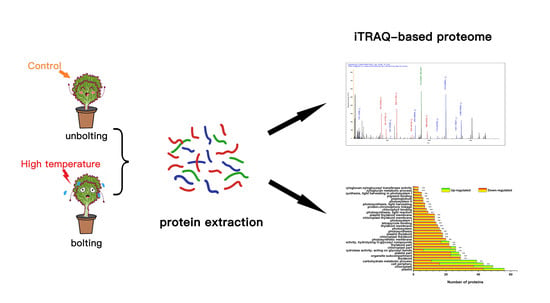
Quantitative Proteomics Analysis of Lettuce (Lactuca sativa L.) Reveals Molecular Basis-Associated Auxin and Photosynthesis with Bolting Induced by High Temperature
Abstract
:1. Introduction
2. Results
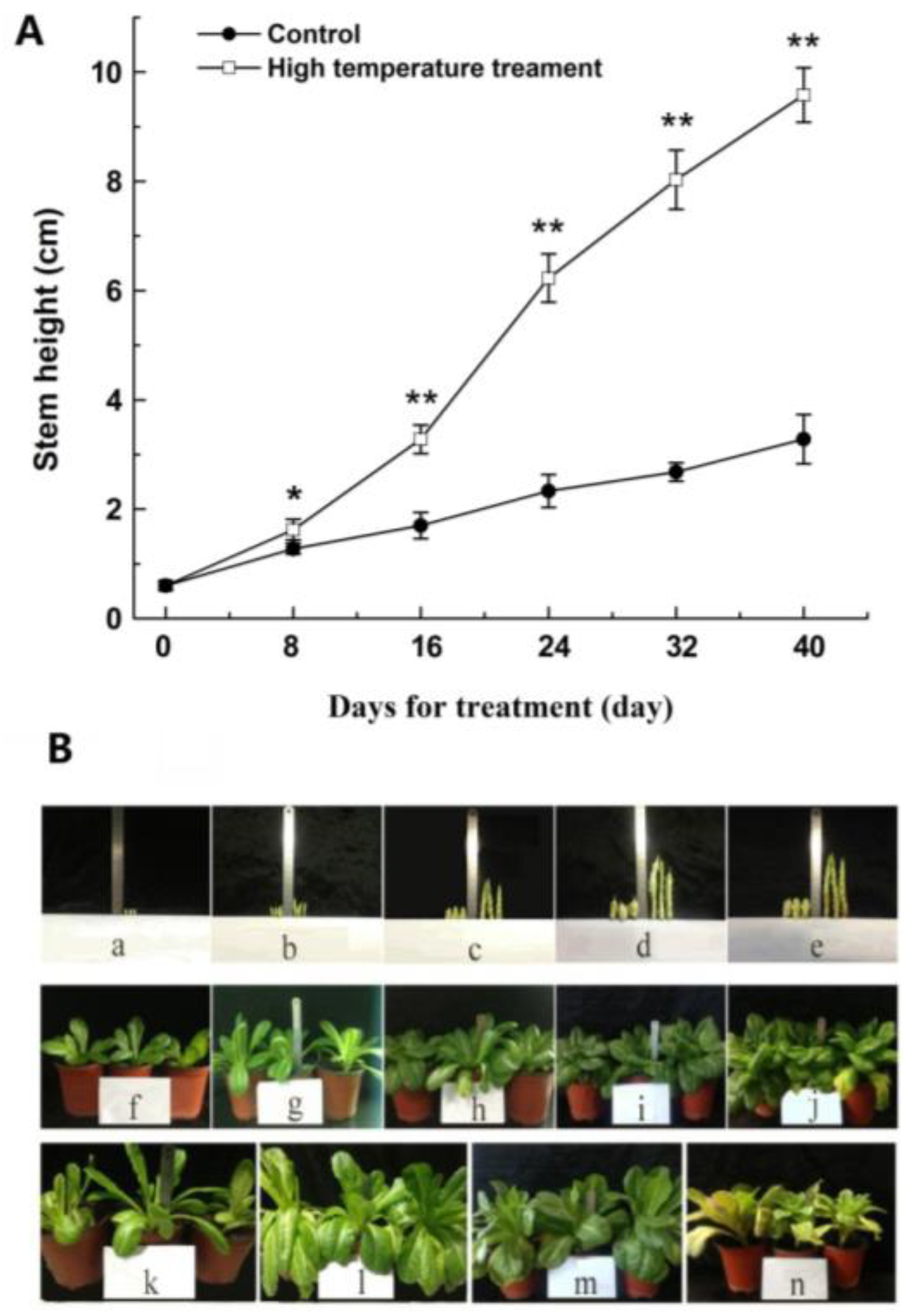
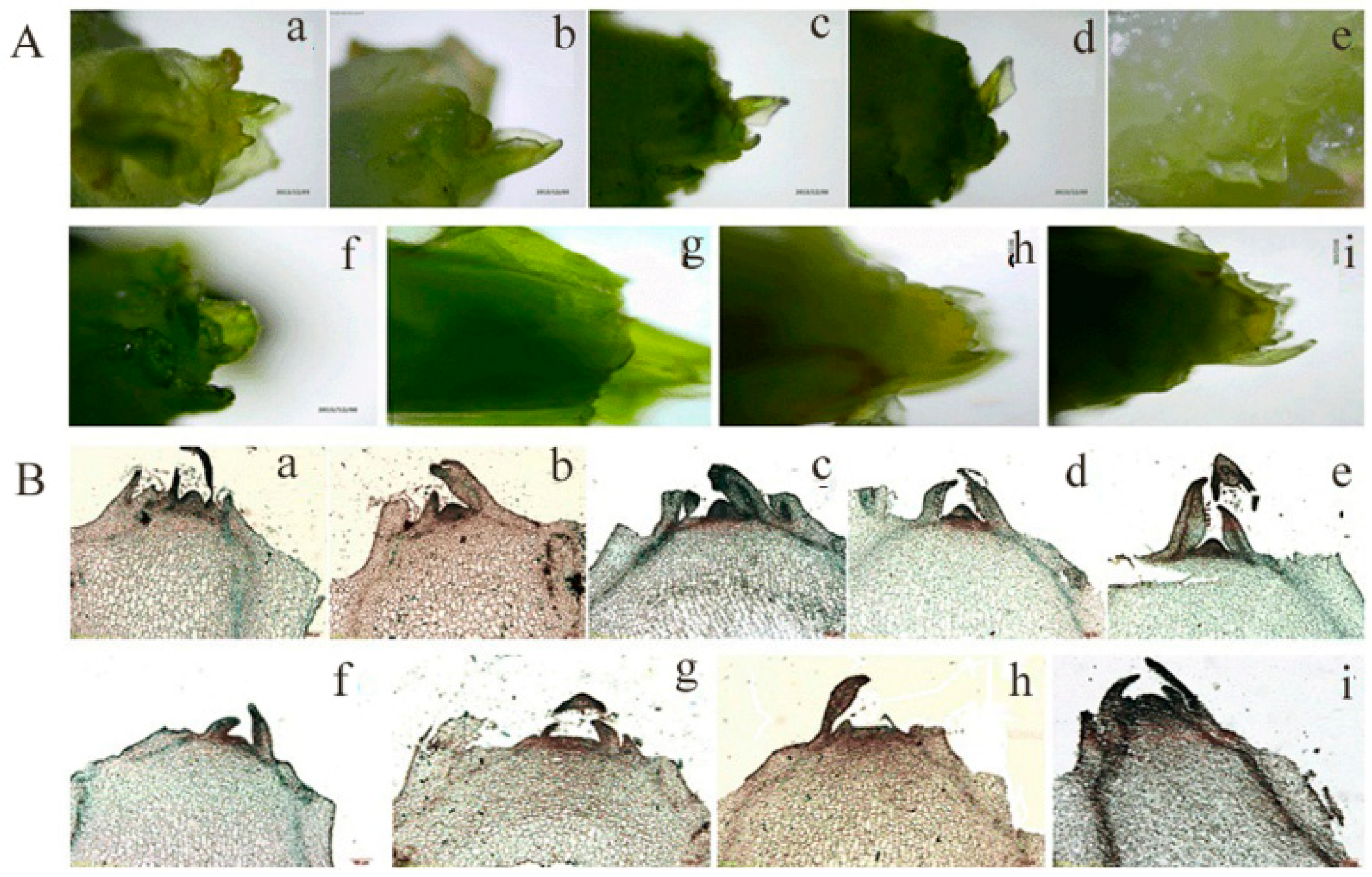
2.1. The Morphological and Physiological Changes of Lettuce Stems During Bolting Induced by High Temperatures
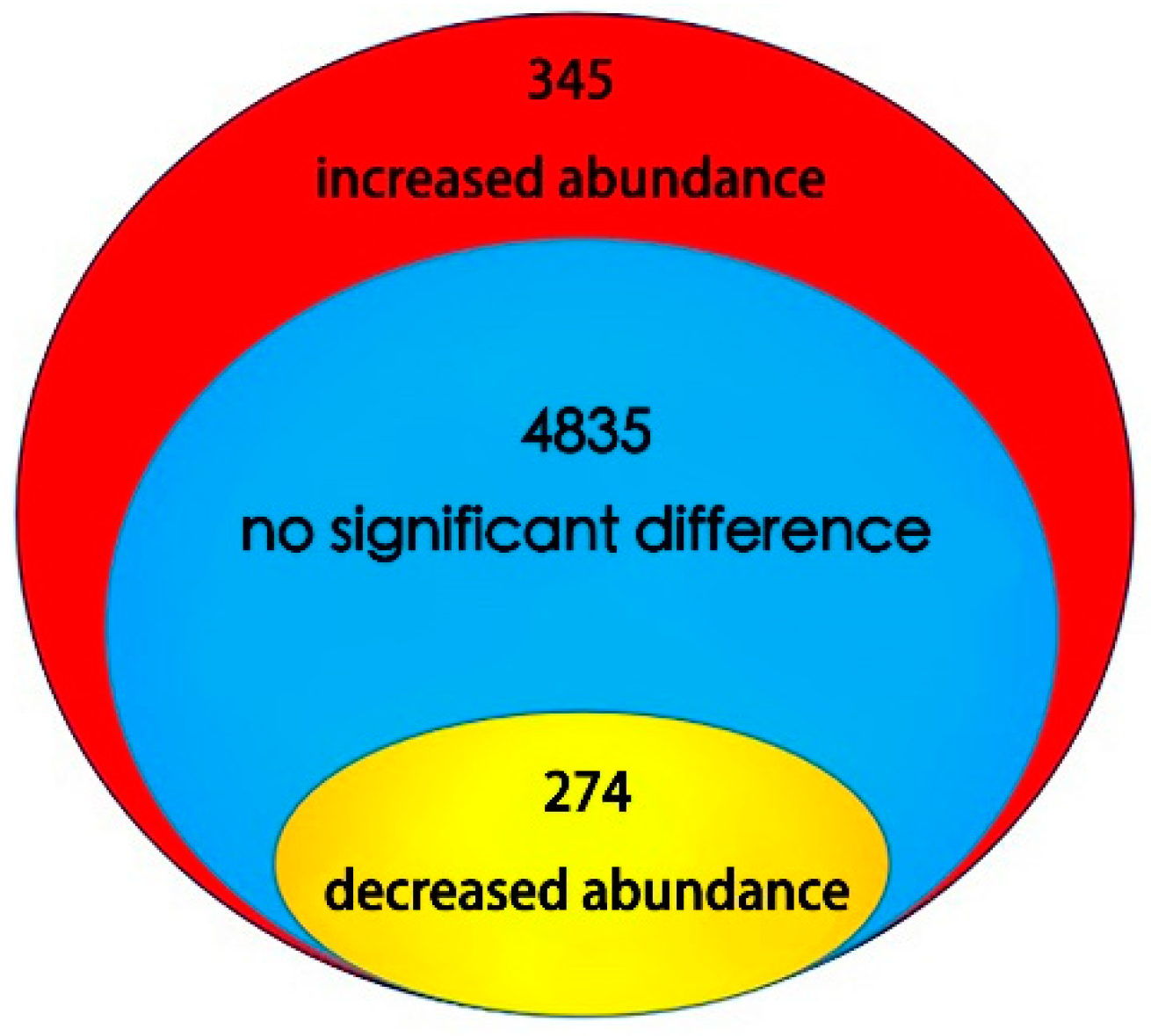
2.2. Identification of Differential Abundance Proteins Using iTRAQ in Lettuce Stems During Bolting Induced by High Temperature
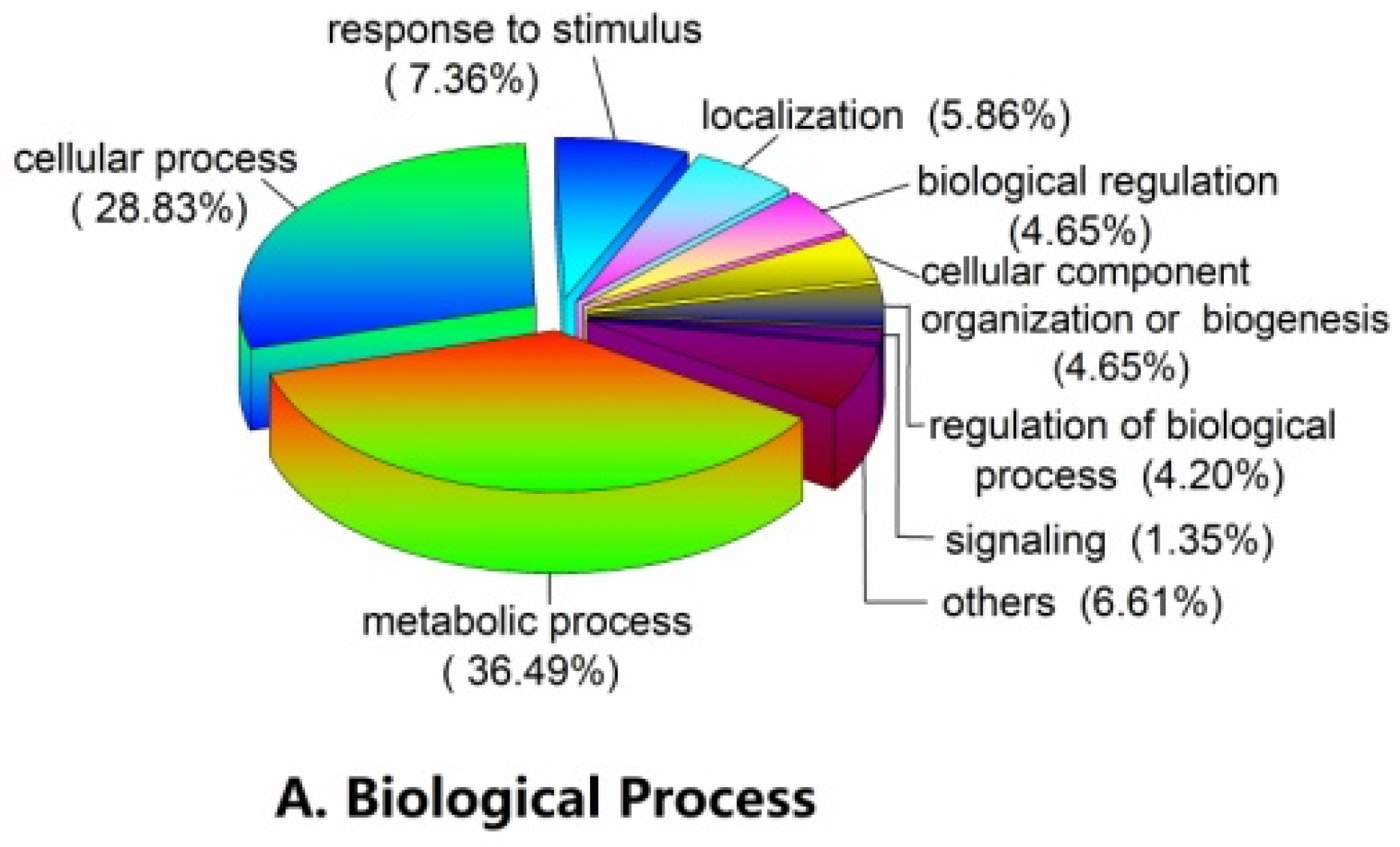
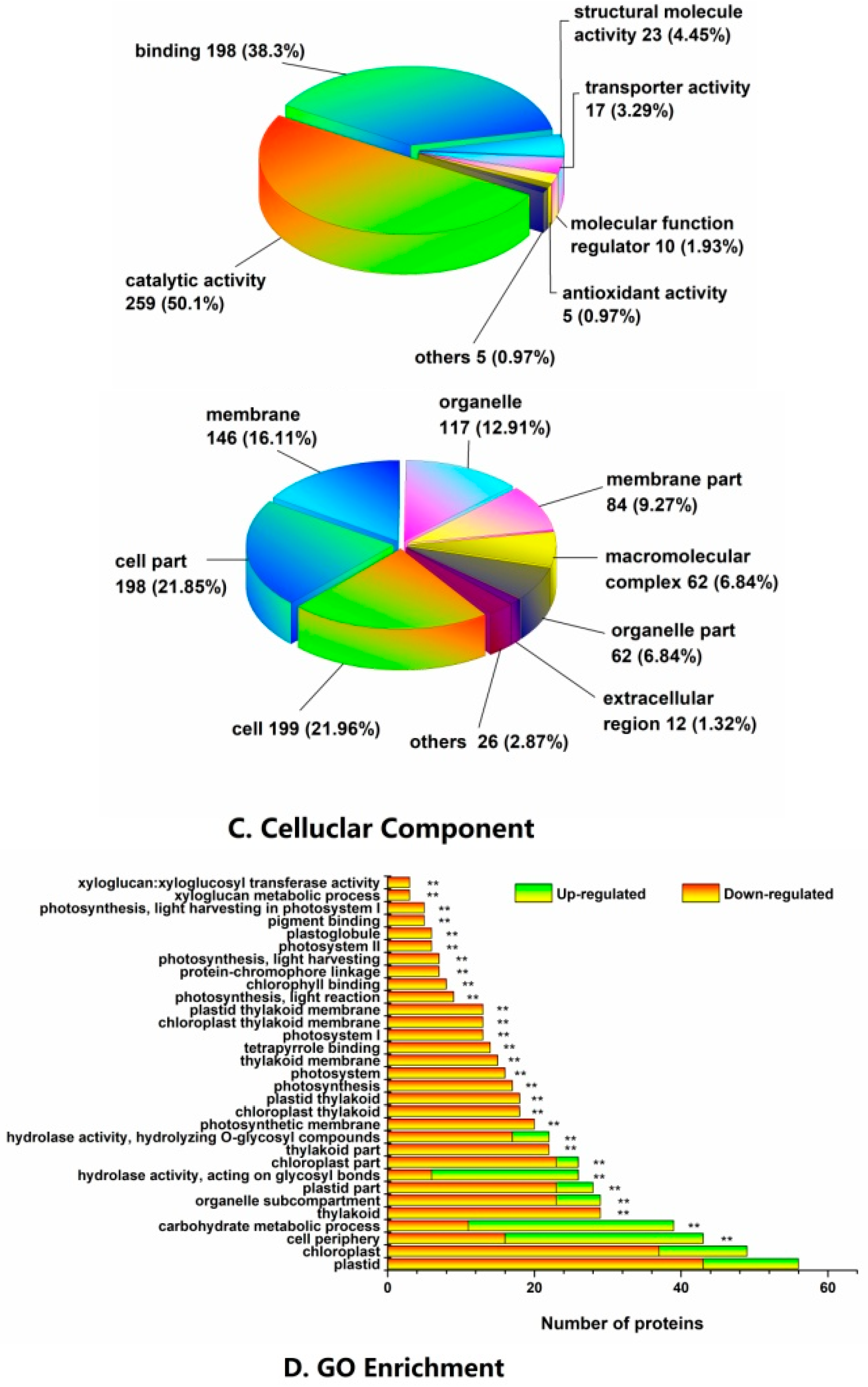
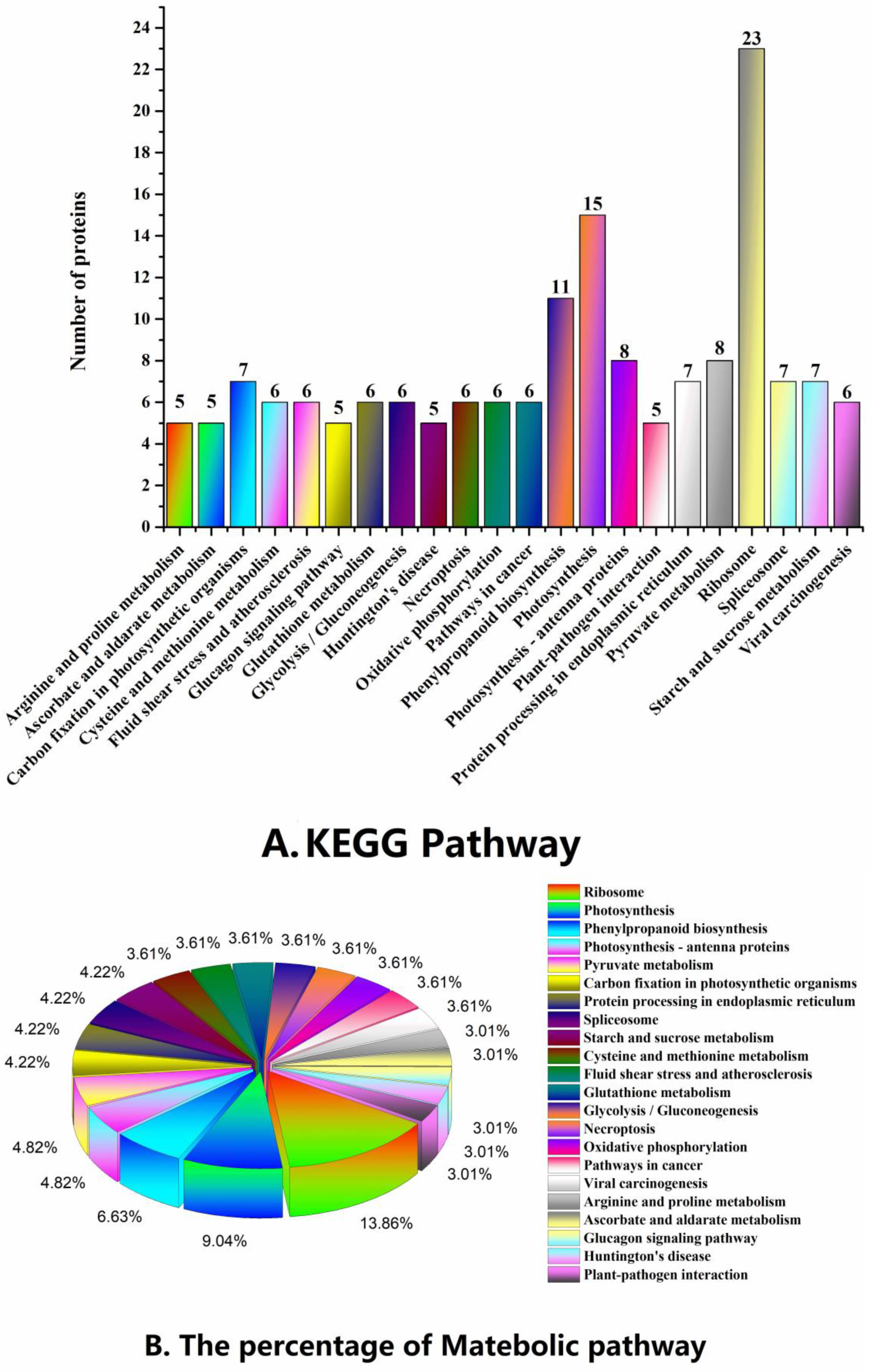
2.3. Functional Classification and Metabolic Pathways of Differential Abundance Proteins
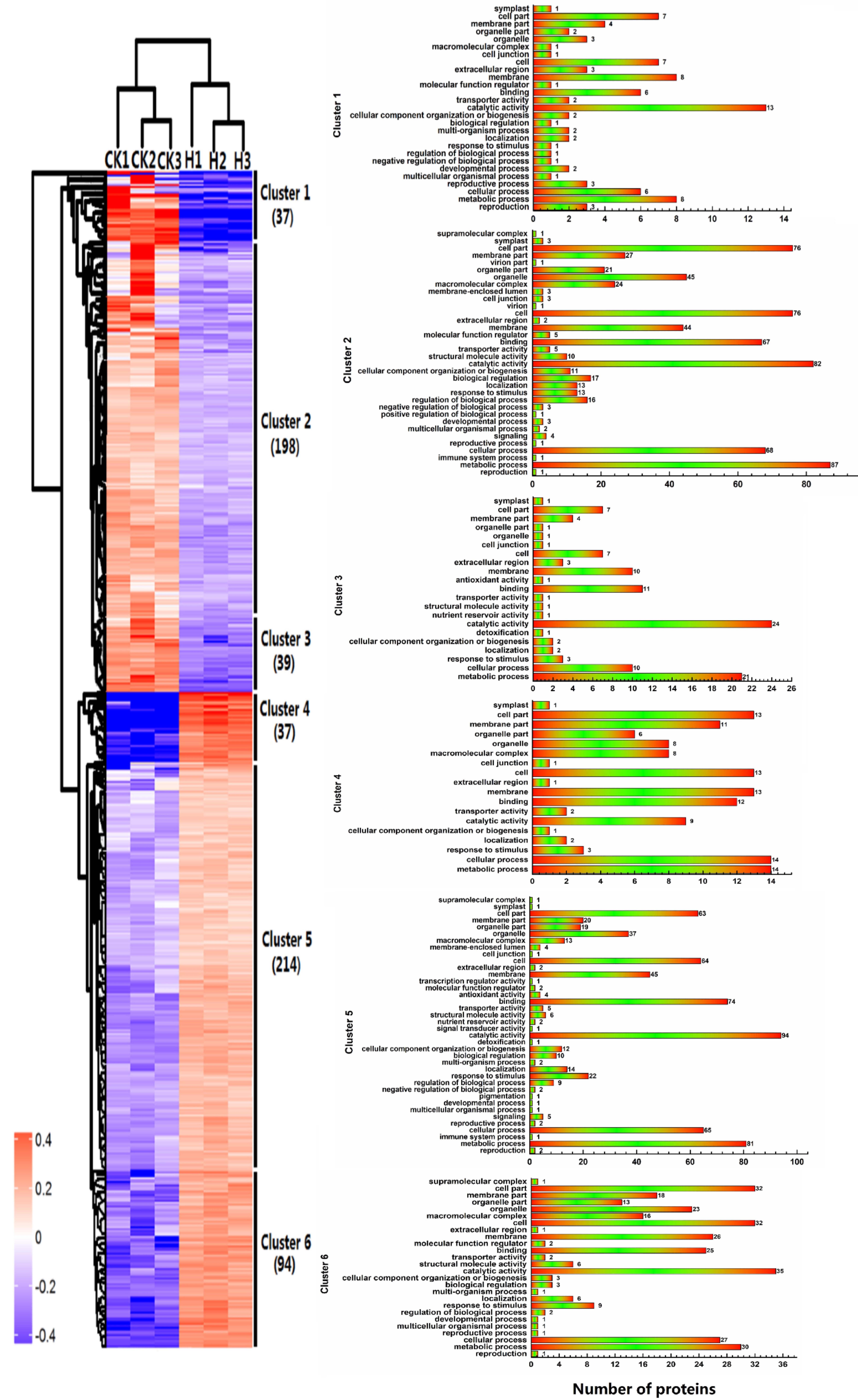
2.4. Hierarchical Clustering of Protein Profiles
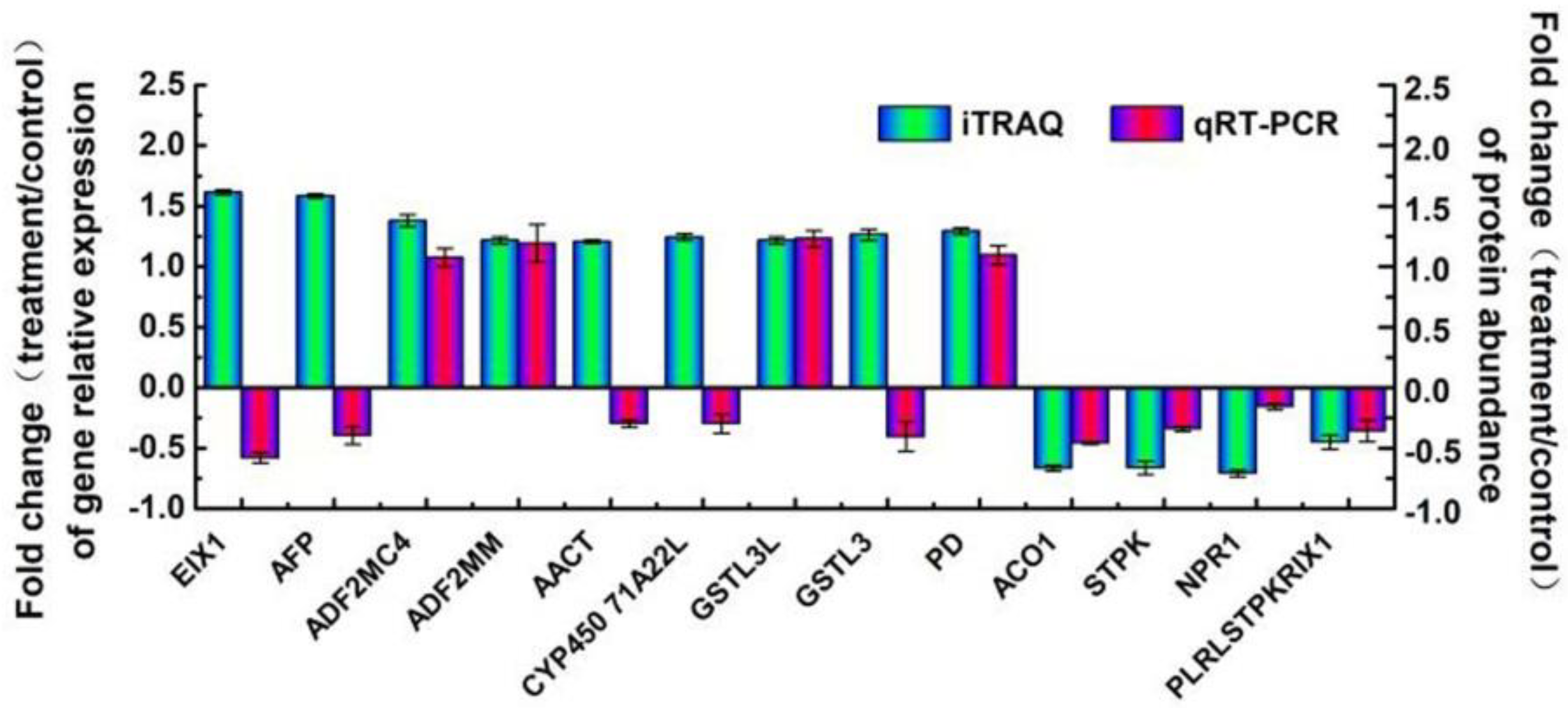
2.5. Expression Levels of Genes Encoding Some Identified Proteins
3. Discussion
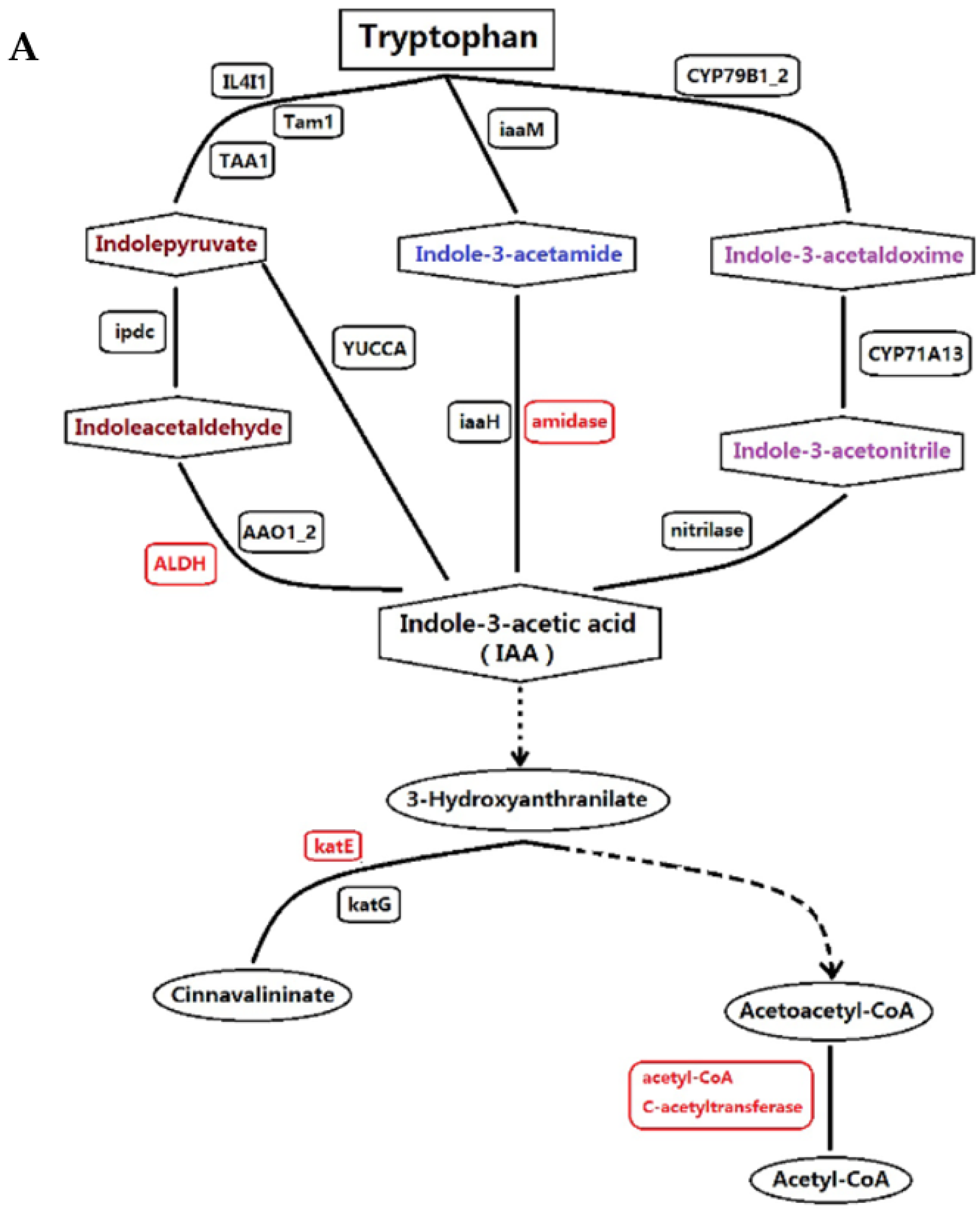
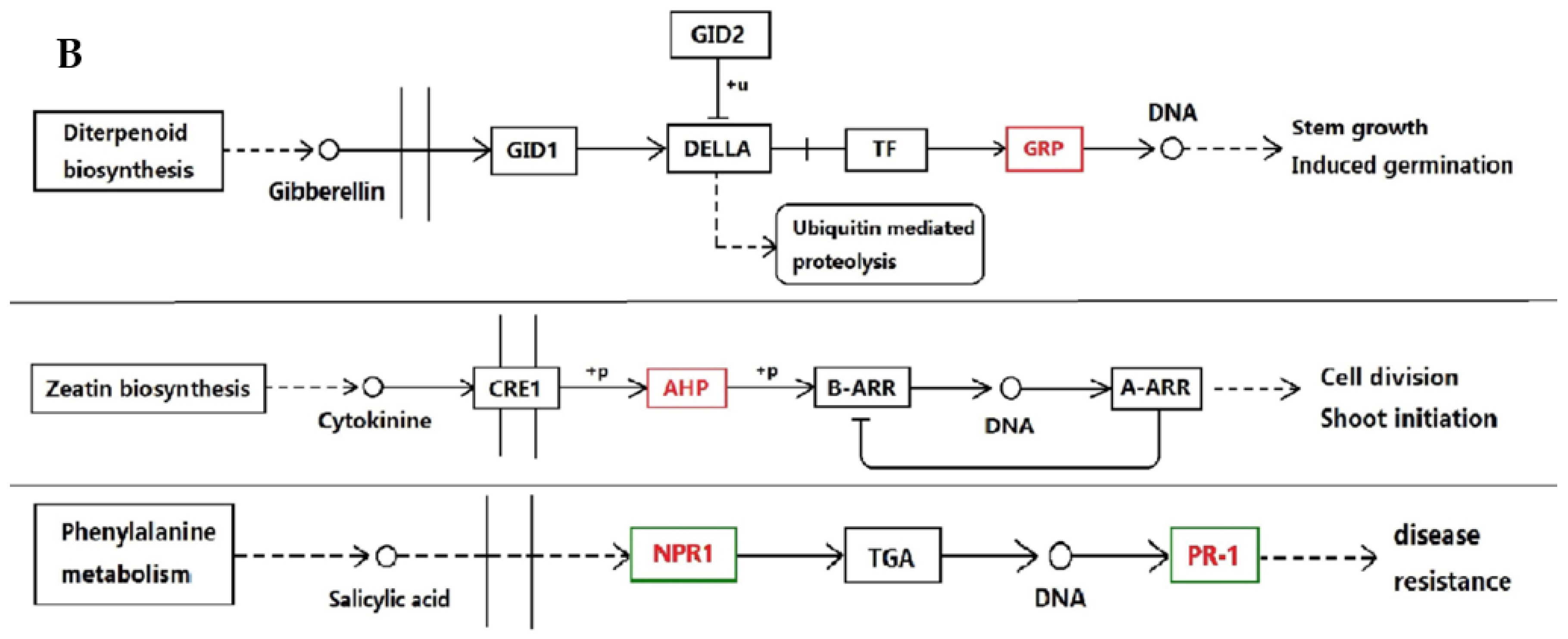
3.1. Proteins Implicated in Hormone Metabolism During Bolting in Lettuce Under a High Temperature
3.2. Proteins Related to Phosphorylation During Bolting in Lettuce Under a High Temperature
3.3. Defense Proteins Play an Important Role in Defense Reaction and Hormone Metabolism During Bolting in Lettuce Under a High Temperature
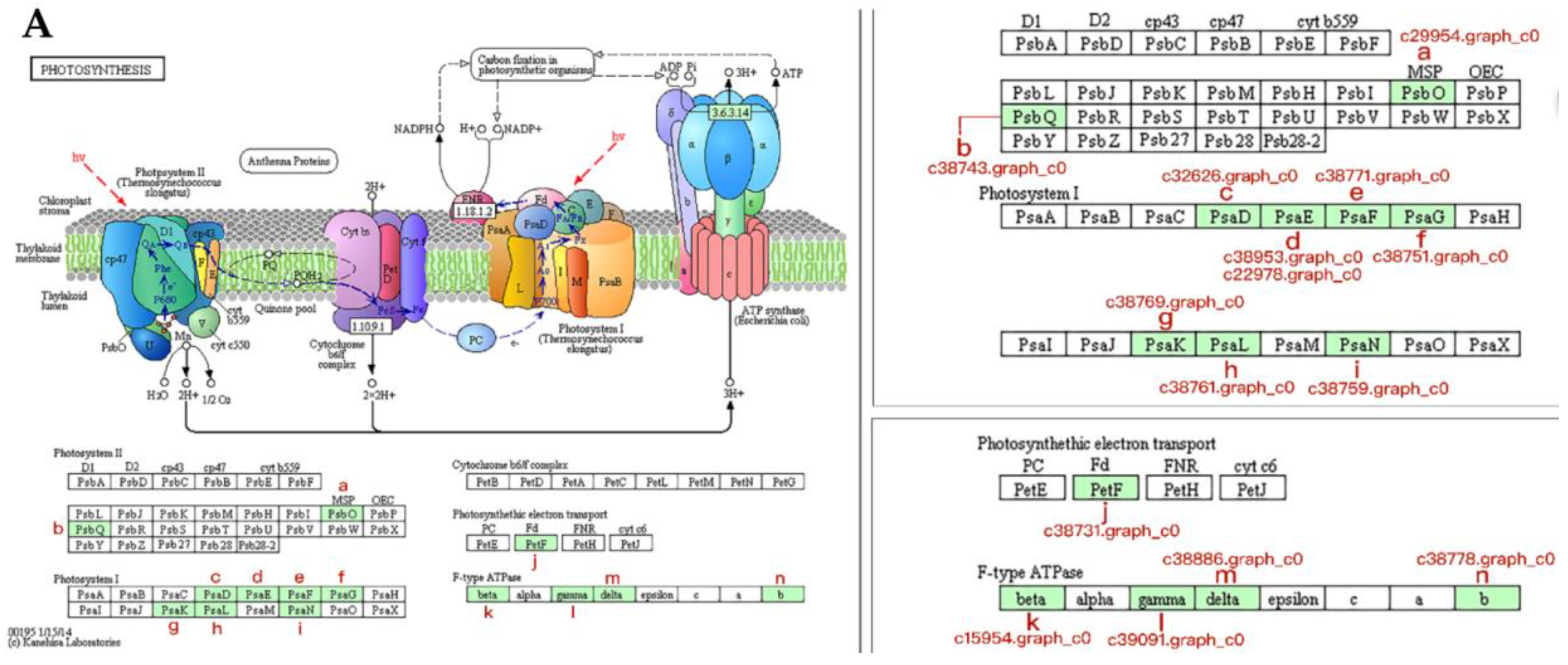
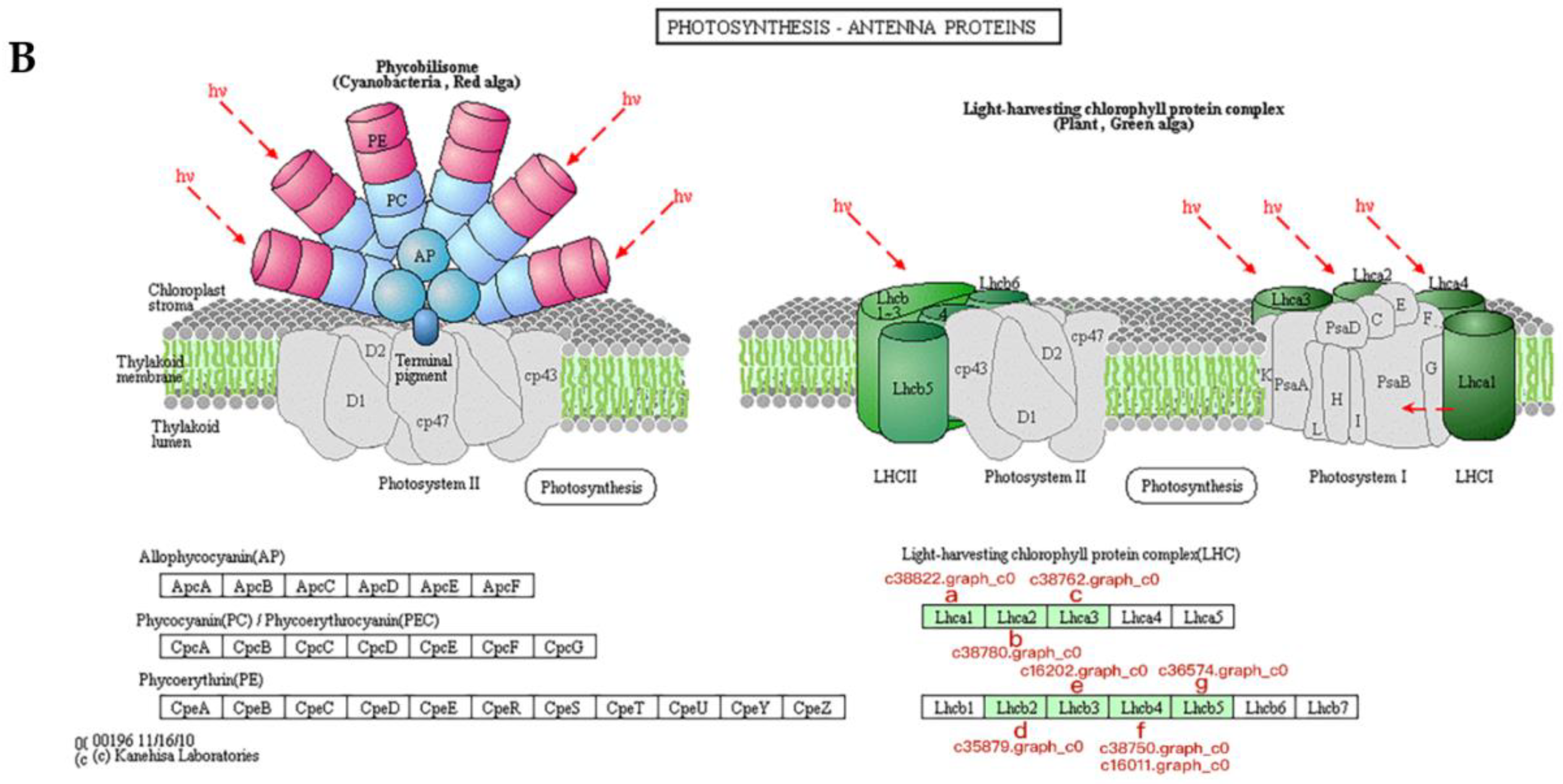
3.5. Proteins Associated with Photosynthesis During Bolting in Lettuce
3.6. Proteins Associated with Expansin During Bolting in Lettuce
4. Materials and Methods
4.1. Plant Materials and Treatment
4.2. Endogenous Hormone Measurement
4.3. Exogenous Auxin (IAA) Treatment
4.4. Protein Extraction
4.5. Protein Digestion and iTRAQ Labeling
4.6. Peptide Fractionation with Strong Cation Exchange (SCX) Chromatography
4.7. Liquid Chromatography (LC)-Electrospray Ionization (ESI) Tandem MS (MS/MS) Analysis
4.8. Database Search and Protein Quantification
4.9. Bioinformatics Analysis of Proteins
