D.G.; Yan, Z.Y. Correlation between Active Components and Endophytic fungi in Salvia miltiorrhiza. Chin. J. Exp. Tradit. Med. Formulae 2013, 23, 66–73. [Google Scholar]
- Zhao, J.L.; Zhou, L.G.; Wu, J.Y. Promotion of Salvia miltiorrhiza hairy root growth and tanshinone production by polysaccharide–protein fractions of plant growth-promoting rhizobacterium Bacillus cereus. Process Biochem. 2010, 45, 1517–1522. [Google Scholar] [CrossRef]
- Tang, K.; Biao, L.I.; Guo, S.X. Analysis of Diversity of Endophytic Fungi in Salvia miltiorrhiza from Henan in China. Chin. Pharm. J. 2015, 50, 570–573. [Google Scholar]
- Yan, Q.; Shi, M.; Ng, J.; Wu, J.Y. Elicitor-induced rosmarinic acid accumulation and secondary metabolism enzyme activities in Salvia miltiorrhiza, hairy roots. Plant Sci. 2006, 170, 853–858. [Google Scholar] [CrossRef]
- Tian, R.; Yang, Q.; Zhou, G.; Tan, J.; Zhang, L.; Fang, C. Taxonomic study on a taxol producing fungus isolated from bark of Taxus chinensis var. mairei. J. Wuhan Botanical Res. 2006, 24, 541–545. [Google Scholar]
- Cao, L.; Huang, J.; Yu, X. Fermentation conditions of Sinopodophyllum hexandrum endophytic fungus on production of podophyllotoxin. Food Ferment. Ind. 2007, 33, 28–32. [Google Scholar]
- Guo, B.; Li, H.; Zhang, L. Isolation of an fungus producting vinbrastine. J. Yunnan Univ. 1998, 20, 214–215. [Google Scholar]
- Shu-Nuo, L.I.; Ya-Dong, L.I. Bolting Flavonoid-Producing Endophytic Fungi from Vaccinium. J. Jilin Agric. Univ. 2009, 31, 587–591. [Google Scholar]
- Wu, J.Y.; Shi, M. Ultrahigh diterpenoid tanshinone production through repeated osmotic stress and elicitor stimulation in fed-batch culture of Salvia miltiorrhiza hairy roots. Appl. Microbiol. Biotechnol. 2008, 78, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ding, G.; Li, B.; Guo, S.X. Transcriptome Analysis of Genes Involved in Dendrobine Biosynthesis in Dendrobium nobile Lindl. Infected with Mycorrhizal Fungus MF23 (Mycena sp.). Sci. Rep. 2017, 7, 316. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yan, H.; Wang, C.; Tian, L.; Wang, A.; Guo, S. Effects of mycorrhizal fungus Mycena sp. on the growth and poly-saccharide properties of Dendrobium officinale. Sci. China Ser. C 2016, 59, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Gao, S.; Di, P.; Chen, J.; Chen, W.; Zhang, L. Methyl jasmonate dramatically enhances the accumulation of phenolic acids in Salvia miltiorrhiza hairy root cultures. Physiol. Plant 2009, 137, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, L.; Gao, S.; Saengking, S.; Di, P.; Chen, J.; Chen, W. The c4h, tat, hppr and hppd Genes Prompted Engineering of Rosmarinic Acid Biosynthetic Pathway in Salvia miltiorrhiza Hairy Root Cultures. PLoS ONE 2011, 6, e29713. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.M.; Wang, C.L.; Chen, X.M.; Zhou, Y.Q.; Wang, Y.Q.; Luo, A.X.; Liu, Z.H.; Guo, S.X. In vitro seed germination and seedling growth of an endangered epiphytic orchid, Dendrobium officinale, endemic to China using mycorrhizal fungi (Tulasnella sp.). Sci. Hortic. 2014, 165, 62–68. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W.; Innis, M.A.; Gelfand, D.H.; Sninsky, J.J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics, 1st ed.; Elsevier: London, UK, 1990; pp. 315–322. [Google Scholar]
- Mozzetti, C.; Ferraris, L.; Tamietti, G.; Matta, A. Variation in enzyme activities in leaves and cell suspension as markers of incompatibility in different Phytophthora-pepper interactions. Physiol. Mol. Plant Pathol. 1995, 46, 95–107. [Google Scholar] [CrossRef]
- Diamondstone, T.I. Assay of tyrosine transaminase activity by conversion of p-hydroxyphenylpyruvate to p-hydroxybenzaldehyde. Anal. Biochem. 1966, 16, 395–401. [Google Scholar] [CrossRef]
- Lamb, C.J.; Rubery, P.H. A spectrophotometric assay for trans-cinnamic acid 4-hydroxylase activity. Anal. Biochem. 1975, 68, 554–561. [Google Scholar] [CrossRef]
- Koopmann, E.; Logemann, E.; Hahlbrock, K. Regulation and functional expression of cinnamate 4-hydroxylase from parsley. Plant Physiol. 1999, 119, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, H.; Tabira, Y.; Ellis, B.E. Methyl jasmonate-induced rosmarinic acid biosynthesis in Lithospermum erythrorhizon cell suspension cultures. Plant Cell Rep. 1993, 12, 706–709. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.S.; Tang, K.; Guo, S.X. The Plant Growth-Promoting Fungus (PGPF) Alternaria sp. A13 Markedly Enhances Salvia miltiorrhiza Root Growth and Active Ingredient Accumulation under Greenhouse and Field Conditions. Int. J. Mol. Sci. 2018, 19, 270. https://doi.org/10.3390/ijms19010270
Zhou LS, Tang K, Guo SX. The Plant Growth-Promoting Fungus (PGPF) Alternaria sp. A13 Markedly Enhances Salvia miltiorrhiza Root Growth and Active Ingredient Accumulation under Greenhouse and Field Conditions. International Journal of Molecular Sciences. 2018; 19(1):270. https://doi.org/10.3390/ijms19010270
Chicago/Turabian StyleZhou, Li Si, Kun Tang, and Shun Xing Guo. 2018. "The Plant Growth-Promoting Fungus (PGPF) Alternaria sp. A13 Markedly Enhances Salvia miltiorrhiza Root Growth and Active Ingredient Accumulation under Greenhouse and Field Conditions" International Journal of Molecular Sciences 19, no. 1: 270. https://doi.org/10.3390/ijms19010270
APA StyleZhou, L. S., Tang, K., & Guo, S. X. (2018). The Plant Growth-Promoting Fungus (PGPF) Alternaria sp. A13 Markedly Enhances Salvia miltiorrhiza Root Growth and Active Ingredient Accumulation under Greenhouse and Field Conditions. International Journal of Molecular Sciences, 19(1), 270. https://doi.org/10.3390/ijms19010270