4.10. Total RNA Extraction and Real-Time PCR
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pajoro, A.; Biewers, S.; Dougali, E.; Valentim, F.L.; Mendes, M.A.; Porri, A. The (r)evolution of gene regulatory networks controlling Arabidopsis plant reproduction; a two decades history. J. Exp. Bot. 2014, 65, 4731–4745. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.L.; Song, M.; Wang, X.J.; Wang, Z.M.; Ren, X.S. Study on physiological and biochemical changes during startup bolting of mustard and cabbage induced by temperature and photoperiod. J. Southwest Univ. 2009, 31, 52–57. [Google Scholar]
- Fukuda, M.; Matsuo, S.; Kikuchi, K.; Mitsuhashi, W.; Toyomasu, T.; Honda, I. Gibberellin metabolism during stem elongation stimulated by high temperature in lettuce. Acta Hortic. 2012, 932, 259–264. [Google Scholar] [CrossRef]
- Achard, P.; Baghour, M.; Chapple, A.; Hedden, P.; Van der Straeten, D.; Genschik, P.; Moritz, T.; Harberd, N.P. The plant stress hormone ethylene controls floral transition via DELLA-dependent regulation ot floral meristem-identity genes. Proc. Natl. Acad. Sci. USA 2007, 104, 6484–6489. [Google Scholar] [CrossRef] [PubMed]
- Chia, T.Y.; Müller, A.; Jung, C.; Mutasa-Göttgens, E.S. Sugar beet contains a large CONSTANS-LIKE gene family including a CO homologue that is independent of the early-bolting (B) gene locus. J. Exp. Bot. 2008, 59, 2735–2748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Guo, M.H.; Tang, X.B.; Jin, D.; Fang, Z.Y. Proteomic and gene expression analyses during bolting-related leaf color change in Brassica rapa. Genet. Mol. Res. 2016, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Tan, J.; Zhuo, C.; Wang, C.Y.; Xiang, B.; Wang, Z.Y. Abscisic acid, H2O2 and nitric oxide interactions mediated cold-induced S-adenosylmethionine synthetase in Medicago sativa subsp. falcata that confers cold tolerance through up-regulating polyamine oxidation. Plant Biotechnol. J. 2014, 12, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, D.; Li, Y.; Li, N. Functional phosphoproteomic analysis reveals that a Serine-62-Phosphorylated isoform of ethylene response factor110 is involved in Arabidopsis bolting. Plant Physiol. 2013, 161, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Strompen, G.; Dettmer, J.; Stierhof, Y.D.; Schumacher, K.; Jurgens, G.; Mayer, U. Arabidopsis vacuolar H-ATPase subunit E isoform 1 is required for Golgi organization and vacuole function in embryogenesis. Plant J. 2010, 41, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Jin, D.; Xu, C.; Zhang, L.; Guo, M.H.; Fang, Z.Y. Regulation of bolting and identification of the α-tubulin gene family in Brassica rapa L. ssp pekinensis. Genet. Mol. Res. 2015, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Andersson, I.; Backlund, A. Structure and function of Rubisco. Plant Physiol. Biochem. 2008, 46, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Ohto, M.; Onai, K.; Furukawa, Y.; Aoki, E.; Araki, T.; Nakamura, K. Effects of sugar on vegetative development and floral transition in Arabidopsis. Plant Physiol. 2001, 127, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Spreitzer, R.J.; Salvucci, M.E. Rubisco: Structure, regulatory interactions, and possibilities for a better enzyme. Annu. Rev. Plant Biol. 2002, 53, 449–475. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Wong, W.S.; Xu, W.Z.; Sun, F.F.; Qing, D.J.; Li, N. Cis-cinnamic acid-enhanced 1 gene plays a role in regulation of Arabidopsis bolting. Plant Mol. Biol. 2011, 75, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, I. Regulation and function of SOC1, a flowering pathway integrator. J. Exp. Bot. 2010, 61, 2247–2254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balanza, V.; Martinez-Fernandez, I.; Ferrandiz, C. Sequential action of FRUITFULL as a modulator of the activity of the floral regulators SVP and SOC1. J. Exp. Bot. 2014, 65, 1193–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.Y.; Chen, Z.J.; Lv, S.S.; Ning, K.; Ji, X.L.; Liu, X.Y.; Wang, Q.; Liu, R.Y.; Fan, S.X.; Zhang, X.L. MADS-Box genes and gibberellins regulate bolting in Lettuce (Lactuca sativa L.). Front. Plant Sci. 2016, 7, 1889–1903. [Google Scholar] [CrossRef] [PubMed]
- Rollins, J.A.; Habte, E.; Templer, S.E.; Colby, T.; Schmidt, J.; von Korff, M. Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley (Hordeum vulgare L.). J. Exp. Bot. 2013, 64, 3201–3212. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Jung, H.J.; Park, J.I.; Yang, T.J.; Nou, I.S. Transcriptome analysis of newly classified bZIP transcription factor of Brassica rapa in cold stress response. Genomics 2014, 104, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Q.; Zhang, Y.; Zou, L.Y.; Li, C.Q.; Yu, B.; Zheng, X.Q.; Zho, Y. An Ensemble Classifier for Eukaryotic Protein Subcellular Location Prediction Using Gene Ontology Categories and Amino Acid Hydrophobicity. PLoS ONE 2012, 7, e31057. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef] [PubMed]
- Wolters, H.; Jürgens, G. Survival of the flexible: Hormonal growth control and adaptation in plant development. Nat. Rev. Genet. 2009, 10, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Villalobos, D.; Díaz-Moreno, S.M.; van der Schuren, A.; Tamaki, T.; Kang, Y.H.; Gujas, B.; Novak, O.; Jaspert, N.; Li, Z.; Wolf, S.; et al. The effects of high steady state auxin levels on root cell elongation in Brachypodium. Plant Cell 2016, 28, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Spartz, A.K.; Sang, H.L.; Wenger, J.P.; Gonzalez, N.; Itoh, H.; Inzé, D.; Peer, W.A.; Murphy, A.S.; Overvoorde, P.J.; Gray, W.M. The SAUR19 subfamily of SMALL AUXIN UP RNA genes promote cell expansion. Plant J. 2012, 70, 978–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilmoth, J.C.; Wang, S.; Tiwari, S.B.; Joshi, A.D.; Hagen, G.; Guilfoyle, T.J.; Alonso, J.M.; Ecker, J.R.; Reed, J.W. NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J. 2005, 43, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.X.; Gan, L.J.; Ng, D.; Zhou, X.; Xia, K. Decreased paniclederived indole-3-acetic acid reduces gibberellin A1 level in the uppermost internode, causing panicle enclosure in male sterile rice Zhenshan 97A. J. Exp. Bot. 2007, 58, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Mockaitis, K.; Estelle, M. Auxin receptors and plant development: A new signaling paradigm. Annu. Rev. Cell Dev. Biol. 2008, 24, 55–80. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Kondo, M.; Nishimura, S.; Koshioka, M.; Tanakadate, S.; Ito, A.; Mander, L. The role of phytohormones in flowering and bolting of Spinach (Spinacia oleracea L.) under mid-night lighting. Acta Hortic. 2006, 711, 247–254. [Google Scholar] [CrossRef]
- Richards, D.E.; King, K.E.; Ait-Ali, T.; Harberd, N.P. HOW GIBBERELLIN REGULATES PLANT GROWTH AND DEVELOPMENT: A molecular genetic analysis of gibberellin signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 67–88. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, H.; Hanada, A.; Kuzuyama, T.; Takagi, M.; Kamiya, Y.; Yamaguchi, S. Contribution of the mevalonate and methylerythritol phosphate pathways to the biosynthesis of gibberellins in Arabidopsis. J. Biol. Chem. 2002, 277, 45188–45194. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.E.; Reid, J.B.; Helliwell, C.A. Cytochromes P450 in gibberellin biosynthesis. Phytochem. Rev. 2006, 5, 405–419. [Google Scholar] [CrossRef]
- Nothmann, J. Effect of growth regulator treatments on heading, bolting, spiralled leaf formation and yield performance of cos lettuce (L. var. roman). J. Hortic. Sci. 1973, 48, 379–386. [Google Scholar] [CrossRef]
- Jiang, X.M.; Dan, L.; Wang, F.J. Effects of exogenous GA3 on flower bud differentiation and flower-ball development of Broccoli (Brassica oleracea var. italica). Plant Physiol. Commun. 2008, 44, 639–642. [Google Scholar]
- Rieu, I.; Ruiz-Rivero, O.; Fernandez-Garcia, N.; Griffiths, J.; Powers, S.J.; Gong, F.; Linhartova, T.; Eriksson, S.; Nilsson, O.; Thomas, S.G.; et al. The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. Plant J. 2007, 53, 488–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, H.; Macleod, G.; Hrabchak, C.; Varmuza, S. New candidate targets of protein phosphatase-1c-gamma-2 in mouse testis revealed by a differential phosphoproteome analysis. Int. J. Androl. 2011, 34, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Li, H.Y.; Shi, Y.S.; Song, Y.C.; Wang, T.Y.; Li, Y. A maize early responsive to dehydration gene, ZmERD4, provides enhanced drought and salt tolerance in Arabidopsis. Plant Mol. Biol. Rep. 2009, 27, 542–548. [Google Scholar] [CrossRef]
- Hardie, D.G. Plant Protein Serine/Threonine Kinases: Classification and Functions. Annu. Rev. Plant Biol. 1999, 50, 97–131. [Google Scholar] [CrossRef] [PubMed]
- Rudrabhatla, P.; Rajasekharan, R. Mutational analysis of stress-responsive peanut dual specificity kinase: Identification of tyrosine residues involved in regulation of protein kinase activity. J. Biol. Chem. 2003, 278, 17328–17335. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, D.; Merchan, F.; Megias, M.; Kondorosi, A.; Sousa, C.; Crespi, M. Ankyrin protein kinases: A novel type of plant kinase gene whose expression is induced by osmotic stress. Plant Mol. Biol. 2003, 51, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Rudrabhatla, P.; Reddy, M.M.; Rajasekharan, R. Genome-wide analysis and experimentation of plant serine/threonine/tyrosine-specific protein kinases. Plant Mol. Biol. 2006, 60, 293–319. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.P.; Skipsey, M.; Edwards, R. Roles for glutathione transferases in plant secondary metabolism. Phytochemistry 2010, 71, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Møldrup, M.E.; Geu-Flores, F.; Olsen, C.E.; Halkier, B.A. Modulation of sulfur metabolism enables efficient glucosinolate engineering. BMC Biotechnol. 2011, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Bartling, D.; Radzio, R.; Steiner, U.; Weiler, E.W. A glutathione S-transferase with glutathione-peroxidase activity from Arabidopsis thaliana; molecular cloning and functional characterization. Eur. J. Biochem. 1993, 216, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.X.; Lagudah, E.S.; Moose, S.P.; Riechers, D.E. Tandemly duplicated safener-induced glutathione S-transferase genes from Triticum tausccii contribute to genome- and organ-specific expression in hexaploid wheat. Plant Physiol. 2002, 130, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.H.; Krishnamurthy, R.; Yeh, K.W. Decreased l-ascorbate content mediating bolting is mainly regulated by the galacturonate pathway in oncidium. Plant Cell Physiol. 2009, 50, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.F.; Lei, J.J.; Cao, B.H.; Chen, G.J.; Chen, C.M. cDNA-AFLP analysis on bolting or flowering of flowering Chinese cabbage and molecular characteristics of BrcuDFR-like /BrcuAXS gene. Mol. Biol. Rep. 2012, 39, 7525–7531. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.P.; Peng, J.L.; Bao, Z.L.; Meng, X.D.; Bonasera, J.M.; Chen, G.Y.; Beer, S.V.; Dong, H.S. Downstream divergence of the ethylene signaling pathway for Harpin-stimulated Arabidopsis growth and insect defense. Plant Physiol. 2004, 136, 3628–3638. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.B.; Tian, J.X.; Du, Q.Z.; Chen, J.H.; Li, Y.; Yang, X.H.; Li, B.L.; Zhang, D.Q. Association genetics and transcriptome analysis reveal a gibberellin-responsive pathway involved in regulating photosynthesis. J. Exp. Bot. 2016, 67, 3325–3338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huerta, L.; Forment, J.; Gadea, J.; Fagoaga, C.; Peña, L.; Perez-Amador, M.A.; García-Martínez, J.L. Gene expression analysis in citrus reveals the role of gibberellins on photosynthesis and stress. Plant Cell Environ. 2008, 31, 1620–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.A.; Khan, M.; Ansari, H.R. Samiullah. Auxin and defoliation effects on photosynthesis and ethylene evolution in mustard. Sci. Hortic. 2002, 96, 43–51. [Google Scholar] [CrossRef]
- Vanneste, S.; Friml, J. Auxin: A trigger for change in plant development. Cell 2009, 136, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Mcqueen-Mason, S.; Durachko, D.M.; Cosgrove, D.J. Two endogenous proteins that induce cell wall extension in plants. Plant Cell 1992, 4, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.C.; Li, L.C.; Cho, H.T.; Susanne, H.B.; Richard, C.M.; Douglas, B. The growing world of expansins. Plant Cell Physiol. 2002, 43, 1436–1444. [Google Scholar]
- Cosgrove, D.J. Loosening of plant cell walls by expansins. Nature 2000, 407, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Wittwer, S.H.; Bukovac, M.J.; Sell, H.M.; Weller, L.E. Some Effects of Gibberellin on Flowering and Fruit Setting. Plant Physiol. 1957, 32, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Ma, H.L.; Calderón-Urrea, A.; Tian, C.X.; Bai, X.M.; Wei, J.M. Anatomical changes to protect organelle integrity account for tolerance to alkali and salt stresses in Melilotus officinalis. Plant Soil 2016, 406, 327–340. [Google Scholar] [CrossRef]
- Swaczynová, J.; Novák, O.; Hauserová, E.; Fuksová, K.; Šíša, M.; Kohout, L.; Strnad, M. New techniques for the estimation of naturally occurring brassinosteroids. J. Plant Growth Regul. 2007, 26, 1–14. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Moriya, Y.; Itoh, M.; Okuda, S.; Yoshizawa, A.C.; Kanehisa, M. KAAS: An automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007, 35, 182–185. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, J.-H.; Zhang, L.-L.; Li, P.-P.; Sun, Y.-C.; Li, J.-K.; Qin, X.-X.; Wang, L.; Qi, Z.-Y.; Xiao, S.; Han, Y.-Y.; et al. Quantitative Proteomics Analysis of Lettuce (Lactuca sativa L.) Reveals Molecular Basis-Associated Auxin and Photosynthesis with Bolting Induced by High Temperature. Int. J. Mol. Sci. 2018, 19, 2967. https://doi.org/10.3390/ijms19102967
Hao J-H, Zhang L-L, Li P-P, Sun Y-C, Li J-K, Qin X-X, Wang L, Qi Z-Y, Xiao S, Han Y-Y, et al. Quantitative Proteomics Analysis of Lettuce (Lactuca sativa L.) Reveals Molecular Basis-Associated Auxin and Photosynthesis with Bolting Induced by High Temperature. International Journal of Molecular Sciences. 2018; 19(10):2967. https://doi.org/10.3390/ijms19102967
Chicago/Turabian StyleHao, Jing-Hong, Li-Li Zhang, Pan-Pan Li, Yan-Chuan Sun, Jian-Ke Li, Xiao-Xiao Qin, Lu Wang, Zheng-Yang Qi, Shuang Xiao, Ying-Yan Han, and et al. 2018. "Quantitative Proteomics Analysis of Lettuce (Lactuca sativa L.) Reveals Molecular Basis-Associated Auxin and Photosynthesis with Bolting Induced by High Temperature" International Journal of Molecular Sciences 19, no. 10: 2967. https://doi.org/10.3390/ijms19102967